Abstract
Purpose
To review comprehensively the available peer-reviewed published articles in the literature on loteprednol suspension, gel, and ointment in the treatment of ocular inflammation and pain following ocular surgery.
Methods
We conducted a PubMed literature search review of all published articles on keywords associated with loteprednol etabonate and ocular surgery.
Results
A total of 59 peer-reviewed articles were found in the literature. The focus of the majority of the articles was on the safety and efficacy of loteprednol etabonate 0.5% in postoperative control of inflammation and pain following cataract surgery. There were only three articles with a remote association between loteprednol etabonate and keratoplasty.
Conclusion
Lotemax® ointment may also have potential as a first-line anti-inflammatory agent of choice in postoperative settings of strabismus and penetrating glaucoma, and following low-risk penetrating keratoplasty procedures.
Keywords: loteprednol, inflammation, pain, keratoplasty, surgery, eye
Introduction
The history of corneal transplantation dates back over 200 years. In 1905, Eduard Zirm performed the first successful full-thickness penetrating keratoplasty (PK).1 Techniques using allografts and xenografts for full-thickness keratoplasty were first performed in the early 1800s; there was a relatively high rate of failure due to endothelial rejection. Over 100 years later, topical and systemic steroids became available. Steroids and improvement in surgical instrumentation in the 1950s led to a steady rise in the survival rates of corneal grafts. In the early 2000s, with the emergence of selective lamellar keratoplasty, the femtosecond laser, modern instrumentation, and in particular newer anti-inflammatories led to better success. All of these improvements allowed physicians and patients a more rapid postoperative recovery due to earlier removal of sutures and a reduction in the rates of corneal graft failure.2 Furthermore, we started to understand the importance of the ocular surface and the significance of healthy limbal stem cells and epithelium. This has led to a change in practice patterns, where we try to get away from preservatives and their effects on goblet cells in the conjunctiva as well as increasing the hydration of the epithelium. This led to better corneal graft success.
A number of articles were found in our literature review on corneal transplants and the use of anti-inflammatory drugs. While the focus of the articles was on reducing graft rejection, most articles discussed the relationship of pain and medications to decrease the pain. Maumenee was the first to report on the mechanism of corneal graft rejection in the 1950s.3 In that work, the mechanism of antigen-antibody reactions was described, as well as immediate and late hypersensitivity responses in an avascular corneal graft.4,5 Follow-up investigative studies analyzed immune reactions following PK. The number of immune cells and in particular macrophages, monocytes, and lymphocytes were shown to correlate directly with the severity of endothelial rejection.5,6 This is why surgeons use immunomodulating medications, particularly topical and/or systemic steroids following PKs. This clearly has improved success from the early days of corneal transplantations. Corticosteroids have a broad mechanism of action. They suppress both the early and late phases of inflammation. One method is by effectively inhibiting phospholipase A2 (used in converting membrane phospholipids to arachidonic acid) in the inflammatory cascade; arachidonic acid is a leading compound in initiation of the inflammatory response. Steroids also decreased the formation of all eicosanoids by reducing the production of cyclooxygenase and lipoxygenase. Despite their noteworthy efficacy in reducing host immune responses, first-generation steroids, ie, dexamethasone, have posed their own unique challenges to patients and physicians who have had to prescribe them routinely to patients from the early days of this procedure. Among some of these issues, exacerbation of ocular surface disease as a result of preservatives within the ocular steroid medications and steroid-induced intraocular pressure (IOP) elevation rank high on the list. Such events reduce the level of adherence to typical steroid drops.7
Nonsteroidal anti-inflammatory drugs (NSAIDs) work solely by inhibiting the cyclooxygenase enzyme and blocking prostaglandin production. NSAIDs are not used in patients following PK for reducing intraocular inflammation or pain. For many years, researchers have sought the triggering pathophysiological events and mechanisms causing rejection and pain post-PK. Increased understanding of the triggering event(s) allows clinicians to target the treatment with more specific drugs. In one such recent discovery, it was shown that majority of corneal resident stromal dendritic cells (DCs) undergo maturation by acquiring high expression of major histocompatibility complex II antigens and costimulatory molecules.8 These donor-derived DCs migrate to host cervical lymph nodes to activate host T cells via the direct pathway. Targeting either the DCs or the host T cells should help to reduce the overall host immune-response rate to the donor cornea following PK. The same authors later found the infiltration of monocular and polymorphonuclear cells may be the mechanism of corneal graft failure. To counter the monocular and polymorphonuclear cell response, an injection of α-melanocyte-stimulating hormone was shown to reduce the number of inflammatory response cells, resulting in an increased survival rate.9 Ma et al showed endostatin (from collagen XVIII), restin (from collagen XV), thrombospondin, and tissue inhibitor of metalloproteinase-3 play a significant role in a series of inflammatory cascades following PK.10 This study also discussed the integral role that healthy limbal stem cells and healthy epithelial basement membrane play in suppressing the impetus for corneal vascularization by decreasing these processes and in the restoration of corneal avascularity.10 Two other studies found corneal neovascularization and eosinophilic infiltration to be additional factors in cases of graft failure.11,12 Inhibition and reversal of such events would certainly extend the survival rate of corneal grafts.
Multiple medications targeting more than one site in the inflammatory pathway may also improve success post-PK. In a series of reports, investigators showed that combinations of two or more systemic immune suppressive agents (oral prednisone, azathioprine, and cyclosporine) led to more successful outcomes in the management of corneal graft failure.13 Other agents may also extend graft longevity or reverse graft rejection: intravenous pulse methylprednisolone, mycophenolate mofetil with Cyclosporine A, topical birch-leaf extract (Betula pendula), rapamycin, and/or systemic tacrolimus have been evaluated as possible candidates to decrease rejection.14–21 The studies showed that pathways leading to T-cell activation and interleukin 1, a potent proinflammatory cytokine, to be critical factors in initiating and maintaining intraocular inflammation following PKs.
These pioneering works helped ophthalmologists to appreciate the contribution that steroids have had in suppressing the inflammatory cascade following PKs. Nearly a half-century after ophthalmologists started to use prednisolone acetate 1% for management of rejection and pain post-PK, a survey published in 2005 by Randleman and Stulting showed that between 37% and 90% of the 396 Cornea Society members responding preferred to use prednisolone acetate 1% in when prescribing anti-inflammatory medications after PK. Another 81%–91% of the surgeons used topical prednisolone for graft rejections. Loteprednol etabonate (LE) had been introduced, and 6%–12% of respondents solely used LE when continuing steroids longer than 6 months after PK.22 Prior to this survey, a similar survey was published in 1992. Rinne and Stulting surveyed 314 members of the Castroviejo Society. Topical prednisolone acetate, alone or in combination, was shown to be the preferred prescription for the routine postoperative immunosuppressive regimen as well as in the prevention and treatment of graft-rejection cases.23 In the US, the currently available topical ophthalmic steroids include: prednisolone acetate 1% (Pred Forte®; Allergan, Irvine, CA, USA), rimexolone suspension 1% (Vexol®; Alcon Laboratories, Fort Worth, TX, USA), fluorometholone 0.1% (FML®, Allergan) and loteprednol acetate 0.2% or 0.5% suspension, gel, or ointment (Alrex® 0.2%, Lotemax® 0.5%; Bausch and Lomb Incorporated, Rochester, NY, USA). Difluprednate ophthalmic emulsion 0.05% (Durezol®; Alcon Laboratories) is also available.
Loteprednol 0.5% is a corticosteroid, like prednisolone. It differs chemically by the absence of number 20 ketone compared to prednisolone. Pivotal trials showed that it was a lower-potency steroid compared to prednisolone, but it may have a role in the routine management of ocular inflammation following PK. It also possesses properties that may offer solutions when using steroids after PK. Loteprednol gel 0.5% (and ointment) is free of preservatives and stays on the ocular surface longer than drops, so it may have a place as well for post-PK patients.
Review of pharmacokinetics and mode of action of loteprednol 0.5% (Lotemax) ointment
In April 2011, the US Food and Drug Administration (FDA) approved LE 0.5% ointment (Lotemax 0.5% ointment) for the treatment of postoperative inflammation and pain following ocular surgery (Figure 1). Ointments allow medication delivery with a base-delivery system that is free of many compounds seen in drops and gels. Additionally, they can be preservative-free. LE 0.5% ointment is preservative-free and does indeed have longer surface contact for drug delivery than drops. Lotemax ointment may have improved long-term tolerability and less epithelial toxicity because it contains mineral oil without benzalkonium chloride (BAK).24 Increased IOP as a result of topical steroids (steroid responder) is a well-documented side reaction described in the literature, which has certainly declined with the advent of Lotemax ointment or suspension.25 As a result, an increasing survival of corneal grafts may be appreciated due to a reduction in endothelial cell loss. The molecular formula of LE is C24H31ClO7 (Figure 2).26 LE is synthesized through structural modifications of prednisolone-related compounds. The ointment also contains inactive ingredients of white petrolatum, mineral oil without the preservative BAK. As a glucocorticoid compound, it inhibits prostaglandin production through several independent mechanisms and reduces the early inflammatory response by suppressing a variety of inciting agents. Additionally, glucocorticoids reduce capillary proliferation, fibroblast proliferation, collagen deposition, and cicatrization. LE is lipid-soluble (ten times greater lipophilicity than dexamethasone) and can penetrate into cells. It is an ester-based corticosteroid as opposed to a ketone-based corticosteroid (carbon-20 position is replaced with chloro methyl ester and the 17α-hydroxyl group with a carbonate moiety), such as dexamethasone or prednisolone. When the ester group is cleaved from loteprednol etabonate, it results in the formation of inactive carboxylic acid metabolites PJ-91 and PJ-90, neither of which binds to the glucocorticoid receptor. Receptor-binding studies have shown that LE has 4.3 times greater binding affinity than dexamethasone for glucocorticoid (type II) receptors and binds competitively to transcortin, a corticosteroid-binding plasma protein. The functional ester group at the C-20 of LE, instead of a ketone group, is believed to result in a reduced propensity for elevation in IOP compared to ketone corticosteroids.27,28 LE undergoes a relatively rapid transformation to an inactive metabolite, which improves its safety profile and reduces the risk of steroid response; this makes LE a good candidate for inflammatory ocular conditions.28 After installation of topical LE, research has shown that plasma levels of LE suspension and its metabolites are below the limit of quantification (1 ng/mL) following ocular administration of LE 0.5% eight times daily for 2 days and/or four times daily for 42 days.29 The study concluded that chronic exposure to LE at a concentration and frequency equal to or greater than the intended therapeutic dose does not result in detectable systemic levels or hypothalamic pituitary axis suppression. The existing literature on the pharmacokinetics and mode of LE ointment action describes these to be similar to the commercially available suspension/gel. Thus, LE ointment has unique chemical properties. It allows for lower toxicity through the inert metabolites seen after enzymatic breakdown. It has also been shown to have less likelihood for steroid response (Figure 3).42
Figure 1.
Loteprednol etabonate 0.5% ointment, Bausch and Lomb Incorporated, Rochester, NY, USA.
Figure 2.
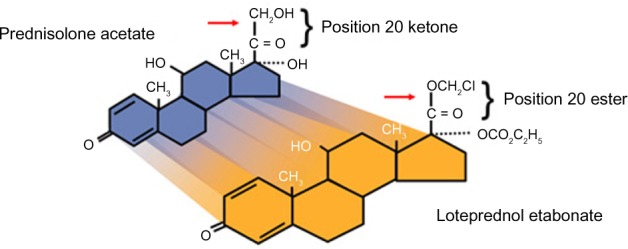
Loteprednol etabonate molecular structure: loteprednol etabonate is synthesized through structural modifications of a prednisolone-related compound.
Notes: Chemical name: chloromethyl 17α-[(ethoxycarbonyl)oxy]-11β-hydroxy-3-oxoandrosta-1,4-diene-17β-carboxylate. Active compound: loteprednol etabonate 5 mg (0.5%). Inactive compounds: mineral oil and white petrolatum.
Figure 3.
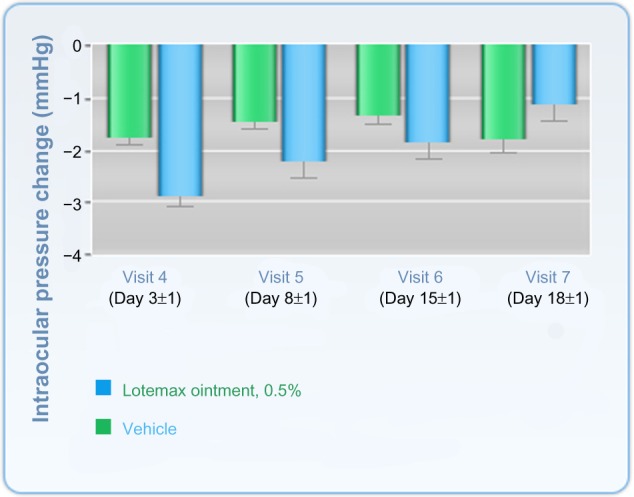
Change in intraocular pressure after treatment with Lotemax® ointment or vehicle.
Notes: Values shown are the mean ± the standard error of the mean. Lotemax 0.5%, Bausch and Lomb Incorporated, Rochester, NY, USA. Copyright © 2011. Reproduced from Dove Medical Press. Comstock TL, Paterno MR, Singh A, Erb T, Davis E. Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol. 2011;5:177–186.42
Abbreviation: Lotemax, Ioteprednol etabonate.
Review of efficacy studies
LE has been available for clinical use for more than a decade. There are three formulations of Lotemax preparations: suspension, gel, and ointment. No randomized comparative controlled study of Lotemax ointment has been published thus far. All clinical studies have investigated the efficacy of Lotemax ophthalmic suspension or the gel rather than the ointment. Loteprednol has been shown to be efficacious in the treatment of inflammation related to seasonal allergic conjunctivitis, giant papillary conjunctivitis, anterior uveitis, dry eye, and cataract surgery. A comprehensive literature review including the keywords “loteprednol etabonate” and “efficacy” (PubMed search 1991–2013) resulted in 103 articles, of which only four were related to corneal transplant surgery.7,22,29 However, corneal transplant surgery has evolved like medication options, so researchers have looked at endothelial survival after Descemet’s stripping automated endothelial keratoplasty (DSAEK).30 This study reported switching some patients to LE during the course of postoperative treatment. The authors approach was to use LE as an alternative to prednisolone acetate. The primary focus of other studies looking at the efficacy of LE was where it is used to treat ocular inflammation, the safety and level of pain control following cataract surgery, and other conditions unrelated to PK surgery (such as vernal or seasonal allergic conjunctivitis).33–36,41 There seems to be a change in practice patterns where corneal surgeons use LE as an option for post-PK and -DSAEK patients. At the same time, we are seeing a movement away from preserved drops in the clinical setting, so the use of LE ointment is occurring, but we are unable to provide any direct citations on the efficacy or safety of Lotemax ointment in treatment of inflammation and pain following PK. However, Lotemax suspension or gel comparative efficacy studies on the control of inflammation and pain following other forms of intraocular surgeries can be indirectly attributed to Lotemax ointment, as it contains the same active ingredient without BAK preservative.
For studies published on Lotemax gel or suspension, we found a multicenter investigator-masked randomized trial by Lane and Holland showing the LE 0.5% (Lotemax) suspension to be equivalent to prednisolone acetate 1.0% (Pred Forte) in control of inflammation following cataract surgery. The efficacy of LE was examined in 88 patients with a 3-week follow-up; there was less IOP fluctuation on days 1 and 3.31 Statistical significance was not published. Both LE and prednisolone acetate medications were well tolerated by patients. In the pivotal FDA trials looking at postoperative patients in a randomized, double-masked, vehicle-controlled evaluation of the efficacy and safety of LE after cataract surgery, the resolution of anterior chamber inflammation was observed in 55% of 203 patients in the LE group and in 28% of the vehicle group (P<0.001) after 14 days. No adverse outcome was observed in either group, and no IOP elevation was noted in the LE group.31 In another randomized study by Stewart et al to evaluate the efficacy and safety of LE drops in controlling ocular inflammation, effective resolution of anterior chamber inflammation was found in 64% of patients in the LE group and in 29% of patients in the control (vehicle) group.32 In evaluating the efficacy of LE in the management of pain following cataract surgery, Comstock and Usner showed 84% control of pain for the LE group and 56% for the placebo group (P<0.005). In controlling discomfort, their study showed 79% efficacy for the LE group and 42% for the placebo group.33 In a prospective randomized study to evaluate the efficacy of LE gel 0.5% in controlling pain and inflammation, Fong et al showed 31.1% of LE-treated patients and 13.9% of vehicle-treated patients had complete resolution of anterior chamber cells at 8 days. There were 407 patients in the study. No adverse reactions to medications were noted. Furthermore, the authors showed 75.7% of LE-treated patients and 45.8% of vehicle-treated patients had no pain (grade 0).34 In comparative studies evaluating LE suspension 0.5%, other corticosteroids, or NSAIDs, Stewart found no statistically significant difference in efficacy between the LE- and fluorometholone acetate 0.1%-treated groups. This was a randomized double-masked study of 30 postoperative cataract patients looking at the resolution of the anterior segment inflammation. No adverse outcome was observed in either group.35 In another randomized comparative study, Oner et al looked at the efficacy of LE 0.5% suspension versus FML 0.1% versus prednisolone acetate 1% (PF 1%) in the treatment of vernal keratoconjunctivitis. The authors showed no statistically significant differences in outcome measures between the LE and PF groups for the 57 enrolled patients. The FML-treated group showed less improvement than the LE and PF groups. The study also showed statistically significant IOP elevation after the third day of treatment with PF. The authors concluded LE was as effective as PF and more effective than FML in treatment of vernal keratoconjunctivitis without side effects.36 Holzer et al published a prospective randomized comparative study of LE suspension 0.5% and ketorolac tromethamine ophthalmic solution 0.5% for treatment of inflammation after cataract surgery, and showed no statistically significant difference in any final outcome parameters between the two groups up to 30 days postoperatively. Postoperative pain was not assessed. The authors noted no adverse side effects in either group.37 A more recent multicenter, randomized, double-masked study by Rajpal et al evaluated the efficacy of LE gel 0.5% compared to vehicle for resolving inflammation and pain following cataract surgery. The authors showed LE gel had superior efficacy in reducing anterior chamber cells and flare in patients following cataract surgery in comparison to the vehicle. The authors concluded that LE gel 0.5% would likely be more suitable in treatment of ocular inflammation following ocular surgery. Because of formulation improvements, including no preservatives and a more physiological pH, the authors felt it may provide added benefit to patients.38 We were unable to identify any publications supporting Lotemax (in any form) as the preferred choice of ocular inflammation following PK surgeries. The Randleman and Stulting survey showed that of the 396 corneal transplant surgeons participating, 37%–90% used topical prednisolone as their primary or preferred routine anti-inflammatory drug of choice following PK. Also, 81%–91% used topical prednisolone acetate 1% (PF) for various manifestations of graft rejection at all time points. Lotemax suspension was used in 12%–26% of cases. That study, as well as others, concluded that topical prednisolone remained the mainstay for the prevention and treatment of corneal graft rejection; however, they pointed out that the role of LE seems to have been expanding.22,39 In a more recent retrospective study of 30 patients by Holland et al to evaluate for a clinical reduction in IOP in known steroid responders using LE 0.5% ophthalmic suspension as second-line rescue therapy after corneal transplantation, the authors concluded that switching to LE from PF was successful in reducing IOP. Reduction of IOP was noted in all 30 patients who were switched from PF to LE. The authors concluded that switching to LE from PF was successful in reducing IOP. The study showed no increase in the rate of allograft rejection. Additionally, they recommended that physicians consider LE 0.5% in the prophylaxis of allograft rejection in steroid responders, due to its lower potential for causing elevated IOP.40 In a prospective randomized multi-centered double-masked trial, Rajpal et al compared LE gel 0.5% to a vehicle and showed LE gel 0.5% to be efficacious and safe in treating postoperative inflammation and pain. The study included 203 patients followed for 18 days after cataract surgery.41 A total of four treatment-emergent serious adverse events were noted during the study; none was felt related to the study drug. In both treatment groups, the mean IOP remained stable, and no cases of steroid response were observed. Finally, in a large randomized, double-masked, parallel-group, vehicle-controlled study of LE, Comstock et al published data on 805 patients assessed for safety and efficacy of LE ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. The study showed LE ointment to be both efficacious and well tolerated in the treatment of ocular inflammation and pain following cataract surgery.42 Mean IOP for all study eyes in both treatment arms (LE and prednisolone) was consistently lower than baseline at all posttreatment visits. This is not surprising, as many studies have shown decreased IOP after cataract surgery.43 One patient in the LE ointment-treated group experienced cystoid macular edema, requiring the patient to exit from the study. The authors, in their conclusion, asserted that the integrated results of all published studies on LE ointment show the medication to be effective and well tolerated in the treatment of postoperative inflammation and pain following ocular surgery.42 Tables 1 and 2 summarize the available published literature on efficacy of Lotemax and intraocular inflammation.
Table 1.
Published clinical studies on Lotemax® and penetrating keratoplasty
| Investigators | Year | Journal | Topic | Design |
|---|---|---|---|---|
| Randleman and Stulting22 | 2006 | Cornea | Corneal graft and steroid | Survey |
| Erdmus et al7 | 2009 | Cornea | IOP and steroid | Retrospective chart review |
| Holland et al40 | 2009 | Cornea | IOP and steroid | Retrospective chart review |
Note: Lotemax, Bausch and Lomb Incorporated, Rochester, NY, USA.
Abbreviation: IOP, intraocular pressure.
Table 2.
Comparative studies on efficacy and safety of Lotemax® 0.5% in resolution of postoperative inflammation
| Investigators | Journal | Year | Total patients | Follow-up | Comparative drug |
|---|---|---|---|---|---|
| Lane and Holland31 | J Cataract Refract Surg | 2013 | 88 | 21 days | PF 1.0% |
| Stewart et al32 | J Cataract Refract Surg | 1998 | 227 | 14 days | Placebo |
| Fong et al34 | Clin Ophthalmol | 2012 | 407 | 14 days | Vehicle |
| Oner et al36 | Jpn J Ophthalmol | 2012 | 60 | 28 days | PF 1.0% and FML 0.1% |
| Holzer et al37 | J Cataract Refract Surg | 2002 | 60 | 30 days | Acular 0.5% |
| Rajpal et al41 | J Cataract Refract Surg | 2013 | 406 | 14 days | Vehicle |
| Comstock et al42 | Clin Ophthalmol | 2011 | 805 | 8 days | Vehicle |
Note: Lotemax, Bausch and Lomb Incorporated, Rochester, NY, USA.
Abbreviations: PF, prednisolone acetate; FML, fluorometholone.
Safety and tolerability of loteprednol ointment
The safety and tolerability profile of Lotemax has been evaluated primarily as a 0.5% ophthalmic suspension (New Drug Application 20–583) in clinical trials. In a clinical evaluation of LE ointment, Comstock et al concluded that the preservative-free ointment formulations of LE may provide a safety advantage over FML ointment and allow physicians a choice of dosage forms in treating ocular inflammation following ocular surgery.42 Howes reported that LE is a corticosteroid designed using the “soft drug” concept of Bodor, and in comparison with other steroids, LE has a superior safety profile, which has been attributed to its soft-drug characteristics.44 Soft drugs are new therapeutic agents that undergo predictable metabolism to inactive metabolites after exerting their therapeutic effect. Hence, they are obtained by building into the molecule, in addition to the activity, the most desired way in which the molecule is to be deactivated and detoxified.30 During early phase FDA toxicity evaluation, rabbits and dogs were followed for 28 days; there were no significant toxicity findings, except for the transient irregular aspect of the ocular surface (in both treated and control groups), felt to be caused by the viscous consistency of the ointment vehicle. No carcinogenic studies have been conducted for LE. LE has not been studied in pregnant or nursing women, but has been found to be teratogenic in animals. As a result, LE should not be used in pregnant or nursing women unless the benefits to the mother clearly outweigh the risk to the fetus or the nursing child. In animal studies, oral administration of LE did not result in fetal growth retardation. The safety of LE ointment in pediatric patients has not been established, and it should be used with caution, as it may interfere with amblyopia treatment. Two large prospective clinical studies evaluated the safety of LE ointment for the FDA clinical analysis (clinical studies 52532 and 52655). When both studies were pooled to create a common database of 405 subjects, analysis for safety was done, and the most common ocular adverse events were anterior chamber inflammation, corneal edema, photophobia, conjunctival hyperemia, eye pain, and iritis. These same events happened with the placebo or vehicle; however, LE had a lower frequency of these events. The studies showed LE was well tolerated by patients receiving the medication. In individuals treated for 28 days or longer with LE suspension, the incidence of significant elevation of IOP ($10 mmHg) was 2% among patients receiving LE and 0.5% among patients receiving placebo. Clinical trials did not show any significant difference in occurrence of serious and nonserious systemic adverse events between LE and placebo. Randomized controlled trials comparing active corticosteroid components looked at the cost-effectiveness of loteprednol compared to other commonly used steroids in the management of ocular inflammation occurring as postoperative inflammation, acute anterior uveitis, giant papillary conjunctivitis, or seasonal allergic conjunctivitis. Forte showed that the total cost of treating ocular inflammation was higher in the prednisolone arm due to the higher probability of elevated IOP and the resources consumed in its management.45 Druzgala et al showed that the levels of LE and its metabolites were highest in the cornea, and so was the ratio of metabolites to unchanged drug, suggesting that the primary site of deactivation of the drug and systemic presence of the drug would be minimal.46 In their review article, Amon and Busin concluded that LE had similar efficacy to rimexolone and difluprednate by offering similar rates in the resolution of ocular inflammation with fewer clinically significant increases in IOP ($10 mmHg); thus, they found LE was a clinically safer ophthalmic corticosteroid.47 Concern continues today, as over the last few decades, about causing significant and intractable steroid-related IOP elevation following PK. When grafts done for a variety of conditions, such as Fuchs’ corneal dystrophy or keratoconus (KCN), need immunosuppression and when IOP rises, it confounds the ability to use pressure-rising steroids, which would inevitably lead to a choice between corneal graft failure or visual field loss. In a retrospective review of 100 patients who underwent PK, Erdurmus et al found that steroid-induced IOP elevation or glaucoma after PK was not unusual in eyes with KCN or Fuchs’ dystrophy (73% in the KCN group and 60.3% in the Fuchs’ dystrophy group).7 LE would be an ideal alternative steroid in such cases. Applying ophthalmic ointment immediately following penetrating intraocular surgery raises some concern. Teaching patients or companions how to apply the ointment is also time-consuming, and doctors vary in how they have patients apply the medication. Additionally, there is a concern about the inadvertent entrance of the ointment into the anterior chamber.48 Optimal care must be taken to have a watertight closure of the incision sites and to instruct the patients to not rub their eyes in cases where LE ointment is considered following the surgery.49 Numerous reports of intraocular ophthalmic ointments have recently appeared in the literature linking the presence of the ointment with the initiation of toxic anterior segment syndrome, glaucoma, and recurrent uveitis.49–54
Patients’ perspectives on loteprednol use
There are no publications on how satisfied patients are when using Lotemax ointment. However, in a two-arm study published in 2013 evaluating the efficacy of Lotemax gel used for treatment of postoperative ocular inflammation and pain, Rajpal et al reported that 85% of patients reported no discomfort on instillation of the gel.41 Furthermore, the study showed that the majority of the 406 patients in both treatment groups had no dryness, itching, or discharge. The authors felt moving away from shaking the bottle (in order to resuspend the drug particles) may increase compliance. The nonsettling nature of the gel formulation as well as the ointment would eliminate the need to shake the bottle.45 A reduced level of BAK and more physiological pH of LE gel resulted in improved patient tolerance, enhanced comfort, and better overall satisfaction. Similarly, Lotemax ointment does not contain BAK, and may provide even further ocular surface tolerance and comfort by containing mineral oil and white petrolatum, an ideal medication for treatment of ocular inflammation and ocular surface disease following PK procedures. As with other ocular ointment formulations containing mineral oil, a transient reduction in visual acuity is expected, and patients need to be informed of such an expected side effect following application of the ointment.
Conclusion
Place in therapy
Following a comprehensive review of published articles on Lotemax 0.5% suspension, gel, and ointment, our conclusion is as follows. The clinical application of Lotemax ointment offers significant advantage over other currently available steroid medications in the treatment of mild-to-moderate ocular inflammatory conditions. Lotemax ointment should be considered as a first-line therapeutic agent in the treatment of ocular inflammation in adult or pediatric patients following cataract surgery when the patients suffer from comorbid ocular surface disease, chronic ocular allergy, dry eyes, or have a history of steroid response or ocular hypertension. The safety and efficacy of the anti-inflammatory application of this drug has well been established in the literature. Lotemax ointment may also have potential as a first-line anti-inflammatory agent of choice in postoperative settings of strabismus, penetrating glaucoma, and following low-risk PK procedures. Lotemax ointment may also have a significant role where patients have arthritis or a tremor and cannot place drops easily. In clinical settings, where steroid ointment needs to be applied chronically in children or low-vision individuals, utmost care should be taken to avoid the onset of amblyopia and adverse health outcomes. Future double-masked randomized clinical trials will establish the safety and efficacy of Lotemax ointment in the treatment of postoperative inflammation following strabismus, glaucoma, and PK surgeries, and will corroborate our asserted clinical application of this drug.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bock J. The jubilee of the first successful optic keratoplasty by Eduard Zim. Wien Klin Wochenschr. 1958;70:381–383. German. [PubMed] [Google Scholar]
- 2.Farid M, Pirouzian A, Steinert RF. Femtosecond laser keratoplasty. Int Ophthalmol Clin. 2013;53:55–64. doi: 10.1097/IIO.0b013e318272d44a. [DOI] [PubMed] [Google Scholar]
- 3.Maumenee AE. Clinical aspects of the corneal homograft reaction. Invest Ophthalmol. 1962;1:244–252. [PubMed] [Google Scholar]
- 4.Parks JJ, Leibowitz HM, Maumenee AE. Immediate hypersensitivity reactions in the cornea of the guinea pig. J Immunol. 1962;89:323–325. [PubMed] [Google Scholar]
- 5.Germuth FG, Maumenee AE, Senterfit LB, Pollack AD. Immunohistologic studies on antigen-antibody reactions in the avascular cornea: I. Reactions in rabbits actively sensitized to foreign protein. J Exp Med. 1962;115:919–928. doi: 10.1084/jem.115.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhard T, Böcking A, Pomjanski N, Sundmacher R. Immune cells in the anterior chamber of patients with immune reactions after penetrating keratoplasty. Cornea. 2002;21:56–61. doi: 10.1097/00003226-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Erdurmus M, Cohen EJ, Yildiz EH, et al. Steroid-induced intraocular pressure elevation or glaucoma after penetrating keratoplasty in patients with keratoconus or Fuchs dystrophy. Cornea. 2009;28:759–764. doi: 10.1097/ICO.0b013e3181967318. [DOI] [PubMed] [Google Scholar]
- 8.Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- 9.Hamrah P, Haskova Z, Taylor AW, Zhang Q, Ksander BR, Dana MR. Local treatment with alpha-melanocyte stimulating hormone reduces corneal allorejection. Transplantation. 2009;88:180–187. doi: 10.1097/TP.0b013e3181ac11ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma DH, Chen HC, Lai JY, et al. Matrix revolution: molecular mechanism for inflammatory corneal neovascularization and restoration of corneal avascularity by epithelial stem cell transplantation. Ocul Surf. 2009;7:128–144. doi: 10.1016/s1542-0124(12)70308-7. [DOI] [PubMed] [Google Scholar]
- 11.Vassileva PI, Hergeldzhieva TG. Avastin use in high risk corneal transplantation. Graefes Arch Clin Exp Ophthalmol. 2009;247:1701–1706. doi: 10.1007/s00417-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 12.Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ, Larkin DF. Effect of allergic conjunctival inflammation on the allogeneic response to donor cornea. Invest Ophthalmol Vis Sci. 2007;48(9):4044–4049. doi: 10.1167/iovs.06-0973. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen P, Barte F, Shinada S, Yiu SC. Management of corneal graft rejection – a case series report and review of the literature. J Clin Exp Ophthalmol. 2010;1:1000103. doi: 10.4172/2155-9570.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill JC, Ivey A. Corticosteroids in corneal graft rejection: double versus single pulse therapy. Cornea. 1994;13:383–388. doi: 10.1097/00003226-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Wacker K, Gründemann C, Kern Y, et al. Inhibition of corneal inflammation following keratoplasty by birch leaf extract. Exp Eye Res. 2012;97:24–30. doi: 10.1016/j.exer.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Sundmacher R, Reinhard T, Heering P. Six years’ experience with systemic cyclosporin A prophylaxis in high-risk perforating keratoplasty patients. A retrospective study. Ger J Ophthalmol. 1992;1:432–436. [PubMed] [Google Scholar]
- 17.Stanojlovic S, Schlickeiser S, Appelt C, et al. Influence of combined treatment of low dose rapamycin and cyclosporin A on corneal allograft survival. Graefes Arch Clin Exp Ophthalmol. 2010;248:1447–1456. doi: 10.1007/s00417-010-1420-z. [DOI] [PubMed] [Google Scholar]
- 18.Dana MR, Yamada J, Streilein JW. Topical interleukin 1 receptor antagonist promotes corneal transplant survival. Transplantation. 1997;63:1501–1507. doi: 10.1097/00007890-199705270-00022. [DOI] [PubMed] [Google Scholar]
- 19.Goslings WR, Yamada J, Dana MR, et al. Corneal transplantation in antibody-deficient hosts. Invest Ophthalmol Vis Sci. 1999;40:250–253. [PubMed] [Google Scholar]
- 20.Yamada J, Dana MR, Zhu SN, Alard P, Streilein JW. Interleukin 1 receptor antagonist suppresses allosensitization in corneal transplantation. Arch Ophthalmol. 1998;116:1351–1357. doi: 10.1001/archopht.116.10.1351. [DOI] [PubMed] [Google Scholar]
- 21.Dana MR, Dai R, Zhu S, Yamada J, Streilein JW. Interleukin-1 receptor antagonist suppresses Langerhans cell activity and promotes ocular immune privilege. Invest Ophthalmol Vis Sci. 1998;39:70–77. [PubMed] [Google Scholar]
- 22.Randleman BJ, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns. Cornea. 2006;25:286–290. doi: 10.1097/01.ico.0000178731.42187.46. [DOI] [PubMed] [Google Scholar]
- 23.Rinne JR, Stulting RD. Current practices in the prevention and treatment of corneal graft rejection. Cornea. 1992;11:326–328. doi: 10.1097/00003226-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Wang TJ, Wang IJ, Ho JD, Chou HC, Lin SY, Huang MC. Comparison of the clinical effects of carbomer-based lipid-containing gel and hydroxypropyl-guar gel artificial tear formulations in patients with dry eye syndrome: a 4-week, prospective, open-label, randomized, parallel-group, noninferiority study. Clin Ther. 2010;32:44–52. doi: 10.1016/j.clinthera.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Razeghinejad MR, Katz LJ. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012;47(2):66–80. doi: 10.1159/000328630. [DOI] [PubMed] [Google Scholar]
- 26.Rachwal S, Druzgala P, Liu ZZ, Vlasak J, Brewster ME, Pop E. Chemistry of loteprednol etabonate and related steroids. II. Reactions at ring C and NMR structural studies of the resulting compounds. Steroids. 1998;63:193–201. doi: 10.1016/s0039-128x(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 27.Druzgala P, Hochhaus G, Bodor N. Soft drugs – 10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate. J Steroid Biochem Mol Biol. 1991;38:149–154. doi: 10.1016/0960-0760(91)90120-t. [DOI] [PubMed] [Google Scholar]
- 28.Albert M, Wu WM, Winwood D, Bodor N. Lipophilicity, solubility and permeability of loteprednol etabonate: a novel, soft anti-inflammatory steroid. J Biopharm Sci. 1991;2:115–125. [Google Scholar]
- 29.Howes J, Novack GD. Failure to detect systemic levels, and effects of loteprednol etabonate and its metabolite, PJ-91, following chronic ocular administration. J Ocul Pharmacol Ther. 1998;14:153–158. doi: 10.1089/jop.1998.14.153. [DOI] [PubMed] [Google Scholar]
- 30.Bodor N, Bauchwald P. Soft drug design: general principles and recent applications. Med Res Rev. 2000;20:58–101. doi: 10.1002/(sici)1098-1128(200001)20:1<58::aid-med3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 31.Lane SS, Holland EJ. Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery. J Cataract Refract Surg. 2013;39:168–173. doi: 10.1016/j.jcrs.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Stewart R, Horwitz B, Howes J, Novack GD, Hart K. Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Inflammation Study Group 1. J Cataract Refract Surg. 1998;24:1480–1489. doi: 10.1016/s0886-3350(98)80170-3. [DOI] [PubMed] [Google Scholar]
- 33.Comstock TL, Usner DW. Effect of loteprednol etabonate ophthalmic suspension 0.5% on post-operative pain and discomfort. E-abstract. 2010; American Society of Cataract and Refractive Surgery Symposium; Boston Convention and Exhibition Center, Boston, MA, USA. April 9–14, 2010. [Google Scholar]
- 34.Fong R, Leitritz M, Siou-Mermet R, Erb T. Loteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicenter trial. Clin Ophthalmol. 2012;6:1113–1124. doi: 10.2147/OPTH.S32643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart RS. Controlled evaluation of fluorometholone acetate and loteprednol etabonate in the treatment of postoperative inflammation following cataract surgery. Invest Ophthalmol Vis Sci. 2004;45(Suppl 1):U159. [Google Scholar]
- 36.Oner V, Türkcü FM, Taş M, Alakuş MF, Işcan Y. Topical loteprednol etabonate 0.5% for treatment of vernal keratoconjunctivitis: efficacy and safety. Jpn J Ophthalmol. 2012;56:312–318. doi: 10.1007/s10384-012-0152-5. [DOI] [PubMed] [Google Scholar]
- 37.Holzer MP, Solomon KD, Sandoval HP, Vroman DT. Comparison of ketorolac tromethamine 0.5% and loteprednol etabonate 0.5% for inflammation after phacoemulsification: prospective randomized double-masked study. J Cataract Refract Surg. 2002;28:93–99. doi: 10.1016/s0886-3350(01)01185-3. [DOI] [PubMed] [Google Scholar]
- 38.Rajpal RK, Siou-Mermet R, Erb T, Comstock T. Resolution of anterior chamber cells and flare with loteprednol etabonate 0.5% gel: new treatment for post-cataract inflammation and pain. E-abstract 1536057. 2013; American Society of Cataract and Refractive Surgery Symposium; Moscone Center/SF Marriott Marquis, San Francisco, CA, USA. April 19–23, 2013. [Google Scholar]
- 39.Barker NH, Henderson TR, Ross CA, Coster DJ, Williams KA. Current Australian practice in the prevention and management of corneal allograft rejection. Clin Experiment Ophthalmol. 2000;28:357–360. doi: 10.1046/j.1442-9071.2000.00335.x. [DOI] [PubMed] [Google Scholar]
- 40.Holland EJ, Djalilian AR, Sanderson JP. Attenuation of ocular hypertension with the use of topical loteprednol etabonate 0.5% in steroid responders after corneal transplantation. Cornea. 2009;28:1139–1143. doi: 10.1097/ICO.0b013e3181a3c52f. [DOI] [PubMed] [Google Scholar]
- 41.Rajpal RK, Roel L, Siou-Mermet R, Erb T. Efficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2013;39:158–167. doi: 10.1016/j.jcrs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Comstock TL, Paterno MR, Singh A, Erb T, Davis E. Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol. 2011;5:177–186. doi: 10.2147/OPTH.S16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34:735–742. doi: 10.1016/j.jcrs.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 44.Howes JF. Loteprednol etabonate: a review of ophthalmic clinical studies. Pharmazie. 2000;55:178–183. [PubMed] [Google Scholar]
- 45.Forte L. Economic evaluation of loteprednol etabonate versus prednisolone in treatment of ocular inflammation. Poster abstract #738320. 2010; American Society of Cataract and Refractive Surgery Symposium; Boston Convention and Exhibition Center, Boston, MA, USA. April 9–14, 2010. [Google Scholar]
- 46.Druzgala P, Wu WM, Bodor N. Ocular absorption and distribution of loteprednol etabonate, a soft steroid, in rabbit eyes. Curr Eye Res. 1991;10:933–937. doi: 10.3109/02713689109020329. [DOI] [PubMed] [Google Scholar]
- 47.Amon M, Busin M. Loteprednol etabonate ophthalmic suspension 0.5%: efficacy and safety for postoperative anti-inflammatory use. Int Ophthalmol. 2012;32:507–517. doi: 10.1007/s10792-012-9589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humayun M, Gottlieb CC MD, Rafuse PE. Intraocular ophthalmic ointment following clear corneal phacoemulsification: clinical implications. J Cataract Refract Surg. 2006;32:2135–2138. doi: 10.1016/j.jcrs.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 49.Aralikatti AK, Needham AD, Lee MW, Prasad S. Entry of antibiotic ointment into the anterior chamber after uneventful phacoemulsification. J Cataract Refract Surg. 2003;29:595–597. doi: 10.1016/s0886-3350(02)01503-1. [DOI] [PubMed] [Google Scholar]
- 50.Riedl M, Maca S, Amon M, Nennadal T, Kruger A, Barisani T. Intraocular ointment after small-incision cataract surgery causing chronic uveitis and secondary glaucoma. J Cataract Refract Surg. 2003;29:1022–1025. doi: 10.1016/s0886-3350(03)00060-9. [DOI] [PubMed] [Google Scholar]
- 51.Chen KH, Lin SY, Li MJ, Cheng WT. Retained antibiotic ophthalmic ointment on an intraocular lens 34 months after sutureless cataract surgery. Am J Ophthalmol. 2005;139:743–745. doi: 10.1016/j.ajo.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 52.Wong JG, Bank A. Surgical removal of intraocular antibiotic ointment after routine cataract phacoemulsification. J Cataract Refract Surg. 2006;32:890–892. doi: 10.1016/j.jcrs.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 53.Chew JJL, Werner L, Mackman G, Mamalis N. Late opacification of a silicone intraocular lens caused by ophthalmic ointment. J Cataract Refract Surg. 2006;32:341–346. doi: 10.1016/j.jcrs.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 54.Werner L, Sher JH, Taylor JR, et al. Toxic anterior segment syndrome and possible association with ointment in the anterior chamber following cataract surgery. J Cataract Refract Surg. 2006;32:227–235. doi: 10.1016/j.jcrs.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 55.The Loteprednol Etabonate Postoperative Inflammation Study Group 2 A double-masked, placebo controlled evaluation of 0.5% loteprednol etabonate in the treatment of post-operative inflammation. Ophthalmology. 1998;105:1780–1786. doi: 10.1016/s0161-6420(98)99054-6. [DOI] [PubMed] [Google Scholar]


