Abstract
The complexity of survival mechanisms in cancer cells from patients remains poorly understood. To obtain a comprehensive picture of tumor cell survival in prostate cancer metastases, we interrogated 5 survival proteins that operate within 3 survival pathways in a cohort of 185 lethal metastatic prostate metastases obtained from 44 patients. The expression levels of BCL-2, BCL-XL, MCL-1, cytoplasmic Survivin, nuclear Survivin, and Stathmin were measured by immunohistochemistry in a tissue microarray. Simultaneous expression of 3 or more proteins occured in 81% of lethal prostate cancer metastasis and BCL-2, cytoplasmic Survivin and MCL-1 were co-expressed in 71% of metastatic sites. An unsupervised cluster analysis separated bone and soft tissue metastases according to patterns of survival protein expression. BCL-2, cytoplasmic Survivin and MCL-1 had significantly higher expression in bone metastases (p < 10−5), while nuclear Survivin was significantly higher in soft tissue metastases (p = 3×10−14). BCL-XL overexpression in soft tissue metastases almost reached significance (p =0.09), while stathmin expression did not (p=0.28). In addition, the expression of MCL-1 was significantly higher in AR-positive tumors. Neuroendocrine differentiation was not associated with specific survival pathways. These studies demonstrate for the first time that bone and soft tissue metastases from the same patient differ significantly in expression of a panel of survival proteins and that with regards to survival protein expression, expression is associated with metastatic site and not patient. Altogether this study suggests that optimal therapeutic inhibition may require combinations of drugs that target both bone as well as soft tissue-specific survival pathways.
Keywords: prostate cancer, metastases, anti-apoptotic pathways, tumor heterogeneity, immunohistochemistry
Introduction
During tumor progression, prostate cancer cells loose susceptibility to a variety of mechanisms that normally promote programmed cell death (1). Multiple survival pathways in prostate cancer cells prevent apoptosis by inhibiting the proteolytic activation of executer caspases, which are a point of convergence for the intrinsic and extrinsic apoptotic cell death pathways (2). Regulated by the ratio of pro-survival to pro-death members of the BCL-2 family, the intrinsic pathway stimulates the release of cytochrome c from mitochondria (3). The expression of BCL-2 family members, BCL-2, BCL-XL and MCL-1 in prostate cancer cells, in vivo, is an important contributor to cell survival (4).
Inhibitors of apoptosis proteins (IAPs), which block caspase activity, interfere with the execution of apoptotic programs (5). Survivin is a structurally unique IAP, which shuttles between the cytoplasm and the nucleus (6). Cytoplasmic and nuclear forms of Survivin are involved in separate cytoprotective pathways in cancer cells. In the cytoplasm, Survivin is stabilized by complex formation with XIAP, another IAP, as well as several cytoplasmic chaperones (7). In C.elegans and yeast nuclei, orthologues of Survivin play key roles in chromosomal segregation and cytokinesis during mitosis (8). In eukaryotic nuclei, two separate pools of Survivin appear to work together in the mitotic spindle checkpoint to stabilize microtubules (9) (10). Altogether, expression levels, activity and subcellular localization of Survivin are regulated by monoubiquitination and phosphorylation by mitotic kinases (11).
Stathmin has recently been used as a surrogate marker of the loss of the tumor suppressor protein, PTEN (12). PTEN loss occurs in approximately 46% of advanced prostate cancers and leads to hyperactivation of the AKT/mTOR pathway, a major survival pathway in the context of cell adhesion and growth factor receptors (12). AKT can inhibit the heterodimerization of BCL-2 family members to reduce apoptosis (13).
BCL-2, BCL-XL, MCL-1 and Survivin expression has been measured in primary prostate cancers and in small cohorts of lymph node and bone metastases (14, 15), (16) and expression has been associated with transgression of the prostate capsule, risk of relapse and metastatic progression (17, 18). However, conclusions from published work are limited because of small cohort sizes with only one sample per patient as well as the small number of survival proteins analyzed in each study. In light of extensive therapeutic development against pro-survival pathways (19) the lack of knowledge of concurrent expression of multiple survival pathways and the heterogeneity across metastatic sites hinders the development of combinatorial therapeutic strategies to kill cancer cells.
To perform a thorough analysis of survival mechanisms in metastatic prostate cancer in patients, we used immunohistochemistry to measure BCL-2, BCL-XL and MCL-1, cytoplasmic and nuclear Survivin and Stathmin as a surrogate measure of PTEN loss. We took advantage of a large cohort of metastatic tissues that were collected in the context of a rapid autopsy program at the University of Washington to analyze the expression of survival pathways in multiple metastases from the same patient and within the same region of the tumors. This study represents the first broad analysis of survival proteins in metastatic prostate cancer in a cohort of sufficient size to detect statistically significant differences between bone and soft tissue metastases. In addition, the design of the study permits distinguishing regulatory effects intrinsic to cancer cells from those of the tumor microenvironment.
MATERIALS AND METHODS
Tissue microarray of patient cancers
Tissues were obtained from the Prostate Cancer Donor Rapid Autopsy Program at the University of Washington (UW) from consented subjects (20). Samples from bone and grossly visible metastatic disease in lymph nodes, liver, lung and other sites were collected in a systematic and consistent fashion on every case (21). A human tissue microarray (UWTMA21) was constructed from 44 patients with 185 metastatic sites of which 121 are bone metastases and 64 are soft tissue metastases (22). The soft tissue metastases include 35 lymph nodes, 19 livers and 10 other soft tissue sites. Each metastatic site is represented by 2 cores.
Immunohistochemistry
The antibodies and conditions used in IHC are summarized in Supplementary Table S1. Briefly, 5-micron thick formalin-fixed and paraffin embed sections were obtained from the tissue microarray (TMA). Slides were baked and deparaffinized. Antigen retrieval for anti-MCL-1 was performed at pH 9.0. For all other antibodies, the antigens were retrieved at pH 6.0 either in the vegetable steamer in a beaker with glass pebbles for 40 min or in a pressure cooker for 10 min to avoid local overheating and core loss. Slides were developed using the Vector labs ABC kit or the EnVision-HRP-enzyme conjugate and 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a substrate. The counterstain was carried out with Hematoxylin 2. Normal mouse or rabbit IgG was used as a control.
Staining Evaluation
TMA cores were scored by 3 observers (C.A., X.Z. or B.S.K.). For all markers, staining was scored based on both intensity and percentage. The intensity of positive staining was graded on a three-tiered scale from 0 – 2+ (0 = no staining, 1+ = weak to moderate staining, 2+ = strong staining) in nuclear and cytoplasmic compartments. When expression was heterogeneous, the percentage of each staining intensity in the core was also scored between 0% to 100%. The staining intensity score was then multiplied by the percentage of positive cells. The total scores were averaged across duplicate cores for further analysis. Because the area of the core is small, most cores have uniform staining and will have total scores of 0, 100 and 200.
Statistical methods
The association between the effect of class (bone/soft tissue) and expression level is measured by the linear mixed effect model, with accounting for dependent non-balanced multiple metastatic sites of the same patient. When expression levels were categorized into ordinal categories, the chi-squared test for trend in proportions was used. Hierarchical clustering was used for the supervised and unsupervised analyses. Spearman’s rank correlation is used for measuring pairwise associations among the biomarkers measured.
RESULTS
Prostate cancer metastases reveal heterogeneous molecular aberrations. Previous studies demonstrated variable expression of the androgen receptor, cell proliferation, neuroendocrine differentiation, and expression of E-cadherin (21, 23). Here we analyzed a cohort of 185 metastases from 44 patients with multiple metastatic samples from each patient to determine expression patterns of multiple survival pathways.
Metastatic tissue samples were collected shortly after death due to metastatic prostate cancer from bone (65.4%), lymph node (18.9%), liver (10.3%), and other sites (5.4%) with visible metastatic growth. Core biopsies of metastatic tissues measuring 1 millimeter in diameter are displayed in a tissue microarray (TMA) and during the staining process fewer than 5% of cores were lost (Figure 1A). Prior to their use for immunohistochemistry, the specificity of antibodies was confirmed by Western blotting with detergent-extracted proteins from prostate cancer cell lines (data not shown). We measured the expression of BCL-2, BCL-XL, MCL-1, cytoplasmic and nuclear Survivin and Stathmin in consecutive sections from the TMA, which facilitated the comparison of expression of multiple proteins in the same region of the tumor. Data from each slide were collected independently by two observers with expertise in the scoring of immunohistochemical stains and discordant results were discussed. If the tumor in the core demonstrated uneven expression, the average intensity of cancer cells in the core was calculated. Most cores demonstrated homogenous expression (Supplementary Figure 1), resulting in overall expression scores in increments of 50 between 0 and 200. Therefore, for statistical analysis, the semi-continuous values were categorized into three ordinal categories of 0, 100 and 200. BCL-2, BCL-XL, MCL-1 and Stathmin were exclusively expressed in the cytoplasm, whereas Survivin was expressed in the cytoplasm and nucleus. Since the functions and association with disease progression of Survivin in the cytoplasm and nucleus differ (24, 25), subcellular compartments were scored separately and in the case of nuclear Survivin, expression was measured with 2 separate antibodies.
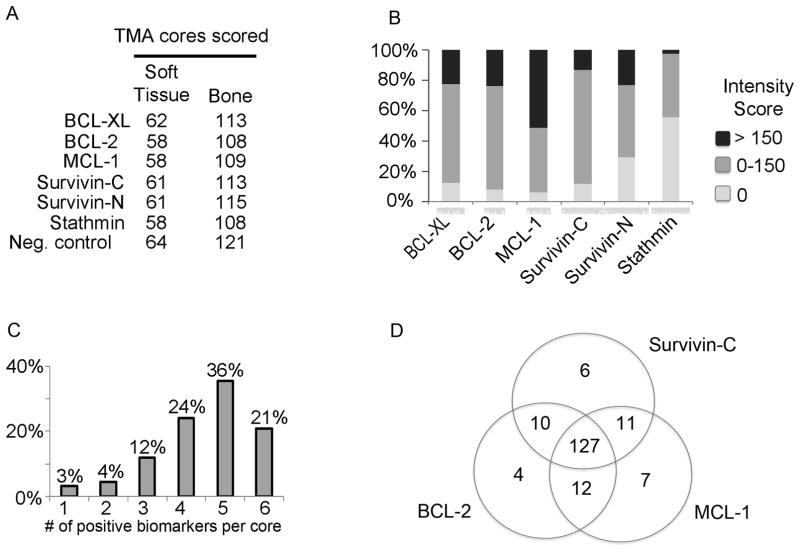
Figure 1. Expression of survival proteins in prostate cancer metastases.
A) Data collection from the tissue microarray (TMA). A tissue microarray of 185 prostate cancer metastases from 44 patients was analyzed by immunohistochemistry. The numbers of bone and soft tissue metastases that were scored are listed for each antibody. B) Proportion of staining intensities for each antibody. Percentages of groups with high (>150), low (≤150) and negative staining intensities are represented within each bar. C) Evaluation of simultaneous expression of survival proteins in prostate cancer metastases. The co-expression of 6 proteins was determined across metastatic sites. The bars depict the percentages of metastasis co-expressing 1 to 6 of survival proteins (BCL-XL, BCL-2, MCL-1, cytoplasmic Survivin, nuclear Survivin and Stathmin). D) Co-expression of cytoplasmic Survivin, BCL-2 and MCL-1 in prostate cancer metastases.
We obtained expression data of BCL-2, BCL-XL, MCL-1, Survivin, and Stathmin from between 167 and 176 of 185 metastatic sites (Figure 1A). As expected, the amounts of expression varied across metastases from the same patient (Supplementary Figure 2). The expression was highest for MCL-1 with scores > 150 in 50% of metastases. BCL-2, BCL-XL and nuclear survivin expression was high (expression score > 150) in 25% and cytoplasmic survivin was highly expressed in 15% of cases (Figure 1B). We were concerned about the accuracy of evaluating the loss of PTEN and potential for high false negative rates because of compromises in tissue quality during the autopsy procedure. Instead, we used Stathmin as a surrogate marker of PTEN loss (12). Stathmin expression was noted in 44% of metastasis, (Figure 1B), which is similar to the reported rate of PTEN loss in prostate cancer lymph node metastases (26).
The expression of Survivin was observed in the cytoplasm and in the nucleus. Nuclear expression was measured with an antibody that binds to both cytoplasmic and nuclear Survivin as well as with an antibody selective for the nuclear form. The measurements with the two antibodies were in good agreement (Spearman’s rank correlation is 0.46, p < 0.0001), supporting specificity for the nuclear Survivin measurement.
Figure 1C depicts the extent of co-expression of BCL-2, BCL-XL, MCL-1, cytoplasmic and nuclear Survivin, and Stathmin. In 81% of metastases, 4 or more of the proteins were co-expressed. In addition, evaluation of those survival pathways with the highest expression in bone metastases demonstrated concurrent expression of BCL-2, cytoplasmic Survivin, and MCL-1 occurred in 71% of metastatic sites (Figure 1D). Together these data demonstrate significant redundancy in survival pathways in prostate cancer metastases.
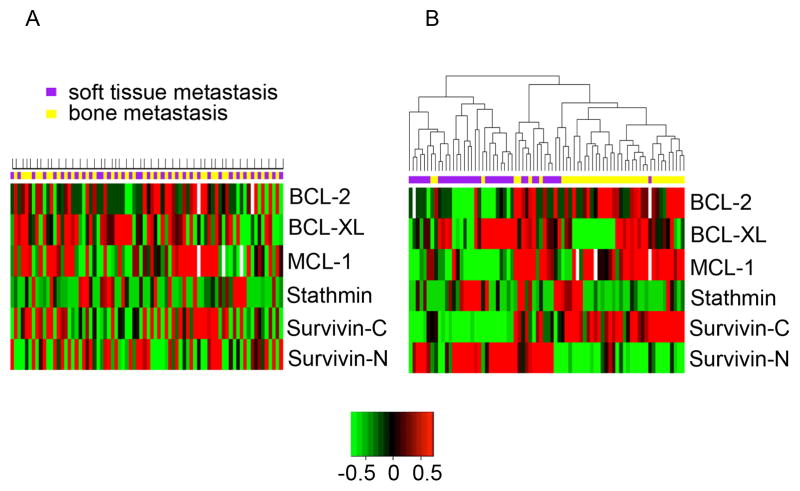
Given the heterogeneity of expression of survival proteins, we performed supervised and unsupervised hierarchal cluster analyses. First, we explored whether patients can be grouped based on expression patterns of the 5 survival proteins. We calculated the average expression across all bone or soft tissue metastases, respectively, from each patient. In a supervised cluster analysis, when grouping bone and soft tissue metastases from each patient together, no clusters were detectable (Figure 2A). Next, we performed an unsupervised cluster analysis. In this case, we observed two separate clusters of bone and soft tissue metastases (Figure 2B). Further subdivision of soft tissue metastases into lymph node, liver, lung or other sites did not reveal additional clusters. The results from this analysis clearly demonstrate that with respect to the survival proteins in our study, metastases from the same site (bone or soft tissue) across patients are more similar to each other than metastases within a patient. This result is in sharp contrast to previous reports of genomic data clustering, which demonstrated clustering of metastatic sites within patients (27).
Figure 2. Cluster analysis of prostate cancer metastasis by protein expression.
The average expression of each marker was calculated separately across bone and soft tissue metastases from 44 patients. A) Supervised cluster analysis of patient metastases. For each patient bone and soft tissue metastases are aligned in consecutive columns. Above each column, bone metastases are indicated in yellow and soft tissue metastases in purple. In addition, patients are indicated by marks at the top. Please note that some patients have only bone or only soft tissue metastases. B) Unsupervised cluster analysis of patient metastases. Bone metastases are marked in yellow, while soft tissue metastases are marked in purple. Note segregation of groups according to metastatic sites in the unsupervised, but not the supervised cluster analysis. White color identifies cores that did not yield data.
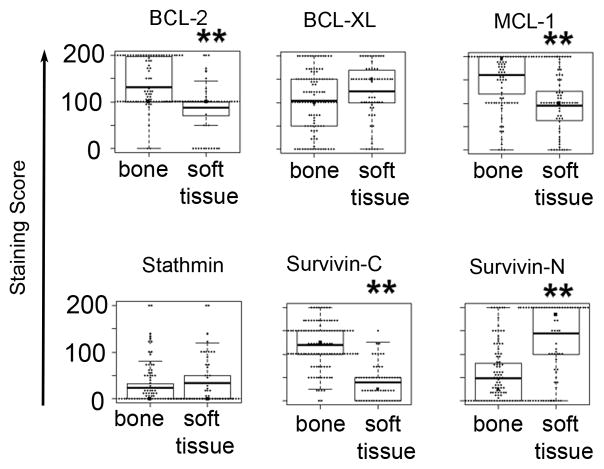
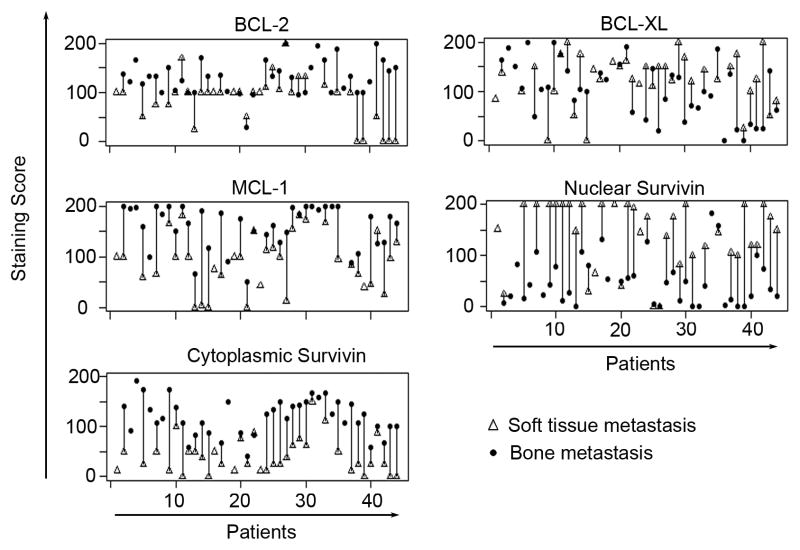
We further analyzed the expression differences of survival proteins between bone and soft tissue metastases and observed highly significant differences for all proteins, except for BCL-XL and Stathmin (Figure 3). To calculate the significance of the difference, we used a mixed effect model, which takes into consideration the dependency of multiple metastases from the same patient. The resulting p-values were < 10−6 for BCL-2, MCL-1, cytoplasmic and nuclear Survivin. In addition, the expression between cytoplasmic and nuclear Survivin was negatively correlated (Spearman’s rank correlation is – 0.42, p < 0,0001) and the greater expression of BCL-XL in soft tissue metastases almost reached significance (p < 0.09). In Figure 4, we compared average expression levels of bone and soft tissue metastases for each patient separately and noted the differences in expression also on a per patient basis.
Figure 3. Protein expression in bone and soft tissue metastases.
Each panel shows box-plots of the distribution of expression values for the indicated protein in bone and soft tissue metastasis in a box and whisker diagram. The boxes represent the 25th–75th percentiles of biomarker expression values in each group. The whisker bars represent the lowest and highest datum within the 1.5 interquartile range. The mean values are indicated by the dark, horizontal line. A double asterisk demarks statistically significant differences between bone and soft tissue expression levels. The p-values are - BCL-2: 1.1×10−6, BCL-XL: 0.0875, MCL-1: 1.5×10−8, Stathmin: 0.288, cytoplasmic Survivin: 3.9×10−16 and nuclear Survivin: 3.2×10−14.
Figure 4. Average expression of survival proteins in bone and soft tissue metastases from each patients.
To visualize the difference in expression of metastases within patients, the average expression in metastatic bone and soft tissue sites is connected by a bar. Note the increased expression in bone metastases (closed circles) for BCL-2, MCL-1 and Survivin-C and increased expression in soft tissue metastases (open triangles) for Survivn-N and marginally also for BCL-XL.
Next we interrogated associations between survival pathways and androgen receptor (AR) expression or neuroendocrine (NE) differentiation. In this cohort, 86.7% of metastases express AR, while 13.3% are AR negative (22). The only significant difference occurred in expression of MCL-1, which was higher in AR positive than in AR negative metastases (p=0.037) (Table 1). NE differentiation was identified by expression of synaptophysin and chromogranin, which was observed in 19 of 161 metastases (11.8 %), of which 9 (47.4%) expressed the AR. However there was no significant difference in survival protein expression in association with NE differentiation.
Table 1.
Survival protein expression by AR and NE status
| A | |||||
|---|---|---|---|---|---|
| NE− (n =163) | NE+ (n =22) | ||||
| mean | sd | mean | sd | p-value | |
| BCL-2 | 115.57 | 49.97 | 123.15 | 79.02 | 0.595 |
| BCL-XL. | 111.62 | 62.86 | 110.75 | 69.88 | 0.609 |
| MCL-1 | 141.79 | 61.23 | 104.47 | 82.99 | 0.082 |
| Survivin-C | 90.91 | 56.61 | 98.81 | 74.36 | 0.470 |
| Survivin-N | 82.90 | 78.58 | 74.29 | 72.25 | 0.633 |
| B | |||||
|---|---|---|---|---|---|
| AR+ (n =144) | AR− (n =22) | ||||
| mean | sd | mean | sd | p-value | |
| BCL-2 | 116.12 | 51.49 | 113.75 | 72.34 | 0.771 |
| BCL-XL. | 113.08 | 61.27 | 116.93 | 68.27 | 0.824 |
| MCL-1 | 143.45 | 61.42 | 89.37 | 73.69 | 0.037 |
| Survivin-C | 90.74 | 59.45 | 85.71 | 62.03 | 0.872 |
| Survivin-N | 90.04 | 78.86 | 58.57 | 67.88 | 0.153 |
The average expression across all metastases was used to determine significant expression differences between A) neuroendocrine and non-neuroendocrine prostate cancers and B) androgen receptor positive and androgen receptor negative prostate cancer. The number of metastasis is indicated in parenthesis. p-values were calculated using the mixed effect model accounting for dependent multiple metastatic sites of the same patient.
Patients were treated with ketoconazole, taxotere, DES and mitoxantrone. Comparing treated and untreated patients, we did not identify a consistent effect of drug treatment on survival protein expression levels (data not shown).
Together, our data demonstrate significant differences in expression of key survival proteins in bone versus soft tissue metastases. Since these constitute pathways of drug resistance, they may need to be abolished to obtain durable treatment responses with targeted therapeutic agents.
Discussion
To obtain a better understanding of the repertoire of survival pathways in metastatic prostate cancer we profiled three parallel, major survival pathways in a tissue microarray of 185 metastases from 44 patients. The survival pathways included (1) the BCL-2 family members, BCL-2, BCL-XL and MCL-1, (2) Survivin, and (3) Stathmin as a surrogate for the loss of PTEN. As expected, prostate cancer metastases express multiple survival pathways simultaneously. Except for 13/185 metastases (7%), all metastatic sites manifested expression of 2 or more survival proteins and 21% expressed all 6 survival proteins. As the efficiency of drugs that inhibit BCL-2/BCL-XL, such as ABT-737, is reduced through expression of MCL-1 and hyperactivation of the AKT pathway (28, 29), recognizing the expression of multiple survival proteins early on will help to guide treatment decisions and enhance treatment responses.
Interestingly, the overall profile of survival pathways displayed dramatic differences between bone and soft tissue metastases. While in bone, BCL-2, cytoplasmic Survivin, and MCL-1 were highly expressed, in soft tissue metastasis, nuclear Survivin and BCL-XL prevailed. A possible reason for the high expression of BCL-2, cytoplasmic Survivin and MCL-1 in bone metastasis is their upregulation by cell surface receptors that are overexpressed in bone compared to soft tissue metastases. For example, we demonstrated that the c-MET receptor, which can enhance MCL-1 expression, is highly expressed in practically all bone metastases, while c-MET expression in soft tissue metastases is significantly diminished (30). Thus the transcriptional regulation of MCL-1 by c-MET (31) could explain upregulation of MCL-1 in bone metastases. Unfortunately, because the RNA is degraded through the decalcification process, this cannot be experimentally proven. Thus, the microenvironmental influence may provide a plausible reason for the discrepant results of cluster analyses based on genomic data, where clustering occurs according to patients (27), and expression of survival proteins, where clustering is according to metastatic site. Altogether the data support a strong microenvironmental influence in the regulation of survival pathways in metastatic prostate cancer through transcriptional rather than genomic deregulation (32).
The tissues in this study were from patients who received multiple systemic therapies against metastatic prostate cancer. Each round of treatment will select for cells with specific pre-existing survival pathways or may induce new mechanisms for survival. With one exception, all patients in our study were treated with anti-androgen therapies and most patients received even more aggressive androgen suppression with ketoconazole. Diethylstilbersterol, Taxetere and mitoxantrone were also commonly used. While heightened expression of survival proteins has been related to the resistance against these treatments (33–35), we did not observe a consistent association between any specific treatment exposure and expression of particular survival proteins. A longitudinal comparison of metastatic disease before versus after exposure to therapy would be required to precisely assess the impact of therapy on expression of survival proteins in the metastatic context.
Altogether, our results suggest that bone and soft tissue metastases differ with regards to survival pathways. The possible regulation of gene expression by the tumor microenvironment highlights the role of targeting the environment as well as the tumor cells to achieve optimal therapeutic responses.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families who were willing to participate in the Prostate Cancer Donor Program, for without them research of this nature would not be possible. We would also like to acknowledge Bruce Montgomery, Paul Lange, Martine Roudier, Peter Nelson and the rapid autopsy teams and students in the Urology Department at the University of Washington. This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington (RLV is a Research Career Scientist) and the Richard M. LUCAS Foundation. Authors acknowledge the following funding sources: BSK-R21CA118592 and W91ZSQ7315N684, RLV and CM - P50CA97186, PO1CA085859, MK and JCR - R21CA152794.
Footnotes
Author contributions:
project organization, data acquisition and analysis: CA, XZ, AV, MEC, MK
experimental design – CKM statistical data analysis - DB
antibody development – JR, MK
patient samples – CSH, LDT, RLV, CM
project concept, coordination, manuscript – BSK, CM, CKM, CA
The authors declare no conflict of interest with the content of the manuscript.
References
- 1.McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006 Jan 1;97(1):18–32. doi: 10.1002/jcb.20634. [Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004 Jan 23;116(2):205–19. doi: 10.1016/s0092-8674(04)00046-7. [Review] [DOI] [PubMed] [Google Scholar]
- 3.Korsmeyer SJ, Wei MC, Saito M, et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000 Dec;7(12):1166–73. doi: 10.1038/sj.cdd.4400783. [Review] [DOI] [PubMed] [Google Scholar]
- 4.Krajewska M, Moss SF, Krajewski S, et al. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996 May 15;56(10):2422–7. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [PubMed] [Google Scholar]
- 5.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002 Jun;3(6):401–10. doi: 10.1038/nrm830. [Review] [DOI] [PubMed] [Google Scholar]
- 6.Zangemeister-Wittke U, Simon HU. An IAP in action: the multiple roles of survivin in differentiation, immunity and malignancy. Cell Cycle. 2004 Sep;3(9):1121–3. [Research Support, Non-U.S. Gov’t Review] [PubMed] [Google Scholar]
- 7.Fortugno P, Beltrami E, Plescia J, et al. Regulation of survivin function by Hsp90. Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):13791–6. doi: 10.1073/pnas.2434345100. [In Vitro Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser AG, James C, Evan GI, et al. Caenorhabditis elegans inhibitor of apoptosis protein (IAP) homologue BIR-1 plays a conserved role in cytokinesis. Curr Biol. 1999 Mar 25;9(6):292–301. doi: 10.1016/s0960-9822(99)80137-7. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 9.Vong QP, Cao K, Li HY, et al. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 2005 Dec 2;310(5753):1499–504. doi: 10.1126/science.1120160. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 10.Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006 Dec;18(6):616–22. doi: 10.1016/j.ceb.2006.08.016. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- 11.Barrett RM, Osborne TP, Wheatley SP. Phosphorylation of survivin at threonine 34 inhibits its mitotic function and enhances its cytoprotective activity. Cell Cycle. 2009 Jan 15;8(2):278–83. doi: 10.4161/cc.8.2.7587. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 12.Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007 May 1;104(18):7564–9. doi: 10.1073/pnas.0702507104. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardai SJ, Hildeman DA, Frankel SK, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004 May 14;279(20):21085–95. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 14.Krajewska M, Krajewski S, Banares S, et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003 Oct 15;9(13):4914–25. [PubMed] [Google Scholar]
- 15.Krajewska M, Krajewski S, Epstein JI, et al. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996 May;148(5):1567–76. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [PMC free article] [PubMed] [Google Scholar]
- 16.Zellweger T, Ninck C, Bloch M, et al. Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer. 2005 Feb 10;113(4):619–28. doi: 10.1002/ijc.20615. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 17.Scherr DS, Vaughan ED, Jr, Wei J, et al. BCL-2 and p53 expression in clinically localized prostate cancer predicts response to external beam radiotherapy. J Urol. 1999 Jul;162(1):12–6. doi: 10.1097/00005392-199907000-00003. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 18.Pollack A, Cowen D, Troncoso P, et al. Molecular markers of outcome after radiotherapy in patients with prostate carcinoma: Ki-67, bcl-2, bax, and bcl-x. Cancer. 2003 Apr 1;97(7):1630–8. doi: 10.1002/cncr.11230. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 19.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008 Oct 27;27(50):6398–406. doi: 10.1038/onc.2008.307. [Research Support, N.I.H., Extramural Review] [DOI] [PubMed] [Google Scholar]
- 20.Roudier MP, True LD, Higano CS, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003 Jul;34(7):646–53. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 21.Roudier MP, True LD, Higano CS, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003 Jul;34(7):646–53. doi: 10.1016/s0046-8177(03)00190-4. [Case Reports Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Morrissey C, Sun S, et al. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS One. 2011;6(11):e27970. doi: 10.1371/journal.pone.0027970. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putzke AP, Ventura AP, Bailey AM, et al. Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. The American journal of pathology. 2011 Jul;179(1):400–10. doi: 10.1016/j.ajpath.2011.03.028. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan DJ, Rexhepaj E, O’Brien SL, et al. Altered cytoplasmic-to-nuclear ratio of survivin is a prognostic indicator in breast cancer. Clin Cancer Res. 2008 May 1;14(9):2681–9. doi: 10.1158/1078-0432.CCR-07-1760. [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy SM, O’Driscoll L, Purcell R, et al. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003 Apr 7;88(7):1077–83. doi: 10.1038/sj.bjc.6600776. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotan TL, Gurel B, Sutcliffe S, et al. PTEN Protein Loss by Immunostaining: Analytic Validation and Prognostic Indicator for a High Risk Surgical Cohort of Prostate Cancer Patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011 Oct 15;17(20):6563–73. doi: 10.1158/1078-0432.CCR-11-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009 May;15(5):559–65. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007 Feb 1;67(3):1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 29.Lin X, Morgan-Lappe S, Huang X, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007 Jun 7;26(27):3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 30.Knudsen BS, Gmyrek GA, Inra J, et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002 Dec;60(6):1113–7. doi: 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Zhau HE, Osunkoya AO, et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A, White TA, MacKenzie AP, et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):17087–92. doi: 10.1073/pnas.1108745108. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Jin S, Abraham V, et al. The Bcl-2/Bcl-X(L)/Bcl-w inhibitor, navitoclax, enhances the activity of chemotherapeutic agents in vitro and in vivo. Mol Cancer Ther. 2011 Dec;10(12):2340–9. doi: 10.1158/1535-7163.MCT-11-0415. [DOI] [PubMed] [Google Scholar]
- 34.Wertz IE, Kusam S, Lam C, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011 Mar 3;471(7336):110–4. doi: 10.1038/nature09779. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 35.Yuan XJ, Whang YE. PTEN sensitizes prostate cancer cells to death receptor-mediated and drug-induced apoptosis through a FADD-dependent pathway. Oncogene. 2002 Jan 10;21(2):319–27. doi: 10.1038/sj.onc.1205054. [Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.