Abstract
Translation termination requires two codon-specific polypeptide release factors in prokaryotes and one omnipotent factor in eukaryotes. Sequences of 17 different polypeptide release factors from prokaryotes and eukaryotes were compared. The prokaryotic release factors share residues split into seven motifs. Conservation of many discrete, perhaps critical, amino acids is observed in eukaryotic release factors, as well as in the C-terminal portion of elongation factor (EF) G. Given that the C-terminal domains of EF-G interacts with ribosomes by mimicry of a tRNA structure, the pattern of conservation of residues in release factors may reflect requirements for a tRNA-mimicry for binding to the A site of the ribosome. This mimicry would explain why release factors recognize stop codons and suggests that all prokaryotic and eukaryotic release factors evolved from the progenitor of EF-G.
Full text
PDF
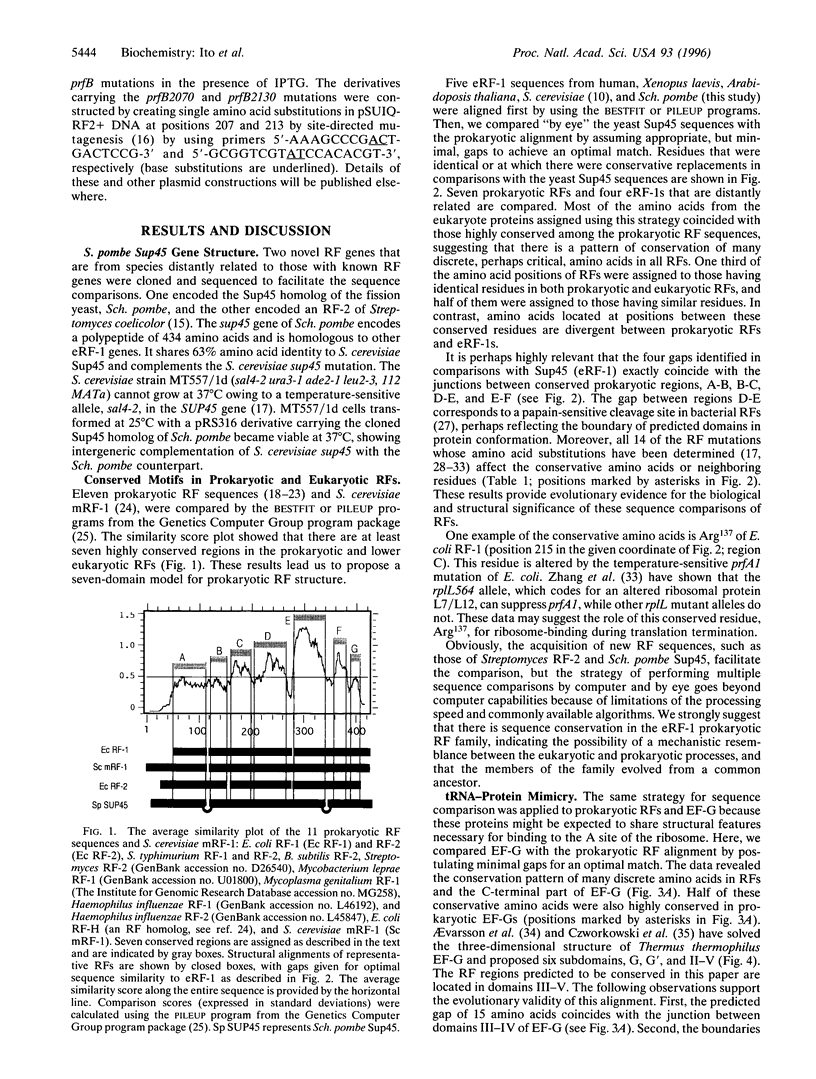

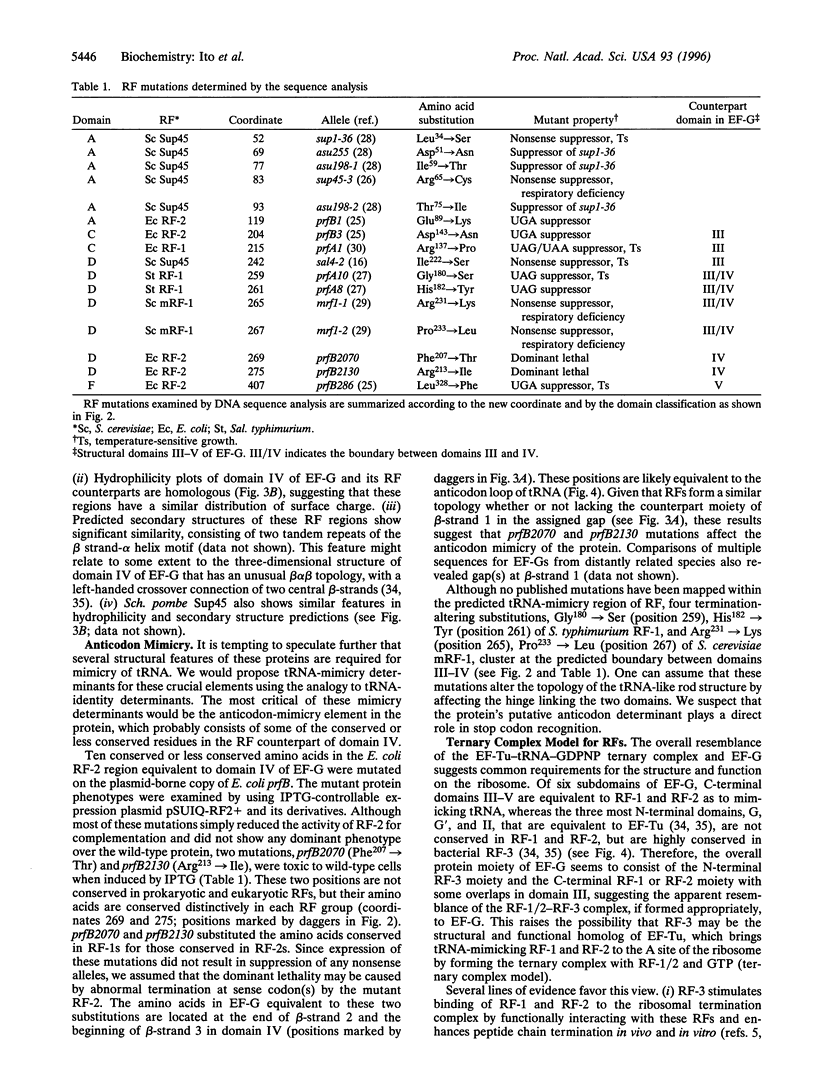
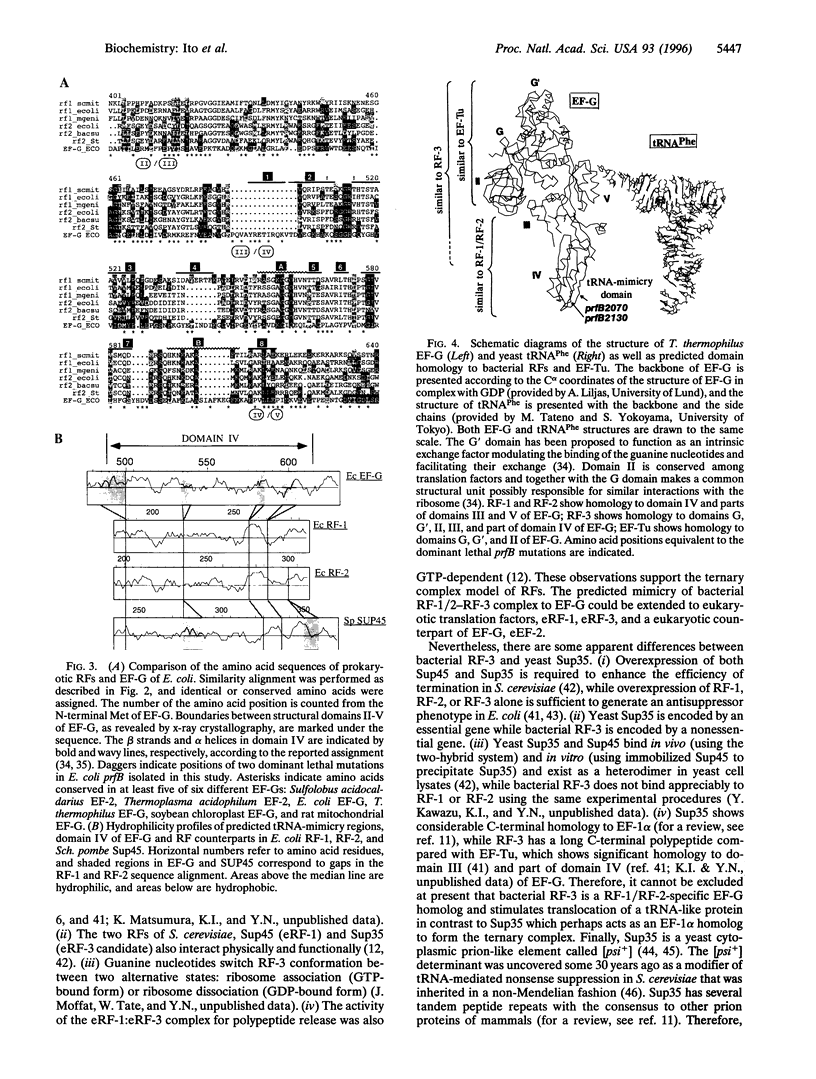

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AEvarsson A., Brazhnikov E., Garber M., Zheltonosova J., Chirgadze Y., al-Karadaghi S., Svensson L. A., Liljas A. Three-dimensional structure of the ribosomal translocase: elongation factor G from Thermus thermophilus. EMBO J. 1994 Aug 15;13(16):3669–3677. doi: 10.1002/j.1460-2075.1994.tb06676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breining P., Piepersberg W. Yeast omnipotent supressor SUP1 (SUP45): nucleotide sequence of the wildtype and a mutant gene. Nucleic Acids Res. 1986 Jul 11;14(13):5187–5197. doi: 10.1093/nar/14.13.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Klein H. A. Characterization of three proteins involved in polypeptide chain termination. Cold Spring Harb Symp Quant Biol. 1969;34:469–477. doi: 10.1101/sqb.1969.034.01.053. [DOI] [PubMed] [Google Scholar]
- Caskey T., Scolnick E., Tompkins R., Goldstein J., Milman G. Peptide chain termination, codon, protein factor, and ribosomal requirements. Cold Spring Harb Symp Quant Biol. 1969;34:479–488. doi: 10.1101/sqb.1969.034.01.054. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Cook R. G., Tate W. P., Caskey C. T. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3616–3620. doi: 10.1073/pnas.82.11.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W. J., Lee C. C., Caskey C. T. Recent advances in peptide chain termination. Mol Microbiol. 1990 Jun;4(6):861–865. doi: 10.1111/j.1365-2958.1990.tb00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czworkowski J., Wang J., Steitz T. A., Moore P. B. The crystal structure of elongation factor G complexed with GDP, at 2.7 A resolution. EMBO J. 1994 Aug 15;13(16):3661–3668. doi: 10.1002/j.1460-2075.1994.tb06675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doel S. M., McCready S. J., Nierras C. R., Cox B. S. The dominant PNM2- mutation which eliminates the psi factor of Saccharomyces cerevisiae is the result of a missense mutation in the SUP35 gene. Genetics. 1994 Jul;137(3):659–670. doi: 10.1093/genetics/137.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna J. A., Cech T. R., Sullenger B. A. Selection of an RNA molecule that mimics a major autoantigenic epitope of human insulin receptor. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2355–2359. doi: 10.1073/pnas.92.6.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. Cloning, genetic characterization, and nucleotide sequence of the hemA-prfA operon of Salmonella typhimurium. J Bacteriol. 1989 Jul;171(7):3948–3960. doi: 10.1128/jb.171.7.3948-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Wang X. Salmonella typhimurium prfA mutants defective in release factor 1. J Bacteriol. 1991 Jul;173(13):4144–4154. doi: 10.1128/jb.173.13.4144-4154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R. D., Adams M. D., White O., Clayton R. A., Kirkness E. F., Kerlavage A. R., Bult C. J., Tomb J. F., Dougherty B. A., Merrick J. M. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995 Jul 28;269(5223):496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- Fraser C. M., Gocayne J. D., White O., Adams M. D., Clayton R. A., Fleischmann R. D., Bult C. J., Kerlavage A. R., Sutton G., Kelley J. M. The minimal gene complement of Mycoplasma genitalium. Science. 1995 Oct 20;270(5235):397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- Frolova L., Le Goff X., Rasmussen H. H., Cheperegin S., Drugeon G., Kress M., Arman I., Haenni A. L., Celis J. E., Philippe M. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature. 1994 Dec 15;372(6507):701–703. doi: 10.1038/372701a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Caskey C. T. Peptide chain termination: effect of protein S on ribosomal binding of release factors. Proc Natl Acad Sci U S A. 1970 Oct;67(2):537–543. doi: 10.1073/pnas.67.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grentzmann G., Brechemier-Baey D., Heurgue V., Mora L., Buckingham R. H. Localization and characterization of the gene encoding release factor RF3 in Escherichia coli. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5848–5852. doi: 10.1073/pnas.91.13.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Jönsson Y. H., Björk G. R., Ikeda H., Nakamura Y. Chromosomal location and structure of the operon encoding peptide-chain-release factor 2 of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5620–5624. doi: 10.1073/pnas.85.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Nakamura Y. Autogenous suppression of an opal mutation in the gene encoding peptide chain release factor 2. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8432–8436. doi: 10.1073/pnas.87.21.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazu Y., Ito K., Matsumura K., Nakamura Y. Comparative characterization of release factor RF-3 genes of Escherichia coli, Salmonella typhimurium, and Dichelobacter nodosus. J Bacteriol. 1995 Oct;177(19):5547–5553. doi: 10.1128/jb.177.19.5547-5553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D. S., Aune K. C., Tate W., Caskey C. T. Characterization of reticulocyte release factor. J Biol Chem. 1977 Jul 10;252(13):4514–4520. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni O., Ito K., Moffat J., Matsumura K., McCaughan K., Nobukuni T., Tate W., Nakamura Y. Identification of the prfC gene, which encodes peptide-chain-release factor 3 of Escherichia coli. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5798–5802. doi: 10.1073/pnas.91.13.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni O., Kawakami K., Nakamura Y. Sequence and functional analysis of mutations in the gene encoding peptide-chain-release factor 2 of Escherichia coli. Biochimie. 1991 Dec;73(12):1509–1516. doi: 10.1016/0300-9084(91)90185-4. [DOI] [PubMed] [Google Scholar]
- Mironova L. N., Samsonova M. G., Zhouravleva G. A., Kulikov V. N., Soom M. J. Reversions to respiratory competence of omnipotent sup45 suppressor mutants may be caused by secondary sup45 mutations. Curr Genet. 1995 Feb;27(3):195–200. doi: 10.1007/BF00326148. [DOI] [PubMed] [Google Scholar]
- Moffat J. G., Timms K. M., Trotman C. N., Tate W. P. Interaction of the release factors with the Escherichia coli ribosome: structurally and functionally-important domains. Biochimie. 1991 Jul-Aug;73(7-8):1113–1120. doi: 10.1016/0300-9084(91)90154-s. [DOI] [PubMed] [Google Scholar]
- Mol C. D., Arvai A. S., Sanderson R. J., Slupphaug G., Kavli B., Krokan H. E., Mosbaugh D. W., Tainer J. A. Crystal structure of human uracil-DNA glycosylase in complex with a protein inhibitor: protein mimicry of DNA. Cell. 1995 Sep 8;82(5):701–708. doi: 10.1016/0092-8674(95)90467-0. [DOI] [PubMed] [Google Scholar]
- Nissen P., Kjeldgaard M., Thirup S., Polekhina G., Reshetnikova L., Clark B. F., Nyborg J. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science. 1995 Dec 1;270(5241):1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- Ogawara H., Urabe H., Ohtaki R., Nakamura Y. Properties of peptide chain release factor 2 from Streptomyces coelicolor A3(2): conserved primary structure but no frameshift regulation. J Bacteriol. 1995 Sep;177(18):5342–5345. doi: 10.1128/jb.177.18.5342-5345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pel H. J., Rep M., Dubbink H. J., Grivell L. A. Single point mutations in domain II of the yeast mitochondrial release factor mRF-1 affect ribosome binding. Nucleic Acids Res. 1993 Nov 25;21(23):5308–5315. doi: 10.1093/nar/21.23.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pel H. J., Rep M., Grivell L. A. Sequence comparison of new prokaryotic and mitochondrial members of the polypeptide chain release factor family predicts a five-domain model for release factor structure. Nucleic Acids Res. 1992 Sep 11;20(17):4423–4428. doi: 10.1093/nar/20.17.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield I., Grant G. M., Akhmaloka, Tuite M. F. Ribosomal association of the yeast SAL4 (SUP45) gene product: implications for its role in translation fidelity and termination. Mol Microbiol. 1992 Dec;6(23):3469–3478. doi: 10.1111/j.1365-2958.1992.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Stansfield I., Jones K. M., Kushnirov V. V., Dagkesamanskaya A. R., Poznyakovski A. I., Paushkin S. V., Nierras C. R., Cox B. S., Ter-Avanesyan M. D., Tuite M. F. The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J. 1995 Sep 1;14(17):4365–4373. doi: 10.1002/j.1460-2075.1995.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield I., Tuite M. F. Polypeptide chain termination in Saccharomyces cerevisiae. Curr Genet. 1994 May;25(5):385–395. doi: 10.1007/BF00351776. [DOI] [PubMed] [Google Scholar]
- Tate W. P., Brown C. M. Translational termination: "stop" for protein synthesis or "pause" for regulation of gene expression. Biochemistry. 1992 Mar 10;31(9):2443–2450. doi: 10.1021/bi00124a001. [DOI] [PubMed] [Google Scholar]
- Ter-Avanesyan M. D., Dagkesamanskaya A. R., Kushnirov V. V., Smirnov V. N. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics. 1994 Jul;137(3):671–676. doi: 10.1093/genetics/137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. B., Murphy J. P., Gallant J. A. Genetic screen for cloned release factor genes. J Bacteriol. 1984 Apr;158(1):362–364. doi: 10.1128/jb.158.1.362-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Rydén-Aulin M., Kirsebom L. A., Isaksson L. A. Genetic implication for an interaction between release factor one and ribosomal protein L7/L12 in vivo. J Mol Biol. 1994 Oct 7;242(5):614–618. doi: 10.1006/jmbi.1994.1611. [DOI] [PubMed] [Google Scholar]
- Zhouravleva G., Frolova L., Le Goff X., Le Guellec R., Inge-Vechtomov S., Kisselev L., Philippe M. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 1995 Aug 15;14(16):4065–4072. doi: 10.1002/j.1460-2075.1995.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





