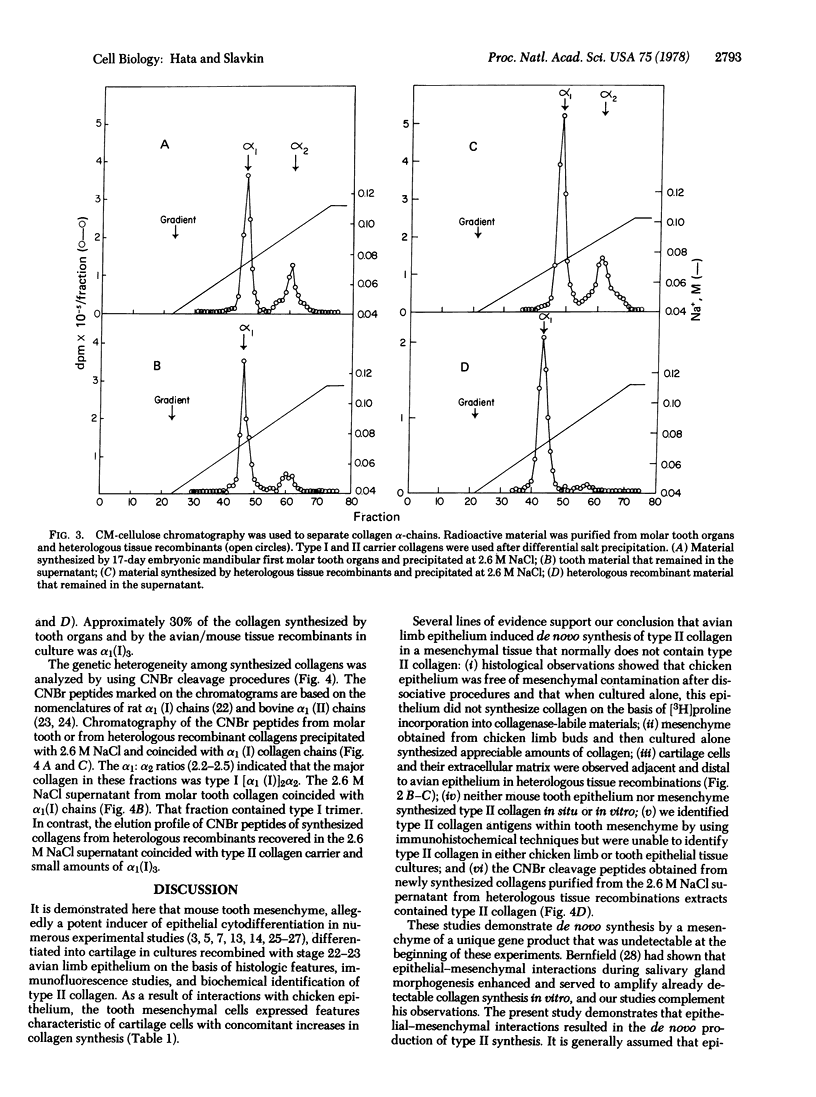
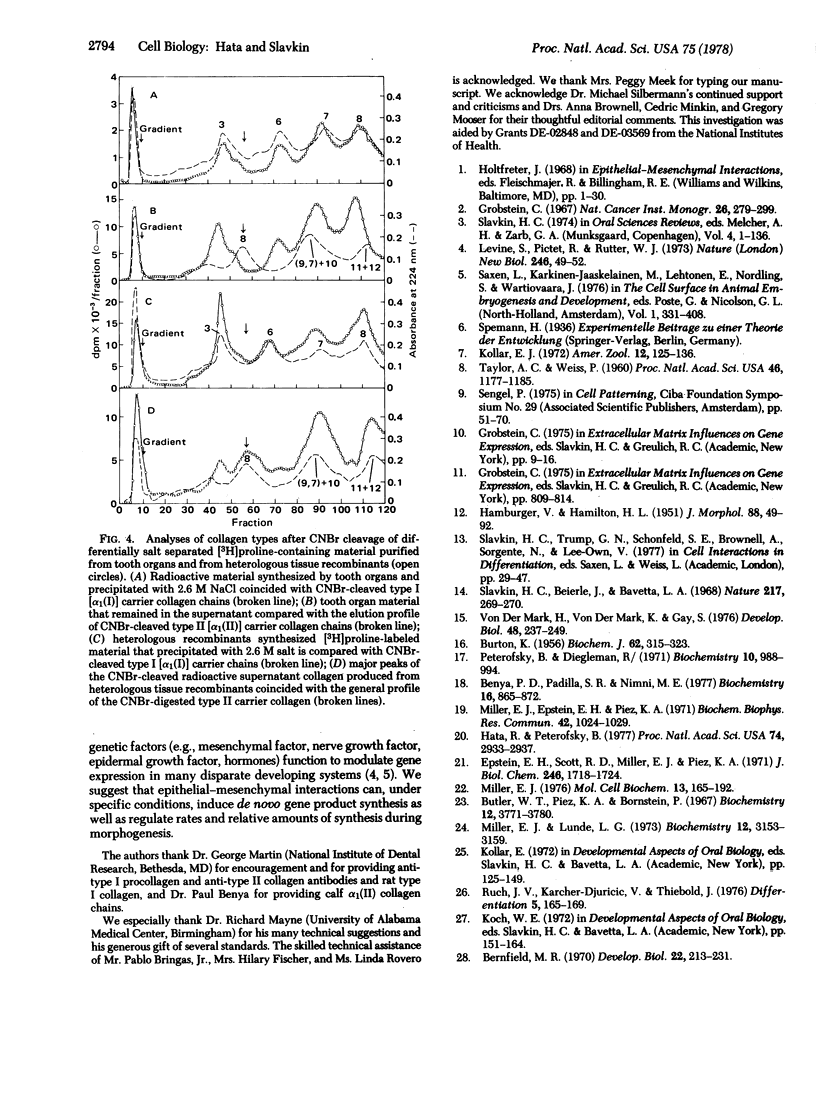
Abstract
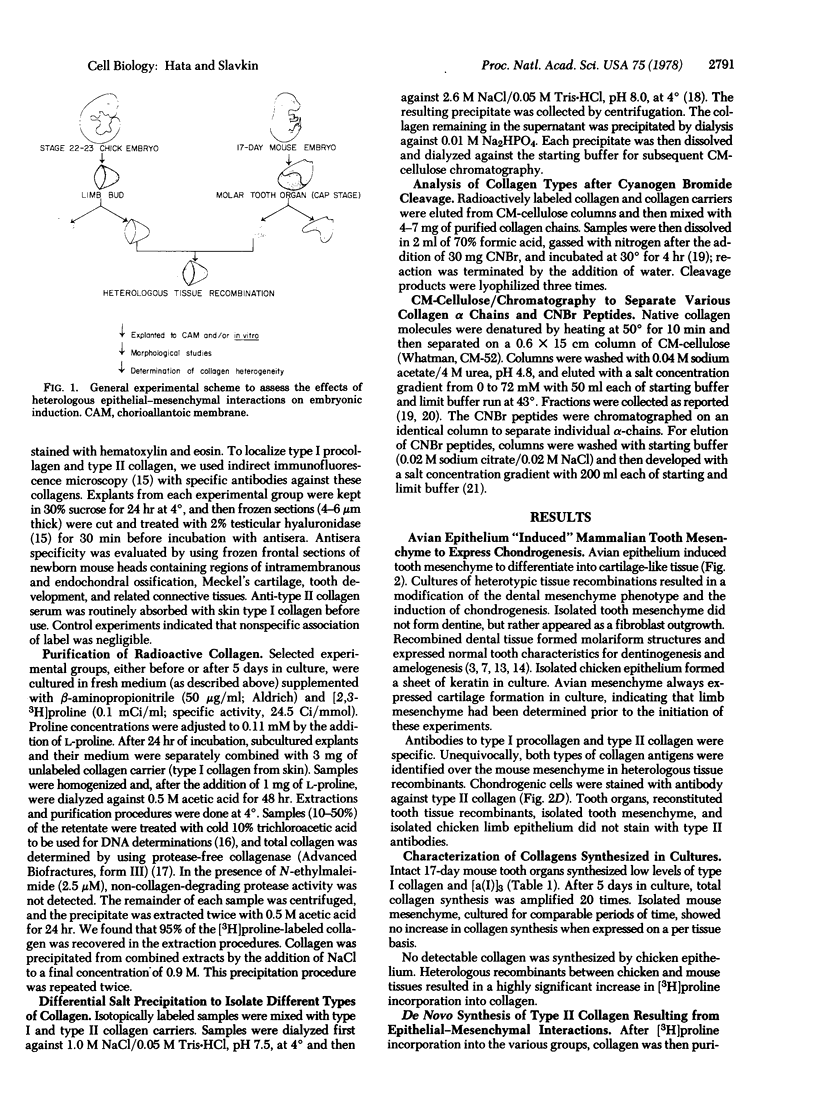
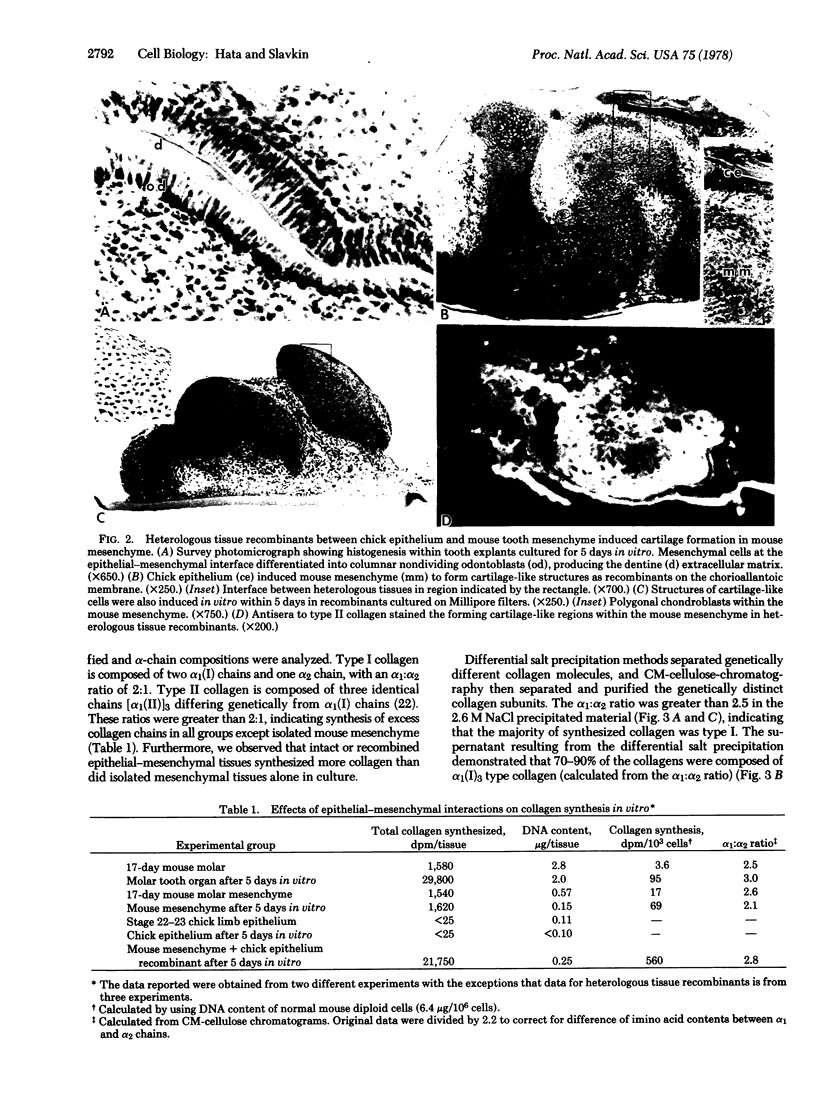
Mesenchymal specification of epithelial cytodifferentiation and morphogenesis has been considered to be a general feature of various epithelial-mesenchymal interacting systems (e.g., salivary gland, mammary gland, feather, hair, and tooth morphogenesis). In contrast, we have demonstrated that a mesenchyme can be induced by a heterologous epithelium to synthesize in quantity a specific gene product(s) unorthodox to the organ from which the mesenchyme was taken. Stage 22-23 avian limb bud epithelium induced 17-day embryonic mouse tooth mesenchyme to differentiate into cartilage. Peptide analysis (cyanogen bromide cleavage after purification of extracted collagen chains) demonstrated that heterologous tissue recombinations produced type II collagen [α(II)]3 (i.e., cartilage-type) in addition to type I collagen [α(I)]2α2. Intact or reconstituted mouse molar tooth organs synthesized type I collagen and type I trimer [α(I)]3 collagen. Immunohistochemical criteria using anti-type II collagen antibodies identified type II collagen in cartilage-like matrix within the mesenchymal component of heterologous tissue recombinants. Cartilage has never been described during in vivo or in vitro tooth tissue differentiation or associated with the pathology of dental papilla mesenchyme. These results support the hypothesis that epithelial-mesenchymal interactions during embryonic development can selectively induce de novo synthesis of unique gene products.
Keywords: induction of collagen types, in vitro chondrogenesis
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. The progeny of rabbit articular chondrocytes synthesize collagen types I and III and type I trimer, but not type II. Verifications by cyanogen bromide peptide analysis. Biochemistry. 1977 Mar 8;16(5):865–872. doi: 10.1021/bi00624a009. [DOI] [PubMed] [Google Scholar]
- Bernfield M. R. Collagen synthesis during epitheliomesenchymal interactions. Dev Biol. 1970 Jun;22(2):213–231. doi: 10.1016/0012-1606(70)90151-x. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Piez K. A., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha-1 chain of rat skin collagen. Biochemistry. 1967 Dec;6(12):3771–3780. doi: 10.1021/bi00864a022. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr, Scott R. D., Miller E. J., Piez K. A. Isolation and characterization of the peptides derived from soluble human and baboon skin collagen after cyanogen bromide cleavage. J Biol Chem. 1971 Mar 25;246(6):1718–1724. [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- Hata R. I., Peterkofsky B. Specific changes in the collagen phenotype of BALB 3T3 cells as a result of transformation by sarcoma viruses or a chemical carcinogen. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2933–2937. doi: 10.1073/pnas.74.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Pictet R., Rutter W. J. Control of cell proliferation and cytodifferentiation by a factor reacting with the cell surface. Nat New Biol. 1973 Nov 14;246(150):49–52. doi: 10.1038/newbio246049a0. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Epstein E. H., Jr, Piez K. A. Identification of three genetically distinct collagens by cyanogen bromide cleavage of insoluble human skin and cartilage collagen. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1024–1029. doi: 10.1016/0006-291x(71)90006-4. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lunde L. G. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973 Aug 14;12(17):3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Ruch J. V., Karcher-Djuricic V., Thiebold J. Cell division and cytodifferentiation of odontoblasts. Differentiation. 1976 Jun 4;5(2-3):165–169. doi: 10.1111/j.1432-0436.1976.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Slavkin H. C., Beierle J., Bavetta L. A. Odontogenesis: cell-cell interactions in vitro. Nature. 1968 Jan 20;217(5125):269–270. doi: 10.1038/217269a0. [DOI] [PubMed] [Google Scholar]
- Slavkin H. C. Embryonic tooth formation. A tool for developmental biology. Oral Sci Rev. 1974;4(0):7–136. [PubMed] [Google Scholar]
- Weiss P., Taylor A. C. RECONSTITUTION OF COMPLETE ORGANS FROM SINGLE-CELL SUSPENSIONS OF CHICK EMBRYOS IN ADVANCED STAGES OF DIFFERENTIATION. Proc Natl Acad Sci U S A. 1960 Sep;46(9):1177–1185. doi: 10.1073/pnas.46.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark H., von der Mark K., Gay S. Study of differential collagen synthesis during development of the chick embryo by immunofluorescence. I. Preparation of collagen type I and type II specific antibodies and their application to early stages of the chick embryo. Dev Biol. 1976 Feb;48(2):237–249. doi: 10.1016/0012-1606(76)90088-9. [DOI] [PubMed] [Google Scholar]