Abstract
Copper (Cu) is an essential metal present at high levels in the CNS. Its role as a co-factor in mitochondrial ATP production and in other essential cuproenzymes is well defined. Menkes and Wilson’s diseases are severe neurodegenerative conditions that demonstrate the importance of Cu transport into the secretory pathway. Brain levels of Cu, which is almost entirely protein bound, exceed extracellular levels by more than a hundred-fold. Cu stored in the secretory pathway is released in a Ca2+-dependent manner and can transiently reach concentrations over 100 µM at synapses. The ability of low µM levels of Cu to bind to and modulate the function of γ-aminobutyric acid type A (GABAA) receptors, N-methyl-D-aspartate (NMDA) receptors and voltage-gated Ca2+ channels contributes to its effects on synaptic transmission. Cu also binds to amyloid precursor protein and prion protein; both proteins are found at synapses and brain Cu homeostasis is disrupted in mice lacking either protein. Especially intriguing is the ability of Cu to affect AMP-activated protein kinase (AMPK), a monitor of cellular energy status. Despite this, few investigators have examined the direct effects of Cu on synaptic transmission and plasticity. Although the variability of results demonstrates complex influences of Cu that are highly method-sensitive, these studies nevertheless strongly support important roles for endogenous Cu and new roles for Cu-binding proteins in synaptic function/plasticity and behavior. Further study of the many roles of Cu in nervous system function will reveal targets for intervention in other diseases in which Cu homeostasis is disrupted.
Cu is a necessity for eukaryotic life and tightly regulated in complex organisms
Copper (Cu) is an essential trace element whose ability to donate and accept electrons gives it great utility in multicellular life. It is thought that microorganisms evolved the ability to utilize Cu when oxygen became more prevalent in the atmosphere and previously insoluble Cu1+ was oxidized to bioavailable Cu2+ (Crichton and Pierre, 2001; Ridge et al., 2008). Cleavage of dioxygen through reduction via Cu allowed these organisms to extract energy from fuels more efficiently. This critical evolutionary juncture coincided with the development of multicellular life (Decker and Terwilliger, 2000; Crichton and Pierre, 2001). Cu has been an essential component of biology through the evolution of complex systems, including the mammalian central nervous system.
Cu serves many functions in biology, but its unique role in oxidation-reduction reactions has been the most extensively studied. An association between Cu and dioxygen-utilizing enzymatic reactions accompanied the appearance of Cu in biological systems. Cu serves as an essential co-factor for about a dozen enzymes (called cuproenzymes) encoded by mammalian genomes. Cuproenzymes are structurally and functionally conserved from yeast and worms to more complex organisms like mice and humans (Andreini et al., 2008). Cytochrome c oxidase, a mitochondrial enzyme, is essential for oxidative phosphorylation and aerobic respiration. Superoxide dismutase, a cytosolic enzyme, detoxifies free radicals. Dopamine-β-monooxygenase (DβM), a secretory pathway enzyme, is essential for catecholamine biosynthesis, and peptidylglycine α-amidating monooxygenase (PAM), another secretory pathway enzyme, is essential for peptide biosynthesis (Fig. 1).
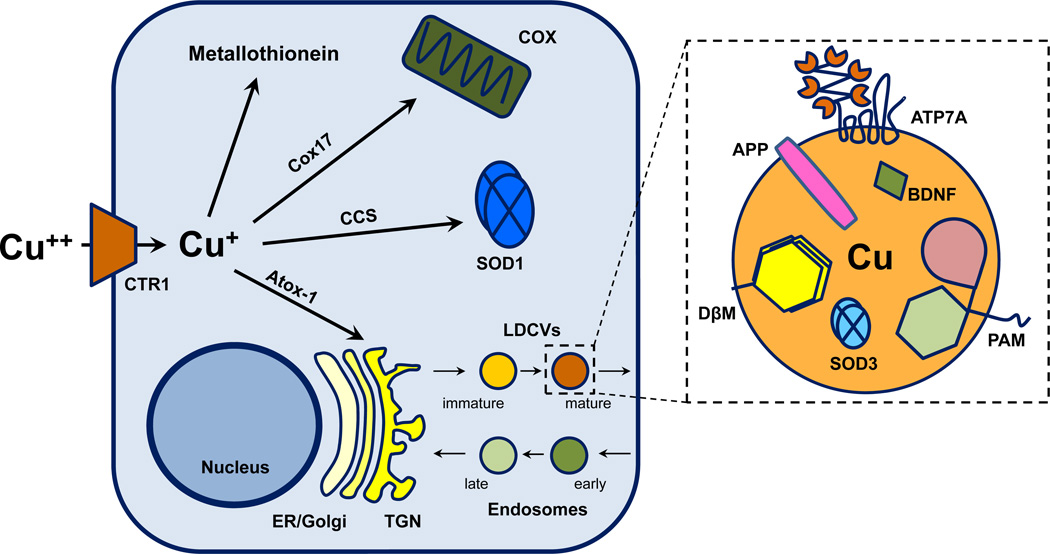
Fig. 1.
Key organelles and proteins involved in cellular Cu homeostasis. (Left) Cu enters cells through the Cu-specific transporter CTR1. Intracellular Cu is buffered by metallothioneins. Designated Cu chaperones deliver Cu to specific cuproenzymes in their respective organelles. Cox17 delivers Cu to COX in mitochondria. SOD1 is the only cytosolic cuproenzyme and receives Cu through CCS. Atox-1 delivers Cu to an ATPase (ATP7A or ATP7B) that transport Cu into the secretory pathway (Right). Cu is present in secretory granules along with cuproenzymes like DβM, extracellular SOD3 and PAM. Based on the localization of ATP7A and ATP7B, Cu is also present in constitutive vesicles and in endocytic compartments. APP and BDNF are also part of the secretory pathway in neurons (see Figs. 3,4). Abbreviations: APP, amyloid precursor protein; Atox-1, antioxidant-1 (a.k.a. HAH1); ATP7A, Menkes disease protein; CCS, Cu chaperone for SOD1; COX, cytochrome c oxidase; Cox17, Cu chaperone for COX; CTR1, Cu transporter 1; DβM, dopamine β-monooxygenase; ER, endoplasmic reticulum; LDCV, large dense-core vesicle; PAM, peptidylglycine α-amidating monooxygenase; SOD1, superoxide dismutase 1; SOD3, superoxide dismutase 3; TGN, trans Golgi network.
However, the advantages of using Cu come at a price. The same reactive properties that give Cu its utility as an enzymatic co-factor pose a risk for the generation of reactive oxygen species and subsequent oxidative damage in uncontrolled settings (Uriu-Adams and Keen, 2005). An intricate network of Cu binding proteins and Cu transporters, whose numbers vastly exceed the number of cuproenzymes, presumably evolved to minimize the potentially detrimental effects of Cu (Andreini et al., 2008). The components of this Cu homeostasis network and their interactions are just beginning to be uncovered (Banci et al., 2010; Lutsenko, 2010; Kim et al., 2010). Importantly, striving to identify the components of this network and elucidate their interactions is paramount in advancing our understanding of and treatments for a range of diseases in which Cu handling and homeostasis are disrupted.
Genetic disorders of Cu homeostasis reveal the consequences of dysregulation
The necessity for Cu and the potential for Cu toxicity are clearly demonstrated through the symptoms associated with genetic disorders of Cu transport and dietary Cu deficiency/toxicity. The two major genetic disorders, Menkes and Wilson’s disease, involve dysfunction of the P-type ATPase transporters ATP7A and ATP7B, respectively. While each protein can transport Cu into the secretory pathway, the pathophysiologies of these two diseases are quite different, reflecting the distinct sites at which these two Cu transporters are expressed.
Menkes Disease
Menkes disease is an X-linked recessive disorder first characterized by John Hans Menkes in 1962 as an inherited form of Cu deficiency (MENKES et al., 1962)[OMIM 309400; for review see (Kodama et al., 1999; de Bie et al., 2007; Kaler, 2011)]. Lack of ATP7A activity in the gut results in diminished Cu uptake and causes Cu deficiency in the rest of the body. Naturally occurring mutations in the human ATP7A gene span the full length of the protein and impair its Cu transport function to varying extents, resulting in a continuum of phenotypic severity (Kaler, 2011). Infants born with Menkes disease typically begin to exhibit developmental delay and failure to thrive a few months after birth. Symptomology associated with Menkes disease results from Cu deficiency in multiple organs and directly reflects deficient activities of specific cuproenzymes. Moreover, symptom severity often shares a close relationship with residual Cu transport activity (Donsante et al., 2007). Symptoms progress until death at around 3 years of age.
ATP7A expression is developmentally and cell-type specifically regulated in the central nervous system. In mice, expression of ATP7A is highest in the perinatal period and declines between 2- and 10-fold into adulthood in a region-dependent manner (Niciu et al., 2006). Neurons of the cortex, hippocampus, olfactory bulb, cerebellum and hypothalamus express ATP7A (Ke et al., 2006; Niciu et al., 2006). Neuronal ATP7A is concentrated in the perinuclear region, overlapping trans-Golgi network markers, and can be detected extending into neurites in vitro (Schlief et al., 2005) and in vivo (Niciu et al., 2006). In non-neuronal cells, ATP7A is expressed in the perinatal period and into adulthood (choroid plexus, ependymal cells and a subset of astrocytes) or in the perinatal period only (cerebral endothelial cells, microglia, optic nerve oligodendrocytes) (Niciu et al., 2006).
Seizures are common in Menkes patients, with occurrence and severity typically increasing through the course of the disease (Kodama et al., 1999; de Bie et al., 2007; Kaler, 2011; Kodama et al., 2011). Brain energy metabolism is disrupted, as evidenced by reductions in cytochrome c oxidase and other genes involved in oxidative phosphorylation (Liu et al., 2005). Since energy metabolism is tightly related to neurotransmission and cellular excitability (Pan et al., 2008; Cloix and Hevor, 2009), disruptions in energy metabolism could influence Menkes-associated seizure susceptibility (Prasad et al., 2011). Deficiencies of both DβM and PAM could contribute to seizure susceptibility through shifts in excitatory-inhibitory balances caused by deficiencies in catecholaminergic and peptidergic neuromodulators, respectively. Norepinephrine has potent anti-convulsive effects (Mason and Corcoran, 1978; Mason and Corcoran, 1979), and destruction of noradrenergic neurons of the locus coeruleus or genetic deletion of DβM lowers the threshold for epileptogenic stimuli in limbic structures (Mishra et al., 1994; Szot et al., 1999). Inhibitory interneurons express and secrete multiple neuropeptides (McDonald and Pearson, 1989; Salio et al., 2006), many of which promote inhibition and/or reduce excitation [for review see (Bajorek et al., 1986; Zadina et al., 1986)]; examples include neuropeptide Y (Bacci et al., 2002), cholecystokinin (Crawley and Corwin, 1994), thyrotropin releasing hormone (Deng et al., 2006) and vasopressin (Raggenbass, 2008). Reduced activity of PAM due to Cu insufficiency leads to tissue-specific impairments in amidation and peptide bioactivity (Bousquet-Moore et al., 2010). Thus Cu deficiency can contribute to Menkes-associated epilepsy through multiple mechanisms.
Wilson’s Disease
Wilson’s disease is an autosomal recessive disorder first characterized by Samuel Alexander Kinnier Wilson in 1912 as an inherited degenerative disease of the liver and brain [OMIM 277900; for review see (Fujiwara et al., 2006; Das and Ray, 2006; de Bie et al., 2007; Pfeiffer, 2007; Lutsenko, 2010)]. Manifestations of Wilson’s disease result from multiple organ dysfunction secondary to Cu overload, rather than Cu deficiency as with Menkes disease, and typically arise in adolescence or adulthood. Dysfunction of the Wilson’s disease protein, ATP7B, results in an inability to remove Cu from the body because hepatocytes cannot excrete it into bile. Cu accumulates in the liver, enters the circulation unbound and deposits in multiple organs. A striking example is Kayser-Fleischer rings, deposits of Cu in the cornea, which are clinically considered pathognomonic for Wilson’s disease (de Bie et al., 2007). Pathophysiology in Wilson’s disease primarily involves excessive oxidative stress directly resulting from Cu overload in the liver and brain. Non-neurological manifestations include symptomology commonly associated with liver failure and hemolytic anemia (Attri et al., 2006; de Bie et al., 2007).
ATP7B is expressed in the brain, but is not as prevalent as ATP7A (Lutsenko et al., 2010). The neuropathology involved in Wilson’s disease is thought to be due to Cu deposition in the brain, which results in neurodegeneration. Changes in lenticular structures associated with basal ganglia circuitry are most common and are detectable by magnetic resonance imaging (Kodama et al., 2011). As such, motor symptoms include parkinsonism, dystonia, ataxia, dysarthria, chorea and oculomotor defects (Das and Ray, 2006; Taly et al., 2007). Seizures and progressive cognitive impairment are also common (Akil and Brewer, 1995; Kodama et al., 2011).
Many Wilson’s disease patients present with psychiatric symptoms (Taly et al., 2007), which may be severe enough for patients to reach diagnostic criteria for Axis I affective/mood and/or anxiety disorders (Pfeiffer, 2007). Progression of Wilson’s disease is associated with changes in personality and behavior and commonly includes increased aggression, irritability, hostility and depressive-like symptoms (Akil and Brewer, 1995). Wilson’s patients are commonly treated for psychiatric symptoms in conjunction with Cu chelation therapy (Taly et al., 2007; Srinivas et al., 2008). Psychiatric manifestations of Wilson’s disease are recapitulated in animal models; in the toxic milk mouse, a mutation in ATP7B results in elevated Cu in the limbic system, striatum and cerebellum, but not in the cortex (Terwel et al., 2011). Toxic milk mice display motor impairment, disrupted learning and memory, and less anxiety-like behavior. This animal model supports the notion that the regional pattern of brain alterations in Wilson’s disease accounts for the prevalence of emotional dysregulation in Wilson’s psychopathology.
Importantly, many of the symptoms of Wilson’s disease can be ameliorated by chelation therapy (Akil and Brewer, 1995; Srinivas et al., 2008; Kodama et al., 2011). Although neurodegeneration occurs eventually, the many neuronal and synaptic effects of elevated Cu no doubt contribute to the reversible psychiatric manifestations associated with Wilson’s disease. While genetic and dietary disorders of Cu homeostasis demonstrate the importance of Cu in the nervous system, it is not clear that the role of Cu as an enzymatic co-factor explains all the symptoms associated with these diseases.
Brain Cu homeostasis: from serum to synapse
Cu uptake in the gut is mediated primarily through CTR1 and, to a lesser extent, divalent metal transporter 1 (DMT1) (Lutsenko, 2010; Kaler, 2011). In enterocytes, CTR1 plays an essential role in the import of Cu from various ingested food sources including legumes, liver, shellfish, nuts and chocolate (Linder and Hazegh-Azam, 1996; Ma and Betts, 2000). In most cells, CTR1 plays an essential role in cellular Cu uptake into the cytosol (Fig. 1). Cu then binds one of several cytosolic chaperones; each chaperone has a designated destination for the Cu it carries. For example, antioxidant-1 (Atox-1, also known as HAH1) delivers Cu directly to P-type ATPase transmembrane transporters ATP7A and ATP7B (Hamza et al., 2001; Lutsenko et al., 2007). The liver acts as the main storage site for Cu; from the liver, Cu can be mobilized to various organs or excreted following secretion into the bile duct (Wijmenga and Klomp, 2004). The Cu-regulated targeting of ATP7B to apical vs. basolateral vesicles determines whether Cu is exported in bile or made accessible to other tissues by secretion into plasma. Cu secreted from the liver is typically loaded into newly synthesized ceruloplasmin, a Cu-dependent secreted ferroxidase. Each ceruloplasmin molecule binds 6 Cu ions, and 75–95% of the Cu in the blood is bound to ceruloplasmin (Hellman and Gitlin, 2002; Bielli and Calabrese, 2002).
Cu entry into the brain requires CTR1 and ATP7A and occurs through two routes. The first route is through the blood-brain barrier, which serves as a regulated junction between the blood and the extracellular fluid of the brain parenchyma. The second is through the blood-cerebral spinal fluid (CSF) barrier, which serves as the junction between the blood and the CSF. CTR1 and ATP7A are expressed in endothelial cells of the blood-brain barrier and in epithelial choroid plexus cells of the blood-CSF barrier (Choi and Zheng, 2009; Kaler, 2011). The values reported for the Cu content of normal human CSF vary widely, but are generally below 1 µM (Table 1). Cu is less abundant in CSF than in serum. This difference is likely influenced by overall protein levels of these two fluids since patients with CNS pathology resulting in increased CSF protein also have increased CSF Cu (Kapaki et al., 1989). Cu in the CSF is significantly elevated in patients with Alzheimer’s disease, Parkinson’s disease, Amyotrophic Lateral Sclerosis (Hozumi et al., 2011), and perhaps Multiple Sclerosis (Kapaki et al., 1989; Melo et al., 2003).
Table 1. Concentration of Cu in normal human cerebrospinal fluid.
Data from several studies are summarized; subjects were all adults. ICP-MS, inductively coupled mass spectroscopy (ICP-MS); HR-ICP-MS, high-resolution inductively coupled plasma-mass spectrometry; AAS, atomic absorption spectrometry; GFAS, graphite furnace absorption spectroscopy. 63.5 µg/l is 1 µM.
| Table 1. Normal/Control CSF concentrations of Cu in humans | ||
|---|---|---|
| [Cu] | Method | Reference |
| 10.2 ug/L | ICP-MS | (Hozumi et al., 2011) |
| 8.67 ug/L | HR-ICP-MS | (Melo et al., 2003) |
| 9.10 ug/L | AAS | (Melo et al., 2003) |
| 22.3 ug/L | HR-ICP-MS | (Gellein et al., 2007) |
| 40.20 ug/L | GFAS | (Kapaki et al., 1989) |
Like other metals such as Zn and Fe, Cu is highly concentrated in the brain (Que et al., 2008). Only the liver, the body’s major organ for Cu storage, and the kidneys have higher Cu concentrations than the brain, where total Cu levels are around 200 µM for each (Linder and Hazegh-Azam, 1996; Lutsenko et al., 2010). Brain Cu levels increase in early post-natal development. Whole brain Cu levels measured by inductively coupled mass spectroscopy rise between 2 and 3.5 fold between infancy and adulthood (~0.8 µg/g at P0; ~28 µg/g at P20–40 wet weight) in Long Evans rats (Saito et al., 1995; Tarohda et al., 2004). The sharp developmental increase in brain Cu corresponds to high levels of ATP7A expression in the postnatal choroid plexus and ependymal cells of the ventricular system (Niciu et al., 2006). That a single injection of Cu or viral transfection of ATP7A into the choroid plexus can rescue the lethal phenotype of mouse models of Menkes disease (Donsante et al., 2011) demonstrates the key role of brain Cu in early development.
Brain Cu levels vary in different regions (Table 2), reflecting varying expression of Cu handling machinery (Niciu et al., 2006) and methodological differences that remain to be resolved. Cu is generally 2-fold more abundant in grey matter than in white matter (Prohaska, 1987). Using atomic absorption spectroscopy, the hypothalamus was found to contain the highest Cu concentration in the rat brain (Rajan et al., 1976). Laser ablation inductively coupled mass spectroscopy revealed an abundance of Cu in the medial geniculate nucleus, superior colliculus, and periaqueductal grey in adult rats (Jackson et al., 2006). High levels of Cu were observed in the lateral nucleus of the amygdala and dorsomedial aspect of the diencephalon. Strain differences can produce 3-fold differences in Cu concentrations in the murine hippocampus (Jones et al., 2008). The Cu content of the human hippocampus is higher than other brain regions (Dobrowolska et al., 2008), with Cu concentrated in the granule and pyramidal cell layers of the dentate gyrus and CA1 area. The functional significance of regional and species-specific Cu distributions is unclear.
Table 2. Concentration of Cu in adult brain.
Data from whole brain and the indicated regions are summarized. 63.5 µg/gm wet weight is 1 µM.
| Table 2. Normal/Control total brain concentrations of Cu in adult mammals by species | ||||
|---|---|---|---|---|
| [Cu] | Region | Species | Method | Reference |
| ~15 µg/g wet weight tissue | Cerebral Cortex | Mouse | GFAS | (Yang et al., 1998) |
| ~50 µg/g wet weight tissue | Hippocampus | Mouse | GFAS | (Yang et al., 1998) |
| ~72 µg/g wet weight tissue | Cerebellum | Mouse | GFAS | (Yang et al., 1998) |
| ~14 µg/g wet weight tissue | Brain stem | Mouse | GFAS | (Yang et al., 1998) |
| 12.7 µg/g dry weight tissue | Cerebral Cortex | Mouse | AAS | (White et al., 1999) |
| 16.4 µg/g dry weight tissue | Cerebellum | Mouse | AAS | (White et al., 1999) |
| 5.45 µg/g wet weight tissue | Whole Brain | Mouse | ICP-MS | (Waggoner et al., 2000) |
| 2.8–10.3 µg/g wet weight tissue | Hippocampus | Mouse (30 BXD strains) | ICP-MS | (Jones et al., 2008) |
| 2.0 µg/g wet weight tissue | Whole Brain | Rat | FAAS | (Critchfield et al., 1993) |
| ~5–14 µg/g dry weight tissue | 7 Regions across 3 ages | Rat (LEA) | ICP-MS | (Saito et al., 1995) |
| 2.79 µg/g wet weight tissue | Average of 18 regions (across 9 points of development) | Rat | ICP-MS | (Tarohda et al., 2004) |
| ~14 (8.74–17.07) µg/g | Average of 8 regions | Lamb | STAT-AAS | (Bakirdere et al., 2010) |
| 3.75 (2.64–5.74) µg/g wet weight tissue | Frontal Lobe | Human | ICP-AES | (Rahil-Khazen et al., 2002) |
| 5.83 (3.46–8.37) µg/g wet weight tissue | Cerebellum | Human | ICP-AES | (Rahil-Khazen et al., 2002) |
The intracellular concentration of brain Cu is 2–3 orders of magnitude higher than its extracellular concentration (Que et al., 2008). Almost all brain Cu is protein bound, and its distribution amongst different subcellular compartments is tightly regulated (Que et al., 2008; Lutsenko et al., 2010). Cu levels are highest in cytosolic and mitochondrial fractions (Rajan et al., 1976; Baerga et al., 1992). Cu is also abundant in synaptic vesicles, especially in the cerebral cortex (Rajan et al., 1976). The abundance of Cu in synaptosomal and endosomal fractions (Saito et al., 1995; Brown et al., 1997; Herms et al., 1999; Brown, 2003; Giese et al., 2005), suggests that Cu release and retrieval are an important part of how Cu is used in the brain. In vitro, synaptosomes can take up Cu (Giese et al., 2005), and membrane depolarization stimulates Cu release (Kardos et al., 1989).
Neuronal activation stimulates the Ca2+-dependent secretion of Cu (Hartter and Barnea, 1988; Schlief et al., 2005), which can reach concentrations of 100–250 µM within synapses (Kardos et al., 1989). As discussed below, Cu can then bind to and influence the function of synaptic and extra-synaptic proteins important to synaptic transmission and neuronal activity (Mathie et al., 2006; Que et al., 2008; Tamano and Takeda, 2011). The regulated release of Cu at synapses could contribute in a unique way to synaptic function and behavior.
Most of the work exploring the role of Cu in synaptic transmission and plasticity has been conducted in the hippocampus. ATP7A is expressed at high levels in the pyramidal neurons and interneurons of the neocortex and hippocampus (Schlief et al., 2005; Niciu et al., 2006), where Cu content is also relatively high (Rajan et al., 1976; Bakirdere et al., 2010). Cu is released from cultured hippocampal neurons in an N-methyl-D-aspartate (NMDA) receptor-dependent manner, and stimulation of NMDA receptors results in the trafficking of ATP7A out of the late Golgi into dendrites (Schlief et al., 2005; Dodani et al., 2011). Since NMDA receptor activity is crucial for different forms of synaptic plasticity and learning and memory (Malenka and Bear, 2004; Yashiro and Philpot, 2008), Cu is very likely to be present at synapses during learning. Despite this, few groups have explored the direct role of Cu in synaptic transmission, plasticity or learning and memory.
Effects of exogenous Cu on synaptic transmission
In order to study the role of Cu in synaptic transmission, many investigators have employed the use of exogenous Cu. Doreulee and colleagues (Doreulee et al., 1997) bath-applied 1–100 µM CuSO4 onto hippocampal slices while monitoring synaptic transmission in the Schaffer collateral-CA1 pathway using extracellular recording methods. They found that 10 µM CuSO4 was needed to depress α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated transmission, while NMDA receptor-mediated signals were sensitive to 1 µM CuSO4. Intracellular recordings confirmed these results. Presynaptic glutamate release and γ-aminobutyric acid (GABA) mediated inhibition were insensitive to 10 µM and 1 µM CuSO4, respectively. CuCl2 (30 µM) had depressing effects on excitatory synaptic transmission, similar to CuSO4, in cultured olfactory bulb neurons (Trombley et al., 1998; Horning and Trombley, 2001). These effects occurred very rapidly and were attributed to direct effects of Cu2+ on voltage-gated ion channel and synaptic receptor functions.
In a recent study, Peters and colleagues (Peters et al., 2011) characterized a biphasic response of cultured rat hippocampal neurons to 10 µM CuCl2. Acute Cu application had a depressive effect, as reported previously in the hippocampus and other brain regions (Xie et al., 1993; Doreulee et al., 1997; Horning and Trombley, 2001); AMPA receptor mediated synaptic transmission was blocked in a concentration-dependent manner and the frequency and amplitude of spontaneous excitatory currents were reduced. Interestingly, the opposite effect was observed when CuCl2 was applied for 3 hours prior to recordings; spontaneous and evoked AMPA receptor and GABAA receptor mediated currents were enhanced. The excitatory effect was mediated by increases in pre-synaptic release of glutamate and post-synaptic expression of AMPA receptor complexes. Both the GluR1 subunit of the AMPA receptor and PSD95 were increased in hippocampal cultures treated with CuCl2 for 3 hours as assessed by Western blot and immunocytochemistry. This effect was not due to compensatory effects following acute synaptic suppression by Cu, since similar long-term effects could not be replicated by selective blockade of AMPA and GABAA receptors. This study provides comprehensive evidence of the profound and complex effects of exogenous Cu on synaptic transmission and strongly supports an important role for endogenous Cu in vivo.
Leiva et al 2000 (Leiva et al., 2000) used extracellular recordings to investigate the effects of exogenous CuSO4 on mixed excitatory and inhibitory signals in acute hippocampal slices. CuSO4 (10 µM) application reduced the amplitude and duration of mixed signals. Bicuculline methiodide and picrotoxin, both of which are GABAA receptor antagonists, had opposite effects on these mixed signals in the Schaffer-CA1 pathway; bicuculline reduced mixed transmission similar to CuSO4, whereas picrotoxin enhanced these signals. These two compounds work through very different mechanisms; bicuculline competitively antagonizes the GABA binding site, whereas picrotoxin acts to non-competitively block the Cl− channel pore of the GABAA receptor (Krishek et al., 1996) (Fig. 2). The effect of CuSO4 and bicuculline together was not greater than either alone, suggesting that Cu works through GABAA receptors to depress synaptic transmission. By contrast, addition of CuSO4 with picrotoxin remarkably enhanced synaptic transmission above what was observed with picrotoxin alone (Leiva et al., 2000). The plethora of GABAA receptor subtypes, which are located on different classes of neurons, different neuronal domains and have different physiological functions and pharmacological profiles complicates interpretation (Zhang et al., 1995; Martina et al., 2001; Farrant and Nusser, 2005). Nevertheless, it is clear that the ability of Cu2+ to bind to GABAA receptors contributes to its complex effects on synaptic transmission (see below).
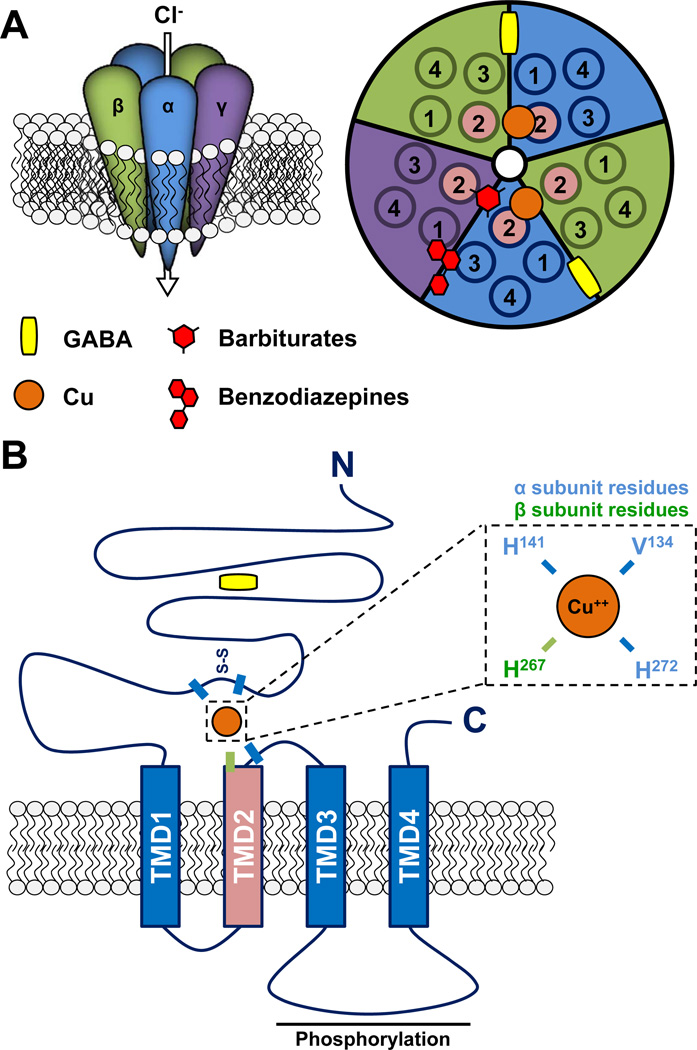
Fig. 2.
GABAA receptor structure and Cu binding. (A) Ionotropic receptors for γ-aminobutyric acid (GABA) are referred to as GABAA receptors and are heteropentameric receptors that are permeable to Cl and thus generally serve an inhibitory function in the adult nervous system. GABAA receptors typically comprise two α, two β, and one γ (or other) subunits, as depicted on the left. On the right, a schematic view of the top of the channel demonstrates the central ion pore (white), five subunits (each with four transmembrane domains; TMD1-4), and approximate binding sites for GABA, Cu, barbiturates and benzodiazepines. (B). Schematic of a single GABAA receptor subunit. Specific residues important for Cu binding are labeled according to subunit type (α and β). Phosphorylation of the cytoplasmic loop between TMD3 and TMD4 regulates trafficking of the GABAA receptor.
Protein targets for synaptically released Cu
While the synaptic effects of Cu are impressive and profoundly complex, very little is known about the molecular mechanisms underlying these effects. Several synaptically localized molecules that bind Cu could mediate non-enzymatic effects of Cu on neuronal signaling.
Glutamate receptors
Receptors for glutamate include a heterogeneous family of tetrameric ligand gated ion channels categorized by their pharmacological profiles as AMPA, kainate and NMDA receptors (Scott and Ehlers, 2004). All ionotropic glutamate receptors are non-specific cation channels that act to depolarize the membrane. Their unique subunit composition contributes to their distinct pharmacological and kinetic properties as well as their ionic permeability. For example, AMPA receptors containing the GluR1 subunit and NMDA receptors are permeable to Ca2+ in addition to Na+ and K+, and thus contribute to Ca2+-dependent intracellular signaling pathways including gating synaptic plasticity (Kullmann et al., 2000).
Weiser and Wienrich (Weiser and Wienrich, 1996) examined the effects of Cu2+ (salt unspecified) on AMPA and NMDA receptor mediated currents in cultured rat cortical neurons. Cu2+ inhibited both receptors independent of membrane voltage, but through distinct mechanisms. The IC50 values for AMPA and NMDA receptors were 4.3 and 15 µM Cu2+, respectively, and near complete blockade was achieved at 100 µM Cu2+. Cu2+ shifted the agonist concentration-response curve toward higher agonist concentrations; the effect was most consistent with a 2 binding site model for Cu2+ inhibition of AMPA receptors. Since the effects of Cu2+ on AMPA receptors were greatly diminished and more rapidly reversed by the reducing agent dithiothreitol (DTT), an oxidative mechanism may be involved. Increasing concentrations of Cu2+ had a similar effect on NMDA receptor agonist concentration-responses. Since the ability of Cu2+ to inhibit NMDA receptor mediated currents was not affected by DTT, the mechanism is thought to be oxidation-independent. Inhibitory effects of Cu2+ on NMDA receptor mediated currents were also observed in acutely isolated olfactory bulb neurons (Trombley and Shepherd, 1996) and in cultured mouse and rat hippocampal neurons (Vlachova et al., 1996); the IC50 values in these studies (30 µM and 0.27 µM CuCl2, respectively), differed significantly. The latter study concluded that Cu2+ inhibition of the NMDA receptor occurs non-competitively and with preference for agonist-bound receptors (Vlachova et al., 1996).
The inhibitory effect of Cu2+ on NMDA receptors was later shown to depend on nitrosylation of the NMDA receptor, which is facilitated in the presence of CuCl2 (Schlief et al., 2005). In a separate set of experiments, Schlief and colleagues found that a high concentration of CuCl2 (200 µM) abrogates NMDA receptor-mediated excitotoxicity of primary rat hippocampal cultured neurons (Schlief et al., 2006). Cultured hippocampal neurons prepared from mice expressing the mutant mottled/brindled Atp7a are more susceptible to NMDA receptor mediated excitotoxic injury. An additional mechanism for Cu2+ inhibition of NMDA receptors involving the prion protein has recently been suggested (described below) (You et al., 2012).
GABAA receptors
GABAA receptors are heteropentameric ionotropic receptors structurally similar to nicotinic acetylcholine receptors (Hevers and Luddens, 1998; Jacob et al., 2008). There are 18 subunits identified to date, and alternative splicing contributes additional diversity. GABAA receptors contain two α subunits (α1-α6), two β subunits (β1-β3) and a single γ(γ1-γ3), δ, ε(ɛ1-ɛ3), θ or π subunit (Fig. 2A). Importantly, subunit composition determines GABAA receptor localization, activity and function within neurons and networks (Wafford et al., 2004; Rudolph and Mohler, 2006). Each subunit has a large extracellular N-terminal domain (NTD) which includes a short cysteine loop and 4 transmembrane domains (TMD; TMD2 lines the channel pore); a long intracellular loop between TMD3 and TMD4 acts as a site for protein-protein interactions and post-translational modifications that regulate trafficking and channel activity (Fig. 2B) (Jacob et al., 2008). The binding of GABA at both interfaces between the NTDs of the α and β subunits leads to channel pore opening and the passage of Cl− ions inhibits action potential activity.
Physiological concentrations of Cu2+ bind to and inhibit GABAA receptor activity (Ma and Narahashi, 1993; Sharonova et al., 1998; Kim and Macdonald, 2003). Unlike Zn2+, which displays subtype preference for binding (α6, EC50 = 1.9 µM; α1 EC50 = 10.4 µM) (Fisher and Macdonald, 1998), Cu2+ binds each human GABAA receptor subtype with equal affinity (EC50 ~ 2.4 µM) (Kim and Macdonald, 2003). Cu2+ and Zn2+ both have inhibitory effects on GABAA receptor function (Narahashi et al., 1994; Trombley et al., 1998; Horning and Trombley, 2001) [for review see (Mathie et al., 2006)]. Cu2+-mediated inhibition of GABAA receptors is reversible (Ma and Narahashi, 1993), but may require chelator-mediated removal of Cu2+ (Sharonova et al., 1998).
Competing inhibitory effects of Cu2+ and Zn2+ on GABAA receptor function strongly suggest that they act through similar mechanisms (Ma and Narahashi, 1993; Narahashi et al., 1994; Fisher and Macdonald, 1998). Although Cu2+ inhibition of GABA-activated currents follows kinetics similar to competitive antagonists, the Cu2+ binding site is distinct from the GABA binding site (Sharonova et al., 1998; Sharonova et al., 2000) (Fig. 2). Cu2+ acts by inhibiting the effects of GABA on channel gating. Cu2+ binding also influences the binding and activity of other GABAA receptor allosteric modulators such as benzodiazepines (Mizuno et al., 1982; Kardos et al., 1984). The Cu2+ binding site includes a Val134-Arg(Gln for α2)-Ala-Glu-Cys-Pro-Met-His141 motif, which includes the Cys-loop in the α1 NTD (Fig. 2B) (Kim and Macdonald, 2003). Val134, Arg/Gln135 and His141 are the most important determinants for Cu2+ binding, but the whole motif is required for the full Cu2+ effect (Fig. 2B). A residue in the β3 subunit (His267 in TMD2) and another residue in the α6 subunit (His273 in the TMD2-TMD3 loop) are important for both Cu2+ and Zn2+ binding (Kim and Macdonald, 2003); not all of the residues essential for Zn2+ binding are required for Cu2+ binding (Fisher and Macdonald, 1998). Taken together, these data suggest that Cu2+ and Zn2+ inhibit GABAA receptor function through overlapping but non-identical binding sites.
The subtype specificity of Cu2+ and Zn2+ binding to GABAA receptors is functionally relevant. GABAA receptors that contain α(1–3) are synaptically localized and mediate phasic or fast inhibition, whereas α(4–6)-containing receptors are extra-synaptic and mediate tonic or slow inhibition (Hevers and Luddens, 1998; Farrant and Nusser, 2005; Jacob et al., 2008). Cu2+ has a stronger maximal effect on α1/α2-containing GABAA receptors than on α4/α6-containing receptors (Kim and Macdonald, 2003). In contrast, Zn2+ has a stronger affinity for α6-containing receptors than for α1-containing receptors but has similar maximal inhibitory efficacies (Fisher and Macdonald, 1998). Subtype-specific localization of GABAA receptors will determine the effect of Cu2+ on any given neuron. For example, in hippocampal pyramidal neurons, α1-containing GABAA receptors localize to parvalbumin-positive axo-somatic synapses while α2-containing receptors localize to cholecystokinin/vasoactive intestinal peptide-positive axo-axonic synapses (Nyiri et al., 2001). α5-containing GABAA receptors are also expressed by hippocampal pyramidal neurons and localize to the base of dendritic spines (Rudolph and Mohler, 2006) (Fig. 3).
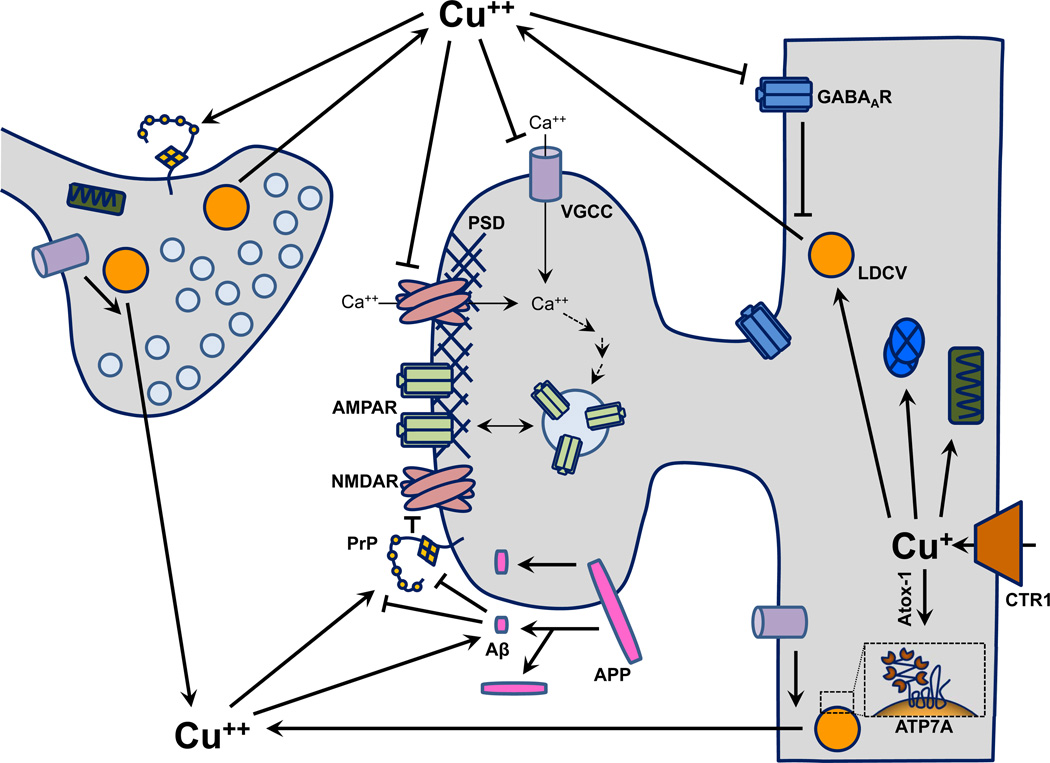
Fig. 3.
Roles of Cu in synaptic function. Cu enters cells through CTR1 and binds to cytosolic chaperones including Atox-1, which delivers Cu to ATP7A. Cu gains entry into the secretory pathway through ATP7A and is stored in LDCVs along with secreted cuproenzymes DβM, SOD3 and PAM. Influx of Ca2+ through NMDARs and VGCCs results in LDCV release and Cu secretion. This calcium signal also facilitates long-term potentiation post-synaptically. Extracellularly, Cu has inhibitory effects on NMDARs, VGCCs and GABAARs. Cu binds PrP and APP, which are located at synapses. Upon binding of Cu, PrP can be internalized or can interact with the NR1 subunit of the NMDA receptor, attenuating ion flux. APP binds Cu at attomolar concentrations, facilitating the multiple cleavages that yield extracellular Aβ, which also binds Cu with high affinity. Aβ can abrogate the inhibitory effect of PrP on the NMDA receptor by sequestering Cu or by binding to PrP. Abbreviations: Aβ, beta amyloid peptide; AMPAR, 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid receptor; APP, amyloid precursor protein; Ca++, calcium; CTR1, Cu transporter 1; GABAAR, γ-aminobutyric acid A receptor; LDCV, large dense-core vesicle; NMDAR, N-methyl-D-aspartate receptor; PrP, prion protein; PSD, post-synaptic density; VGCC, voltage-gated Ca++ channel.
Amyloid precursor protein (APP)
APP, whose proteolytic cleavage yields the pathological Aβ peptide, is implicated in Alzheimer’s disease pathology. The extracellular domain of APP binds Cu2+ [applied as Cu(NO3)2] with attomolar affinity (Atwood et al., 2000) while the intracellular domain is critical to APP trafficking to and from the plasma membrane (Guo et al., 2012). APP is concentrated in the endoplasmic reticulum of neurons, but endogenous APP immunoreactivity can be appreciated in dendritic as well as somatic domains of cortical, hippocampal and cerebellar neurons (Guo et al., 2012). Cu2+-glycine (1 µM) promotes the proteolytic cleavage of APP to yield Aβ, and Aβ-mediated neuronal toxicity is enhanced in the presence of Cu2+ (Huang et al., 1999). A connection between APP and Cu homeostasis became apparent when cortical Cu levels in mice lacking APP were shown to increase to approximately 150% of wildtype levels (White et al., 1999). APP over-expression results in a reduction of total brain Cu by 14% (Maynard et al., 2002). High levels of CuCl2 (150 µM) promote cell surface expression of APP and its distribution into neurites, but had no effect on APP proteolytic processing in cultured cortical neurons (Acevedo et al., 2011). APP clearly plays an important but poorly understood role in Cu homeostasis; its dendritic localization and avidity for Cu2+ suggest a role for APP in Cu-mediated modulation of synaptic transmission and plasticity (Fig. 3).
Prion protein (PrP)
PrP, another Cu-binding transmembrane protein, is involved in spongiform encephalopathies such as Creutzfeldt-Jakob disease [for review see (Vassallo and Herms, 2003; Zomosa-Signoret et al., 2008). PrP is pre- and post-synaptically expressed in neurons and contains 5 sites that bind Cu2+ with high nM to low µM affinity (Fig. 3) (Herms et al., 1999; Zomosa-Signoret et al., 2008). The binding of Cu2+ to PrP triggers internalization of the metalloprotein complex. Genetic deficiency of PrP in mice results in a reduction of synaptosomal Cu to 60% of wildype levels, but total Cu levels remain unchanged (Herms et al., 1999; Giese et al., 2005). However, reduced total Cu levels in PrP null mice have also been reported (Brown, 2003); this discrepancy may be age-dependent or result from methodological differences. The electrophysiological phenotype of these mice included impaired LTP and reduced GABAergic inhibition in the hippocampus (Collinge et al., 1994). Interestingly, the synaptic plasticity deficit of PrP null mice corresponds with impaired spatial learning and memory consolidation (Criado et al., 2005). While PrP clearly plays a role in Cu homeostasis, the details and significance of its role are not yet understood (Zomosa-Signoret et al., 2008) (Fig. 3). The Cu binding capacity of APP and PrP are not only highly suggestive of their role in Cu homeostatic disease states, but also implicate a pathophysiological role for disrupted Cu homeostasis in Alzheimer’s and prion-related diseases.
A Cu-mediated interaction between the neurotoxic Aβ peptide, PrP, and NMDA receptors was recently revealed using cultured rat hippocampal neurons (You et al., 2012). Application of either Aβ peptide (1 µM) or a Cu-specific chelator [bathocuproine disulphonate (BCS); 10 µM] similarly enhanced steady-state NMDA receptor currents, which contributed to neuronal death assayed by TUNEL staining. Co-application of Aβ peptide and BCS lacked any additive effect, suggesting action through a common mechanism. Genetic or proteolytic elimination of PrP increased baseline NMDA receptor steady-state current and abolished the enhancing effects of both Aβ peptide and BCS, laying the foundation for a regulatory role of PrP. The Cu-dependent co-immunoprecipitation of PrP with the obligate NR1 subunit of the NMDA receptor revealed a potential mechanism. The NR1 subunit contains a binding site for the co-agonist Gly, whose affinity for the NMDA receptor was enhanced in the absence of PrP. Together these data paint a compelling picture of PrP-dependent regulation of NMDA receptors that is pathologically disrupted in the presence of Aβ through the sequestration of Cu (Fig. 3). The authors highlight the implications of these results for neurodegenerative diseases, especially Alzheimer’s disease. Additionally, these results suggest a new direct mechanism by which Cu deficiency can contribute to hyperexcitability to precipitate seizure activity. This study also provides notable mechanistic insight into the complex influences of Cu on synaptic transmission and plasticity given the well-established role of NMDA receptor function in gating synaptic plasticity and learning and memory (see below) (Zajaczkowski et al., 1997).
TrkB/Brain-derived neurotrophic factor (BDNF)
Brain-derived neurotrophic factor (BDNF), a potent stimulant of neuronal and synaptic growth, is important in brain development, learning and memory [for review see (Minichiello, 2009)]. BDNF is released upon activity-dependent neuronal stimulation and binds to tropomyosin-receptor kinase B (TrkB) receptors (Fig. 4), inducing their autophosphorylation and activation. TrkB receptors expressed on cultured cortical neurons are phosphorylated/activated in response to exposure to CuCl2 for 15 min (Hwang et al., 2007). A maximal response is observed with 10 µM CuCl2, and Cu is more effective than several other metals, including Zn. CuCl2 enhances proBDNF release and proteolytic processing to yield mature BDNF. Both effects depend on the activity of matrix metalloproteinases 2 and 9 (MMP2, MMP9), extracellular Zn-dependent endopeptidases. An interesting aside is that BDNF signaling enhances hippocampal and cortical post-synaptic NMDA receptor activity (Madara and Levine, 2008), which could lead to further Cu release and TrkB activation. Thus, under certain conditions Cu could positively influence LTP through TrkB signaling (Fig. 4).
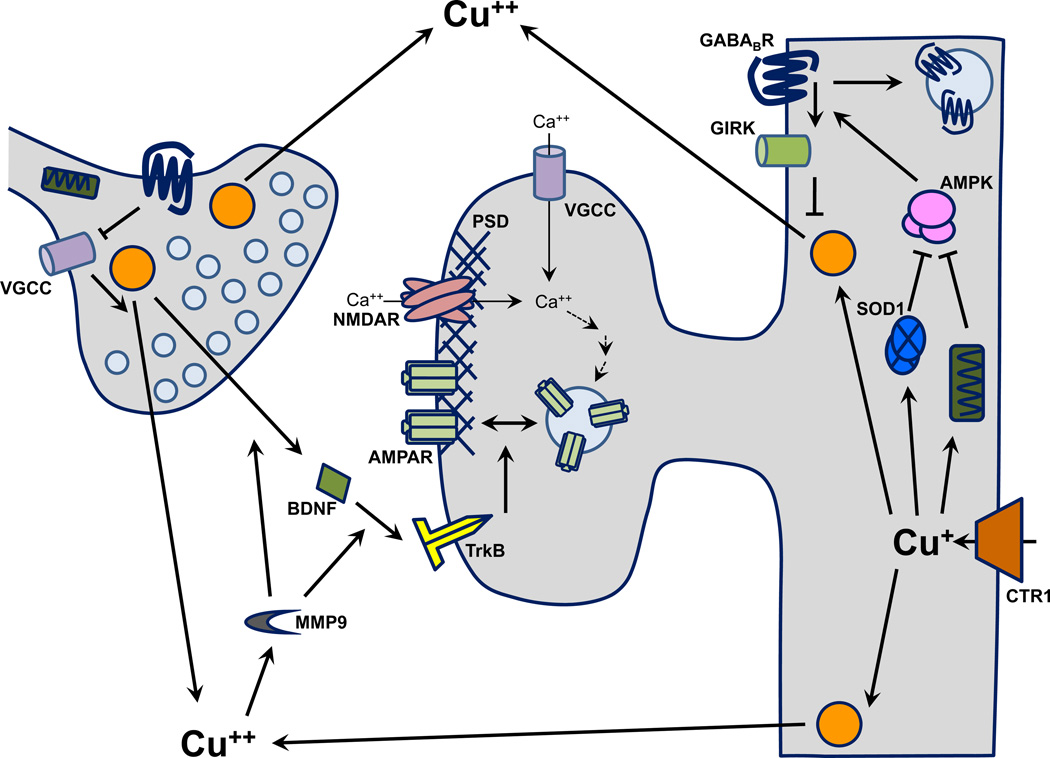
Fig. 4.
Potential mechanisms for Cu effects on synaptic transmission. After uptake, Cu bound to CCS is delivered to cytosolic SOD1 and Cu bound to Cox17 is delivered to mitochondrial COX. AMPK, a cellular energy sensor, is activated by superoxide and high AMP levels; SOD1 lowers superoxide levels and COX synthesizes ATP, reducing AMPK activity. AMPK phosphorylates GABAB receptors, which facilitates the downstream GIRK channel activation that mediates slow inhibition. Prolonged activation of NMDARs has the opposite effect on slow inhibition by promoting internalization of GABAB receptors via protein phosphatase 2 (not shown). Cu is also delivered to LDCVs via Atox-1 and ATP7A. Cu stored in vesicles is released through Ca2+-dependent mechanisms. Extracellular Cu stimulates MMP9, which facilitates the activation and release of BDNF. BDNF can bind to and activate TrkB receptors, which can enhance NMDAR-mediated currents and facilitate LTP. Abbreviations: AMP, adenosine monophosphate; AMPK, AMP-activated kinase; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; COX, cytochrome c oxidase; GABABR, γ-amino butyric acid B receptor; GIRK, G protein-coupled inward rectifying potassium; MMP9, matrix metalloproteinase 9; SOD1, superoxide dismutase 1; TrkB, tropomyosin receptor kinase B.
Matrix metalloproteinases
MMPs contribute to synaptic plasticity through additional mechanisms. MMP-9 activation is activity-dependent in the hippocampus and is necessary and sufficient for hippocampal LTP and learning and memory (Nagy et al., 2006; Bozdagi et al., 2007; Nagy et al., 2007). Moreover, MMP-9 deficient mice lack hippocampal LTP, but respond to LTP induced by bath application of recombinant MMP-9 (Nagy et al., 2006). These results were later corroborated with an MMP-9-dependent increase in dendritic spine volume which typically accompanies increases in synaptic strength (Wang et al., 2008). This effect of MMP-9 is mediated through its cleavage of ICAM-5, a post-synaptic integrin and negative regulator of synaptic strength and growth (Conant et al., 2010). Thus, activation of MMP-9 during LTP reduces the inhibitory effects of ICAM-5 on synaptic strength and growth. Neuronal, but not glial, MMP-9 is specifically activated by 10 µM CuCl2 (Hwang et al., 2007), but it remains to be determined whether this MMP/ICAM-5/LTP pathway requires or is modulated by the release and extracellular availability of Cu (Fig. 4).
Adenosine monophosphate (AMP)-activated protein kinase (AMPK)
As discussed above, extracellular Cu can affect cytosolic signal transduction pathways through its effects on NMDA receptors and MMPs. AMPK is a sensor used by eukaryotic cells to regulate cellular energy; in response to the AMP/ATP ratio, AMPK acts on multiple downstream pathways to increase net ATP production (Fig. 4). Cytosolic AMPK exists as a heterotrimer comprised of a catalytic α, and regulatory β and γ subunits (Spasic et al., 2009; Green et al., 2011). AMPK becomes activated via binding of AMP or by phosphorylation by upstream kinases such as Ca/calmodulin-dependent protein kinase kinase β (CaMKKβ) (Green et al., 2011). CaMKKβ is expressed in neuronal tissue and is activated by cytosolic Ca2+ supplied through the NMDA receptor (Thornton et al., 2011). Dietary Cu deficiency promotes AMPK activation and mitochondrial impairment in rat cerebellar tissue (Gybina and Prohaska, 2008). Interestingly, this effect occurs without a change in the AMP:ATP ratio, suggesting that Cu status influences AMPK activity through a parallel pathway. The Cu chaperone for superoxide dismutase (CCS; Fig. 1) is up-regulated in the Cu deficient cerebellum (Gybina and Prohaska, 2008), and SOD1 expression reduces superoxide-mediated activation of AMPK in cell cultures (Han et al., 2010). Thus AMPK activity is mediated through multiple Cu-dependent mechanisms.
AMPK has many downstream targets whose effects are primarily aimed at enhancing ATP synthesis and limiting cellular energy expenditure (Spasic et al., 2009). In neurons, important energy consumers include the maintenance of ion gradients and neuronal membrane activity. As such, AMPK activity limits neuronal activity through GABAB receptors (Fig. 4). GABAB receptors are heterodimeric G protein-coupled receptors whose extracellular binding of GABA induces inhibitory effects at pre- and post-synaptic sites (Terunuma et al., 2010a). Post-synaptically, GABAB receptors are coupled to inward rectifying K+ (GIRK) channels whose activity shunts propagating excitatory signals. In hippocampal neurons, AMPK activation with AMP or metformin prolongs GIRK channel activity via phosphorylation at Ser783 of the GABAB receptor 2 subunit (Kuramoto et al., 2007). However, others have shown that prolonged activation of NMDA receptors promotes internalization and degradation of GABAB receptors, presumably through protein phosphatase 2-mediated dephosphorylation of the same site (Terunuma et al., 2010b). This relationship is reciprocal, since GABAB receptor-mediated inhibition is a particularly important regulator of NMDA receptor mediated Ca2+ signals critical to LTP (Morrisett et al., 1991). The fact that Cu can influence the NMDA receptor/AMPK/GABAB receptor pathway at multiple points speaks to the likelihood that Cu plays an intricate role in modulating synaptic physiology and therefore behavior.
Roles of Cu in synaptic plasticity and behavior
Cu and synaptic plasticity
Exogenous Cu has consistently been shown to inhibit hippocampal long-term potentiation (LTP), a form of synaptic plasticity hypothesized to be important for learning and memory (Malenka, 2003; Lynch, 2004). Doreulee and colleagues (Doreulee et al., 1997) found that the depressing effects of Cu on NMDA receptors had consequences for LTP, which was prevented by 1 µM CuSO4 in the bath. Goldschmith and colleagues (Goldschmith et al., 2005) confirmed the inhibitory effects using brain slices generated from rats receiving elevated levels of dietary Cu (8–12 mg/day) for 20–25 days. Years later, the same groups tested the effects of chronically injected Cu (1 mg/kg, i.p.) on hippocampal LTP with similar results (Leiva et al., 2009). It was reported in both studies that Cu levels were more than 10-fold higher in the brains of Cu supplemented rats than in control rats, raising concerns of Cu toxicity. Nevertheless, it was concluded that elevated levels of Cu suppress LTP at Schaffer-CA1 synapses.
NMDA receptor-independent inhibition of LTP by Cu occurs in acute slices of mouse CA1 and CA3 regions of the hippocampus (Salazar-Weber and Smith, 2011). In this case, Cu2+ may block LTP through inhibition of high-voltage gated Ca2+ channels, which are sensitive to CuCl2 at an IC50 of ~1 µMin acutely dissociated rat cortical (Castelli et al., 2003) and olfactory bulb neurons (Horning and Trombley, 2001). In particular, L-type voltage-gated Ca2+ channels, which are located post-synaptically on dendrites and dendritic spines and contribute to Ca2+ influx during LTP induction (Kullmann et al., 2000; Malenka and Bear, 2004), are sensitive to 10 µM CuCl2 (Korte et al., 2003). Interestingly, this interaction may also depend on PrP (Korte et al., 2003). Thus Cu may impair LTP through multiple mechanisms (Figs. 3,4).
The physiological roles of Cu in the hippocampus are clearly complex. Acute CuCl2 application to hippocampal slices has no effect on paired-pulse facilitation (Doreulee et al., 1997; Salazar-Weber and Smith, 2011). However, NMDA receptor-independent LTP induction in the presence of 5 µM CuCl2 enhances paired pulse-facilitation and paired-pulse depression in CA1 and CA3 regions of the hippocampus, respectively (Salazar-Weber and Smith, 2011). To determine the effect of Cu on established LTP, Leiva and colleagues (Leiva et al., 2003) waited until 90 min after LTP induction to bath apply 10 µM CuSO4, which decreased synaptic efficacy as observed previously. Upon washout, the synaptic response returned at a substantially larger size. While these findings are difficult to interpret, they clearly demonstrate separate activity-dependent synaptic effects of Cu that have complex influences on synaptic plasticity. In a separate set of experiments performed by the same group, paired pulses were applied to Schaffer collaterals at intervals ranging from 20 to 300 ms in hippocampal slices generated from rats on normal or chronic Cu supplemented diets (Goldschmith et al., 2005). Paired pulse facilitation decreased in both groups following LTP induction, representing enhancement of pre-synaptic release probability. However, this change was blunted in Cu supplemented animals, and paired pulse ratios were reduced in Cu supplemented animals at baseline. These results are consistent with enhanced pre-synaptic release probability at baseline with Cu supplementation and support an alternative interpretation of occluded rather than impaired LTP. The contrast between the synaptic effects of Cu applied in vitro and in vivo are difficult to reconcile, but together may suggest a dual effect of Cu on synaptic plasticity in the hippocampus, where Cu prevents the induction of new LTP and may reinforce LTP that has already been established.
These findings are consistent with biochemical evidence of the inhibitory effect of Cu2+ on key molecules involved in LTP induction. The NMDA receptor acts as a coincidence detector for synaptic activity and post-synaptic excitability. Unlike the majority of its glutamate receptor counterparts, NMDA receptors are permeable to Ca2+, which triggers a cascade of signaling events that ultimately lead to changes in post-synaptic receptor expression and/or pre-synaptic release probability (Malenka, 2003; Yashiro and Philpot, 2008). Schlief and colleagues (Schlief et al., 2005) found that NMDA receptor activation promoted the movement of ATP7A into dendrites and the ATP7A-dependent release of Cu from primary hippocampal neurons. Importantly, the effect of NMDA receptor activation on ATP7A expression was Cu-independent since the experiment was conducted in the presence of the Cu-specific chelator BCS. Together these data demonstrate that the relationship between NMDA receptor activity and Cu homeostasis is bidirectional, and this relationship strongly supports a physiological role for Cu in synaptic plasticity and learning and memory.
Cu and behavior
Despite the consistent and striking observations made to this point in vitro, a direct behavioral effect of Cu on hippocampal-dependent learning and memory has not yet been demonstrated. Leiva and colleagues 2009 (Leiva et al., 2009) showed that the same chronic Cu injection regimen had no effect on performance in the Morris water maze task, a cognitive behavioral task testing working and long-term spatial (hippocampal-dependent) memory. Neither dietary Cu supplementation (1 ppm CuSO4 in the drinking water) nor Cu chelation (using 0.1% D-penicillamine dissolved in the drinking water) had an effect on shuttle-box avoidance learning by rats (Fujiwara et al., 2006). Dietary Cu supplementation alone is anxiolytic and reduced sensorimotor gating performance in the same study. Along the same lines, mild dietary Cu restriction (0.6 ppm food Cu for 9–10 weeks) in mice has an anxiogenic effect in the elevated zero maze (Bousquet-Moore et al., 2010). The same dietary Cu restriction regimen also enhances sensitivity to pentylenetetrazol-induced seizures, consistent with the seizure-prone Menkes phenotype. Dietary supplementation with Zn, which competes with Cu for uptake in the gut, impaired fear learning and memory in normal mice and spatial learning and memory in an Alzheimer’s mouse model (Railey et al., 2010; Railey et al., 2011). Consistent with the idea that Zn supplementation induces Cu deficiency, the deficits in fear learning were alleviated with Cu supplementation (Railey et al., 2010). Thus the strength of dietary challenge can weigh heavily on behavioral assessment of the role of Cu in brain function.
The mammalian hippocampus possesses a uniquely high density of NMDA receptors, specifically at the Schaffer-CA1 synapse (Meoni et al., 1998). As such, LTP at Schaffer-CA1 synapses is characteristically reliant on NMDA receptor-mediated signaling. However, LTP in other brain regions such as the cortex and amygdala does not rely as heavily on NMDA receptors, and may thus respond differently to the levels of Cu available. NMDA receptor-independent LTP may also respond differently in the presence of Cu even within the hippocampus. Therefore, the roles of Cu in synaptic plasticity and learning and memory are complex and likely region-specific, which may account for the array of symptoms observed in disorders involving disrupted Cu homeostasis including Alzheimer’s disease, prion disease, and Wilson’s disease [for review see (Uriu-Adams and Keen, 2005)].
Conclusions
The arrival of Cu on the biological scene before the need for a nervous system indicates that Cu was available as the complex neuronal signaling pathways we see today evolved. The reactivity of this metal and its unique association with metabolic processes dependent on molecular oxygen, combined with its presence at synapses suggest many roles for endogenous Cu in normal brain function. Studies performed to date reveal a complex network of interconnected pathways through which Cu can both serve as a signal itself and modulate signaling through multiple pathways. Additional studies will undoubtedly reveal more Cu-responsive pathways than the limited studies done to date. Common neurological diseases like Alzheimer's disease, with cognitive and behavioral symptomology, involve pathology in which Cu homeostasis is disrupted. Thus, there is a need for further investigation into the complex network of Cu signaling and pathways responsive to Cu in the brain. Advancement of our understanding of these mechanisms will help identify new molecular targets for neurological and psychiatric diseases, many of which currently have few and poor treatment options, and will provide great insight into the basic mechanisms through which humans interact with and experience the world.
Reference List
- Acevedo KM, Hung YH, Dalziel AH, Li QX, Laughton K, Wikhe K, Rembach A, Roberts B, Masters CL, Bush AI, Camakaris J. Copper promotes the trafficking of the amyloid precursor protein. J. Biol. Chem. 2011;286:8252–8262. doi: 10.1074/jbc.M110.128512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil M, Brewer GJ. Psychiatric and behavioral abnormalities in Wilson's disease. Adv. Neurol. 1995;65:171–178. [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J. Proteome. Res. 2008;7:209–216. doi: 10.1021/pr070480u. [DOI] [PubMed] [Google Scholar]
- Attri S, Sharma N, Jahagirdar S, Thapa BR, Prasad R. Erythrocyte metabolism and antioxidant status of patients with Wilson disease with hemolytic anemia. Pediatr. Res. 2006;59:593–597. doi: 10.1203/01.pdr.0000203098.77573.39. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Scarpa RC, Huang X, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI. Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1-42. J. Neurochem. 2000;75:1219–1233. doi: 10.1046/j.1471-4159.2000.0751219.x. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Differential modulation of synaptic transmission by neuropeptide Y in rat neocortical neurons. Proc. Natl. Acad. Sci. U. S. A. 2002;99:17125–17130. doi: 10.1073/pnas.012481899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerga ID, Maickel RP, Green MA. Subcellular distribution of tissue radiocopper following intravenous administration of 67Cu-labeled Cu-PTSM. Int. J. Rad. Appl. Instrum. B. 1992;19:697–701. doi: 10.1016/0883-2897(92)90104-7. [DOI] [PubMed] [Google Scholar]
- Bajorek JG, Lee RJ, Lomax P. Neuropeptides: anticonvulsant and convulsant mechanisms in epileptic model systems and in humans. Adv. Neurol. 1986;44:489–500. [PubMed] [Google Scholar]
- Bakirdere S, Kizilkan N, Yaman M. Determination of Zinc, Copper, Iron, and Manganese in Different Regions of Lamb Brain. Biol. Trace Elem. Res. 2010 doi: 10.1007/s12011-010-8804-0. [DOI] [PubMed] [Google Scholar]
- Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465:645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- Bielli P, Calabrese L. Structure to function relationships in ceruloplasmin: a 'moonlighting' protein. Cell Mol. Life Sci. 2002;59:1413–1427. doi: 10.1007/s00018-002-8519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Moore D, Prohaska JR, Nillni EA, Czyzyk T, Wetsel WC, Mains RE, Eipper BA. Interactions of peptide amidation and copper: novel biomarkers and mechanisms of neural dysfunction. Neurobiol. Dis. 2010;37:130–140. doi: 10.1016/j.nbd.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR. Prion protein expression modulates neuronal copper content. J. Neurochem. 2003;87:377–385. doi: 10.1046/j.1471-4159.2003.02046.x. [DOI] [PubMed] [Google Scholar]
- Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, von BA, Schulz-Schaeffer W, Giese A, Westaway D, Kretzschmar H. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- Castelli L, Tanzi F, Taglietti V, Magistretti J. Cu2+, Co2+, and Mn2+ modify the gating kinetics of high-voltage-activated Ca2+ channels in rat palaeocortical neurons. J. Membr. Biol. 2003;195:121–136. doi: 10.1007/s00232-003-0614-2. [DOI] [PubMed] [Google Scholar]
- Choi BS, Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009;1248:14–21. doi: 10.1016/j.brainres.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloix JF, Hevor T. Epilepsy, regulation of brain energy metabolism and neurotransmission. Curr. Med. Chem. 2009;16:841–853. doi: 10.2174/092986709787549316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Whittington MA, Sidle KC, Smith CJ, Palmer MS, Clarke AR, Jefferys JG. Prion protein is necessary for normal synaptic function. Nature. 1994;370:295–297. doi: 10.1038/370295a0. [DOI] [PubMed] [Google Scholar]
- Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166:508–521. doi: 10.1016/j.neuroscience.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Criado JR, Sanchez-Alavez M, Conti B, Giacchino JL, Wills DN, Henriksen SJ, Race R, Manson JC, Chesebro B, Oldstone MB. Mice devoid of prion protein have cognitive deficits that are rescued by reconstitution of PrP in neurons. Neurobiol. Dis. 2005;19:255–265. doi: 10.1016/j.nbd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Crichton RR, Pierre JL. Old iron, young copper: from Mars to Venus. Biometals. 2001;14:99–112. doi: 10.1023/a:1016710810701. [DOI] [PubMed] [Google Scholar]
- Critchfield JW, Carl FG, Keen CL. Anticonvulsant-induced changes in tissue manganese, zinc, copper, and iron concentrations in Wistar rats. Metabolism. 1993;42:907–910. doi: 10.1016/0026-0495(93)90068-y. [DOI] [PubMed] [Google Scholar]
- Das SK, Ray K. Wilson's disease: an update. Nat. Clin. Pract. Neurol. 2006;2:482–493. doi: 10.1038/ncpneuro0291. [DOI] [PubMed] [Google Scholar]
- de Bie P, Muller P, Wijmenga C, Klomp LW. Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. J. Med. Genet. 2007;44:673–688. doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker H, Terwilliger N. Cops and robbers: putative evolution of copper oxygen-binding proteins. J. Exp. Biol. 2000;203:1777–1782. doi: 10.1242/jeb.203.12.1777. [DOI] [PubMed] [Google Scholar]
- Deng PY, Porter JE, Shin HS, Lei S. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J. Physiol. 2006;577:497–511. doi: 10.1113/jphysiol.2006.118141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolska J, Dehnhardt M, Matusch A, Zoriy M, Palomero-Gallagher N, Koscielniak P, Zilles K, Becker JS. Quantitative imaging of zinc, copper and lead in three distinct regions of the human brain by laser ablation inductively coupled plasma mass spectrometry. Talanta. 2008;74:717–723. doi: 10.1016/j.talanta.2007.06.051. [DOI] [PubMed] [Google Scholar]
- Dodani SC, Domaille DW, Nam CI, Miller EW, Finney LA, Vogt S, Chang CJ. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5980–5985. doi: 10.1073/pnas.1009932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Tang J, Godwin SC, Holmes CS, Goldstein DS, Bassuk A, Kaler SG. Differences in ATP7A gene expression underlie intrafamilial variability in Menkes disease/occipital horn syndrome. J. Med. Genet. 2007;44:492–497. doi: 10.1136/jmg.2007.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Yi L, Zerfas PM, Brinster LR, Sullivan P, Goldstein DS, Prohaska J, Centeno JA, Rushing E, Kaler SG. ATP7A gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a Menkes disease mouse model. Mol. Ther. 2011;19:2114–2123. doi: 10.1038/mt.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreulee N, Yanovsky Y, Haas HL. Suppression of long-term potentiation in hippocampal slices by copper. Hippocampus. 1997;7:666–669. doi: 10.1002/(SICI)1098-1063(1997)7:6<666::AID-HIPO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. The role of an alpha subtype M2-M3 His in regulating inhibition of GABAA receptor current by zinc and other divalent cations. J. Neurosci. 1998;18:2944–2953. doi: 10.1523/JNEUROSCI.18-08-02944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Iso H, Kitanaka N, Kitanaka J, Eguchi H, Ookawara T, Ozawa K, Shimoda S, Yoshihara D, Takemura M, Suzuki K. Effects of copper metabolism on neurological functions in Wistar and Wilson's disease model rats. Biochem. Biophys. Res. Commun. 2006;349:1079–1086. doi: 10.1016/j.bbrc.2006.08.139. [DOI] [PubMed] [Google Scholar]
- Gellein K, Roos PM, Evje L, Vesterberg O, Flaten TP, Nordberg M, Syversen T. Separation of proteins including metallothionein in cerebrospinal fluid by size exclusion HPLC and determination of trace elements by HR-ICP-MS. Brain Res. 2007;1174:136–142. doi: 10.1016/j.brainres.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Giese A, Buchholz M, Herms J, Kretzschmar HA. Mouse brain synaptosomes accumulate copper-67 efficiently by two distinct processes independent of cellular prion protein. J. Mol. Neurosci. 2005;27:347–354. doi: 10.1385/JMN:27:3:347. [DOI] [PubMed] [Google Scholar]
- Goldschmith A, Infante C, Leiva J, Motles E, Palestini M. Interference of chronically ingested copper in long-term potentiation (LTP) of rat hippocampus. Brain Res. 2005;1056:176–182. doi: 10.1016/j.brainres.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Green MF, Anderson KA, Means AR. Characterization of the CaMKKbeta-AMPK signaling complex. Cell Signal. 2011;23:2005–2012. doi: 10.1016/j.cellsig.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Li H, Gaddam SS, Justice NJ, Robertson CS, Zheng H. Amyloid Precursor Protein Revisited: NEURON-SPECIFIC EXPRESSION AND HIGHLY STABLE NATURE OF SOLUBLE DERIVATIVES. J. Biol. Chem. 2012;287:2437–2445. doi: 10.1074/jbc.M111.315051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gybina AA, Prohaska JR. Copper deficiency results in AMP-activated protein kinase activation and acetylCoA carboxylase phosphorylation in rat cerebellum. Brain Res. 2008;1204:69–76. doi: 10.1016/j.brainres.2008.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I, Faisst A, Prohaska J, Chen J, Gruss P, Gitlin JD. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6848–6852. doi: 10.1073/pnas.111058498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang Q, Song P, Zhu Y, Zou MH. Redox regulation of the AMP-activated protein kinase. PLoS. One. 2010;5:e15420. doi: 10.1371/journal.pone.0015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartter DE, Barnea A. Evidence for release of copper in the brain: depolarization-induced release of newly taken-up 67copper. Synapse. 1988;2:412–415. doi: 10.1002/syn.890020408. [DOI] [PubMed] [Google Scholar]
- Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- Herms J, Tings T, Gall S, Madlung A, Giese A, Siebert H, Schurmann P, Windl O, Brose N, Kretzschmar H. Evidence of presynaptic location and function of the prion protein. J. Neurosci. 1999;19:8866–8875. doi: 10.1523/JNEUROSCI.19-20-08866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol. Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Horning MS, Trombley PQ. Zinc and copper influence excitability of rat olfactory bulb neurons by multiple mechanisms. J. Neurophysiol. 2001;86:1652–1660. doi: 10.1152/jn.2001.86.4.1652. [DOI] [PubMed] [Google Scholar]
- Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci. 2011;303:95–99. doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Huang X, Cuajungco MP, Atwood CS, Hartshorn MA, Tyndall JD, Hanson GR, Stokes KC, Leopold M, Multhaup G, Goldstein LE, Scarpa RC, Saunders AJ, Lim J, Moir RD, Glabe C, Bowden EF, Masters CL, Fairlie DP, Tanzi RE, Bush AI. Cu(II) potentiation of alzheimer abeta neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- Hwang JJ, Park MH, Koh JY. Copper activates TrkB in cortical neurons in a metalloproteinase-dependent manner. J. Neurosci. Res. 2007;85:2160–2166. doi: 10.1002/jnr.21350. [DOI] [PubMed] [Google Scholar]
- Jackson B, Harper S, Smith L, Flinn J. Elemental mapping and quantitative analysis of Cu, Zn, and Fe in rat brain sections by laser ablation ICP-MS. Anal. Bioanal. Chem. 2006;384:951–957. doi: 10.1007/s00216-005-0264-6. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LC, Beard JL, Jones BC. Genetic analysis reveals polygenic influences on iron, copper, and zinc in mouse hippocampus with neurobiological implications. Hippocampus. 2008;18:398–410. doi: 10.1002/hipo.20399. [DOI] [PubMed] [Google Scholar]
- Kaler SG. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapaki E, Segditsa J, Papageorgiou C. Zinc, copper and magnesium concentration in serum and CSF of patients with neurological disorders. Acta Neurol. Scand. 1989;79:373–378. doi: 10.1111/j.1600-0404.1989.tb03803.x. [DOI] [PubMed] [Google Scholar]
- Kardos J, Kovacs I, Hajos F, Kalman M, Simonyi M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci. Lett. 1989;103:139–144. doi: 10.1016/0304-3940(89)90565-x. [DOI] [PubMed] [Google Scholar]
- Kardos J, Samu J, Ujszaszi K, Nagy J, Kovacs I, Visy J, Maksay G, Simonyi M. Cu2+ is the active principle of an endogenous substance from porcine cerebral cortex which antagonizes the anticonvulsant effect of diazepam. Neurosci. Lett. 1984;52:67–72. doi: 10.1016/0304-3940(84)90352-5. [DOI] [PubMed] [Google Scholar]
- Ke BX, Llanos RM, Wright M, Deal Y, Mercer JF. Alteration of copper physiology in mice overexpressing the human Menkes protein ATP7A. Am. J. Physiol Regul. Integr. Comp Physiol. 2006;290:R1460–R1467. doi: 10.1152/ajpregu.00806.2005. [DOI] [PubMed] [Google Scholar]
- Kim BE, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab. 2010;11:353–363. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Macdonald RL. An N-terminal histidine is the primary determinant of alpha subunit-dependent Cu2+ sensitivity of alphabeta3gamma2L GABA(A) receptors. Mol. Pharmacol. 2003;64:1145–1152. doi: 10.1124/mol.64.5.1145. [DOI] [PubMed] [Google Scholar]
- Kodama H, Fujisawa C, Bhadhprasit W. Pathology, clinical features and treatments of congenital copper metabolic disorders--focus on neurologic aspects. Brain Dev. 2011;33:243–251. doi: 10.1016/j.braindev.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Kodama H, Murata Y, Kobayashi M. Clinical manifestations and treatment of Menkes disease and its variants. Pediatr. Int. 1999;41:423–429. doi: 10.1046/j.1442-200x.1999.01095.x. [DOI] [PubMed] [Google Scholar]
- Korte S, Vassallo N, Kramer ML, Kretzschmar HA, Herms J. Modulation of L-type voltage-gated calcium channels by recombinant prion protein. J. Neurochem. 2003;87:1037–1042. doi: 10.1046/j.1471-4159.2003.02080.x. [DOI] [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. A functional comparison of the antagonists bicuculline and picrotoxin at recombinant GABAA receptors. Neuropharmacology. 1996;35:1289–1298. doi: 10.1016/s0028-3908(96)00089-5. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F, Walker MC. The role of mammalian ionotropic receptors in synaptic plasticity: LTP, LTD and epilepsy. Cell Mol. Life Sci. 2000;57:1551–1561. doi: 10.1007/PL00000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva J, Gaete P, Palestini M. Copper interaction on the long-term potentiation. Arch. Ital. Biol. 2003;141:149–155. [PubMed] [Google Scholar]
- Leiva J, Palestini M, Infante C, Goldschmidt A, Motles E. Copper suppresses hippocampus LTP in the rat, but does not alter learning or memory in the morris water maze. Brain Res. 2009;1256:69–75. doi: 10.1016/j.brainres.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Leiva J, Palestini M, Tetas M, Lopez J. Copper sensitivity in dorsal hippocampus slices. Arch. Ital. Biol. 2000;138:175–184. [PubMed] [Google Scholar]
- Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996;63:797S–811S. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- Liu PC, Chen YW, Centeno JA, Quezado M, Lem K, Kaler SG. Downregulation of myelination, energy, and translational genes in Menkes disease brain. Mol. Genet. Metab. 2005;85:291–300. doi: 10.1016/j.ymgme.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol. 2010;14:211–217. doi: 10.1016/j.cbpa.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Bhattacharjee A, Hubbard AL. Copper handling machinery of the brain. Metallomics. 2010;2:596–608. doi: 10.1039/c0mt00006j. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Ma J, Betts NM. Zinc and copper intakes and their major food sources for older adults in the 1994-96 continuing survey of food intakes by individuals (CSFII) J. Nutr. 2000;130:2838–2843. doi: 10.1093/jn/130.11.2838. [DOI] [PubMed] [Google Scholar]
- Ma JY, Narahashi T. Differential modulation of GABAA receptor-channel complex by polyvalent cations in rat dorsal root ganglion neurons. Brain Res. 1993;607:222–232. doi: 10.1016/0006-8993(93)91510-y. [DOI] [PubMed] [Google Scholar]
- Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J. Neurophysiol. 2008;100:3175–3184. doi: 10.1152/jn.90880.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. The long-term potential of LTP. Nat. Rev. Neurosci. 2003;4:923–926. doi: 10.1038/nrn1258. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Pare D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J. Neurophysiol. 2001;86:2887–2895. doi: 10.1152/jn.2001.86.6.2887. [DOI] [PubMed] [Google Scholar]
- Mason ST, Corcoran ME. Forebrain noradrenaline and Metrazol-induced seizures. Life Sci. 1978;23:167–171. doi: 10.1016/0024-3205(78)90266-7. [DOI] [PubMed] [Google Scholar]
- Mason ST, Corcoran ME. Depletion of brain noradrenaline, but not dopamine, by intracerebral 6-hydroxydopamine potentiates convulsions induced by electroshock. J. Pharm. Pharmacol. 1979;31:209–211. doi: 10.1111/j.2042-7158.1979.tb13480.x. [DOI] [PubMed] [Google Scholar]
- Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Ther. 2006;111:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. Overexpression of Alzheimer's disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002;277:44670–44676. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci. Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- Melo TM, Larsen C, White LR, Aasly J, Sjobakk TE, Flaten TP, Sonnewald U, Syversen T. Manganese, copper, and zinc in cerebrospinal fluid from patients with multiple sclerosis. Biol. Trace Elem. Res. 2003;93:1–8. doi: 10.1385/BTER:93:1-3:1. [DOI] [PubMed] [Google Scholar]
- MENKES JH, Aleter M, STEIGLEDER GK, WEAKLEY DR, SUNG JH. A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics. 1962;29:764–779. [PubMed] [Google Scholar]
- Meoni P, Mugnaini M, Bunnemann BH, Trist DG, Bowery NG. [3H]MK-801 binding and the mRNA for the NMDAR1 subunit of the NMDA receptor are differentially distributed in human and rat forebrain. Brain Res. Mol. Brain Res. 1998;54:13–23. doi: 10.1016/s0169-328x(97)00289-1. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Burger RL, Bettendorf AF, Browning RA, Jobe PC. Role of norepinephrine in forebrain and brainstem seizures: chemical lesioning of locus ceruleus with DSP4. Exp. Neurol. 1994;125:58–64. doi: 10.1006/exnr.1994.1006. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Ogawa N, Mori A. Super-high-affinity binding site for [3H]diazepam in the presence of Co2+, Ni2+, Cu2+, or Zn2+ Neurochem. Res. 1982;7:1487–1493. doi: 10.1007/BF00965091. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Mott DD, Lewis DV, Swartzwelder HS, Wilson WA. GABAB-receptor-mediated inhibition of the N-methyl-D-aspartate component of synaptic transmission in the rat hippocampus. J. Neurosci. 1991;11:203–209. doi: 10.1523/JNEUROSCI.11-01-00203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn. Mem. 2007;14:655–664. doi: 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Ma JY, Arakawa O, Reuveny E, Nakahiro M. GABA receptor-channel complex as a target site of mercury, copper, zinc, and lanthanides. Cell Mol. Neurobiol. 1994;14:599–621. doi: 10.1007/BF02088671. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Ma XM, El Meskini R, Ronnett GV, Mains RE, Eipper BA. Developmental changes in the expression of ATP7A during a critical period in postnatal neurodevelopment. Neuroscience. 2006;139:947–964. doi: 10.1016/j.neuroscience.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur. J. Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Pan JW, Williamson A, Cavus I, Hetherington HP, Zaveri H, Petroff OA, Spencer DD. Neurometabolism in human epilepsy. Epilepsia. 2008;49(Suppl 3):31–41. doi: 10.1111/j.1528-1167.2008.01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Munoz B, Sepulveda FJ, Urrutia J, Quiroz M, Luza S, De Ferrari GV, Aguayo LG, Opazo C. Biphasic effects of copper on neurotransmission in rat hippocampal neurons. J. Neurochem. 2011;119:78–88. doi: 10.1111/j.1471-4159.2011.07417.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RF. Wilson's Disease. Semin. Neurol. 2007;27:123–132. doi: 10.1055/s-2007-971173. [DOI] [PubMed] [Google Scholar]
- Prasad AN, Levin S, Rupar CA, Prasad C. Menkes disease and infantile epilepsy. Brain Dev. 2011;33:866–876. doi: 10.1016/j.braindev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Prohaska JR. Functions of trace elements in brain metabolism. Physiol Rev. 1987;67:858–901. doi: 10.1152/physrev.1987.67.3.858. [DOI] [PubMed] [Google Scholar]
- Que EL, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- Raggenbass M. Overview of cellular electrophysiological actions of vasopressin. Eur. J. Pharmacol. 2008;583:243–254. doi: 10.1016/j.ejphar.2007.11.074. [DOI] [PubMed] [Google Scholar]
- Rahil-Khazen R, Bolann BJ, Myking A, Ulvik RJ. Multi-element analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES) J. Trace Elem. Med. Biol. 2002;16:15–25. doi: 10.1016/S0946-672X(02)80004-9. [DOI] [PubMed] [Google Scholar]
- Railey AM, Groeber CM, Flinn JM. The effect of metals on spatial memory in a transgenic mouse model of Alzheimer's disease. J. Alzheimers. Dis. 2011;24:375–381. doi: 10.3233/JAD-2011-101452. [DOI] [PubMed] [Google Scholar]
- Railey AM, Micheli TL, Wanschura PB, Flinn JM. Alterations in fear response and spatial memory in pre- and post-natal zinc supplemented rats: remediation by copper. Physiol Behav. 2010;100:95–100. doi: 10.1016/j.physbeh.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Rajan KS, Colburn RW, Davis JM. Distribution of metal ions in the subcellular fractions of several rat brain areas. Life Sci. 1976;18:423–431. doi: 10.1016/0024-3205(76)90220-4. [DOI] [PubMed] [Google Scholar]
- Ridge PG, Zhang Y, Gladyshev VN. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS. One. 2008;3:e1378. doi: 10.1371/journal.pone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Saito T, Itoh T, Fujimura M, Saito K. Age-dependent and region-specific differences in the distribution of trace elements in 7 brain regions of Long-Evans Cinnamon (LEC) rats with hereditary abnormal copper metabolism. Brain Res. 1995;695:240–244. doi: 10.1016/0006-8993(95)00819-c. [DOI] [PubMed] [Google Scholar]
- Salazar-Weber NL, Smith JP. Copper Inhibits NMDA Receptor-Independent LTP and Modulates the Paired-Pulse Ratio after LTP in Mouse Hippocampal Slices. Int. J. Alzheimers. Dis. 2011;2011:864753. doi: 10.4061/2011/864753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- Schlief ML, Craig AM, Gitlin JD. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J. Neurosci. 2005;25:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief ML, West T, Craig AM, Holtzman DM, Gitlin JD. Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14919–14924. doi: 10.1073/pnas.0605390103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DB, Ehlers MD. Glutamate Receptors, Ionotropic. In: Chief á-á, William JL, Lane MD., editors. Encyclopedia of Biological Chemistry. New York: Elsevier; 2004. pp. 213–219. [Google Scholar]
- Sharonova IN, Vorobjev VS, Haas HL. High-affinity copper block of GABA(A) receptor-mediated currents in acutely isolated cerebellar Purkinje cells of the rat. Eur. J. Neurosci. 1998;10:522–528. doi: 10.1046/j.1460-9568.1998.00057.x. [DOI] [PubMed] [Google Scholar]
- Sharonova IN, Vorobjev VS, Haas HL. Interaction between copper and zinc at GABA(A) receptors in acutely isolated cerebellar Purkinje cells of the rat. Br. J. Pharmacol. 2000;130:851–856. doi: 10.1038/sj.bjp.0703392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic MR, Callaerts P, Norga KK. AMP-activated protein kinase (AMPK) molecular crossroad for metabolic control and survival of neurons. Neuroscientist. 2009;15:309–316. doi: 10.1177/1073858408327805. [DOI] [PubMed] [Google Scholar]
- Srinivas K, Sinha S, Taly AB, Prashanth LK, Arunodaya GR, Janardhana Reddy YC, Khanna S. Dominant psychiatric manifestations in Wilson's disease: a diagnostic and therapeutic challenge. J. Neurol. Sci. 2008;266:104–108. doi: 10.1016/j.jns.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Szot P, Weinshenker D, White SS, Robbins CA, Rust NC, Schwartzkroin PA, Palmiter RD. Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J. Neurosci. 1999;19:10985–10992. doi: 10.1523/JNEUROSCI.19-24-10985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly AB, Meenakshi-Sundaram S, Sinha S, Swamy HS, Arunodaya GR. Wilson disease: description of 282 patients evaluated over 3 decades. Medicine (Baltimore) 2007;86:112–121. doi: 10.1097/MD.0b013e318045a00e. [DOI] [PubMed] [Google Scholar]
- Tamano H, Takeda A. Dynamic action of neurometals at the synapse. Metallomics. 2011;3:656–661. doi: 10.1039/c1mt00008j. [DOI] [PubMed] [Google Scholar]
- Tarohda T, Yamamoto M, Amamo R. Regional distribution of manganese, iron, copper, and zinc in the rat brain during development. Anal. Bioanal. Chem. 2004;380:240–246. doi: 10.1007/s00216-004-2697-8. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Pangalos MN, Moss SJ. Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv. Pharmacol. 2010a;58:113–122. doi: 10.1016/S1054-3589(10)58005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Vargas KJ, Wilkins ME, Ramirez OA, Jaureguiberry-Bravo M, Pangalos MN, Smart TG, Moss SJ, Couve A. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc. Natl. Acad. Sci. U. S. A. 2010b;107:13918–13923. doi: 10.1073/pnas.1000853107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Loschmann YN, Schmidt HH, Scholer HR, Cantz T, Heneka MT. Neuroinflammatory and behavioural changes in the Atp7B mutant mouse model of Wilson's disease. J. Neurochem. 2011;118:105–112. doi: 10.1111/j.1471-4159.2011.07278.x. [DOI] [PubMed] [Google Scholar]
- Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D. AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem. J. 2011;434:503–512. doi: 10.1042/BJ20101485. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Horning MS, Blakemore LJ. Carnosine modulates zinc and copper effects on amino acid receptors and synaptic transmission. Neuroreport. 1998;9:3503–3507. doi: 10.1097/00001756-199810260-00031. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Differential modulation by zinc and copper of amino acid receptors from rat olfactory bulb neurons. J. Neurophysiol. 1996;76:2536–2546. doi: 10.1152/jn.1996.76.4.2536. [DOI] [PubMed] [Google Scholar]
- Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol. Aspects Med. 2005;26:268–298. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Vassallo N, Herms J. Cellular prion protein function in copper homeostasis and redox signalling at the synapse. J. Neurochem. 2003;86:538–544. doi: 10.1046/j.1471-4159.2003.01882.x. [DOI] [PubMed] [Google Scholar]
- Vlachova V, Zemkova H, Vyklicky L., Jr Copper modulation of NMDA responses in mouse and rat cultured hippocampal neurons. Eur. J. Neurosci. 1996;8:2257–2264. doi: 10.1111/j.1460-9568.1996.tb01189.x. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Macaulay AJ, Fradley R, O'Meara GF, Reynolds DS, Rosahl TW. Differentiating the role of gamma-aminobutyric acid type A (GABAA) receptor subtypes. Biochem. Soc. Trans. 2004;32:553–556. doi: 10.1042/BST0320553. [DOI] [PubMed] [Google Scholar]
- Waggoner DJ, Drisaldi B, Bartnikas TB, Casareno RL, Prohaska JR, Gitlin JD, Harris DA. Brain copper content and cuproenzyme activity do not vary with prion protein expression level. J. Biol. Chem. 2000;275:7455–7458. doi: 10.1074/jbc.275.11.7455. [DOI] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser T, Wienrich M. The effects of copper ions on glutamate receptors in cultured rat cortical neurons. Brain Res. 1996;742:211–218. doi: 10.1016/s0006-8993(96)01009-8. [DOI] [PubMed] [Google Scholar]
- White AR, Reyes R, Mercer JF, Camakaris J, Zheng H, Bush AI, Multhaup G, Beyreuther K, Masters CL, Cappai R. Copper levels are increased in the cerebral cortex and liver of APP and APLP2 knockout mice. Brain Res. 1999;842:439–444. doi: 10.1016/s0006-8993(99)01861-2. [DOI] [PubMed] [Google Scholar]
- Wijmenga C, Klomp LW. Molecular regulation of copper excretion in the liver. Proc. Nutr. Soc. 2004;63:31–39. doi: 10.1079/pns2003316. [DOI] [PubMed] [Google Scholar]
- Xie X, Gerber U, Gahwiler BH, Smart TG. Interaction of zinc with ionotropic and metabotropic glutamate receptors in rat hippocampal slices. Neurosci. Lett. 1993;159:46–50. doi: 10.1016/0304-3940(93)90795-m. [DOI] [PubMed] [Google Scholar]
- Yang MS, Wong HF, Yung KL. Determination of endogenous trace metal contents in various mouse brain regions after prolonged oral administration of aluminum chloride. J. Toxicol. Environ. Health A. 1998;55:445–453. doi: 10.1080/009841098158359. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Tsutsui S, Hameed S, Kannanayakal TJ, en L, P E, bers JD, Lipton SA, Stys PK, Zamponi GW. ABeta neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1737–1742. doi: 10.1073/pnas.1110789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadina JE, Banks WA, Kastin AJ. Central nervous system effects of peptides, 1980–1985: a cross-listing of peptides and their central actions from the first six years of the journal Peptides. Peptides. 1986;7:497–537. doi: 10.1016/0196-9781(86)90020-3. [DOI] [PubMed] [Google Scholar]
- Zajaczkowski W, Frankiewicz T, Parsons CG, Danysz W. Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology. 1997;36:961–971. doi: 10.1016/s0028-3908(97)00070-1. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Lee HJ, Rocheleau T, ffrench-Constant RH, Jackson MB. Subunit composition determines picrotoxin and bicuculline sensitivity of Drosophila gamma-aminobutyric acid receptors. Mol. Pharmacol. 1995;48:835–840. [PubMed] [Google Scholar]
- Zomosa-Signoret V, Arnaud JD, Fontes P, varez-Martinez MT, Liautard JP. Physiological role of the cellular prion protein. Vet. Res. 2008;39:9. doi: 10.1051/vetres:2007048. [DOI] [PubMed] [Google Scholar]