Abstract
Objective
Early parenting experiences likely shape children’s brain development, with consequences potentially extending into adulthood. Parents’ affective disorders and expressions of positive affect could exert an influence on affect-related circuitry. The current study evaluated how maternal depression and maternal warmth assessed in early childhood and early adolescence were related to boys’ reward function during early adulthood.
Method
Participants were 120 boys at socioeconomic risk for emotional problems. Mothers’ history of depression during the child’s lifetime was measured when boys were 42 months old and 10/11 years old. Maternal warmth was observed during mother–child interactions at 18 and 24 months and at 10 and 11 years.
Results
Maternal warmth during early childhood was associated with less activation in the medial prefrontal cortex (mPFC) when anticipating and experiencing reward loss. Maternal warmth during early adolescence was associated with less activation in the mPFC when winning rewards and greater activation in the caudate when experiencing loss. The association between maternal warmth during early childhood and early adolescence and reward function in the striatum and mPFC was stronger for boys exposed to maternal depression relative to boys who were not.
Conclusions
The experience of warmth and affection from mothers may be a protective factor for reward function in boys exposed to maternal depression, possibly by engaging vulnerable neural reward systems through affiliation.
Keywords: maternal depression, reward, warmth
Healthy brain development occurs in the context of supportive and warm family relationships. Given the critical role of parental warmth in affective development1,2 and the role of neural reward circuitry in mental health, it is important to understand the development of the brain’s reward regions within the context of a child’s family. One important family characteristic, maternal depression, has garnered a large amount of interest in the field of developmental psychopathology because of its strong, consistent association with children’s emotional problems.3
Developmental theories have proposed several mechanisms by which exposure to maternal depression—beyond inherited tendencies toward depression— may be associated with maladaptive brain development in offspring.4 One posited mechanism is through depression’s compromising effect on caregiving quality. Depressed mothers show reliable differences in how they interact with their offspring, compared to non-depressed mothers across development5–7 and expressing less maternal warmth to offspring may disrupt both positive and negative affective systems.4,8 Reward function may be particularly altered as children of depressed mothers show disrupted positive affect.9 Indeed, 2 functional magnetic resonance imaging (fMRI) studies have shown that adolescents with a maternal history of depression show less activation in reward regions in response to rewards.8,10 Electroencephalography (EEG) studies during earlier developmental periods indicate that exposure to maternal depression is associated with altered patterns of brain function.11
Although mothers with a history of depression tend to show less positive affect when interacting with their children, individual differences in warmth exist among depressed mothers and some depressed mothers maintain warm mother–child relationships.5 Children who have been exposed to maternal depression may be more likely to be affected in their neural development by variations in maternal warmth relative to youth without this history, perhaps because of a need for these positive interactions to buffer negative influences associated with maternal depression, such as stressful life environments.4
Developmental timing may influence the effect of family climate on child outcomes. Maternal depression during early childhood (rather than later in development) appears to have the most robust effect on child outcomes, such as temperament, however maternal depression during the transition to adolescence also has been linked to affective development.5 Maternal warmth is important for affective development during early childhood, when temperament is stabilizing and self-regulation is developing.12 In addition to the importance of peers during adolescence, maternal warmth continues to be important during adolescence,13 when there is developmental sensitivity because of changes associated with puberty, including maturation of neural reward-related regions.14 Maternal warmth (i.e., high levels of maternal positive affect) has been associated with both positive and negative child behavior across development, including child social skills and affect regulation,2,5–7
Function of reward circuitry plays an important role in depression15,16 and other disorders.17 Reward regions are activated by social cues, including positive affect from a loved one,18,19 suggesting that early family experiences likely play a role in the development of reward regions. Critical regions in reward circuitry include the striatum, which is linked to positive affect in healthy and depressed adolescents, and the medial prefrontal cortex (mPFC), which is implicated in affect regulation and valuating one’s own performance in obtaining a reward.15,16, 20 Low striatal and high medial prefrontal cortex (mPFC) activation in response to reward have been demonstrated in adolescent and adult depression21,22 and observed in adolescents with a familial history of depression.8,10 Neural processing of loss is also important, particularly for adolescents exposed to a depressed mother—as these adolescents may have missed out on rewarding experiences because of less frequently occurring positive mother–child interactions or interactions of poorer quality.
Similar brain regions are activated in response to non-social and social rewards (i.e., striatum and mPFC).18 Monetary reward paradigms are an established method of evaluating reward function and have been linked to various social influences, such as child maltreatment.23 As reward function is involved in the development of affective disorders, identifying early experiences that may affect reward function merits consideration.
The current project evaluated maternal depression and maternal warmth on reward function in boys. We included a sample of low-income boys, examining exposure to maternal depression and maternal warmth during 2 important developmental periods: toddlerhood and early adolescence as they represent important and rapid periods of affective development.12,14 We focus on low-income families as rates of depression are higher among parents in low-income families relative to middle-income families and as the combination of depressive symptoms and high levels of stress experienced by low-income families can lead to compromised caregiving.24 We focus on boys as depression processes have been understudied in boys relative to girls, even though depression has devastating consequences for boys.25
We hypothesized that maternal history of depression would be associated with less striatal activation and greater mPFC activation to reward, and that greater maternal warmth would be associated with greater striatal and less mPFC activation in response to winning or losing rewards. We predicted that maternal warmth would be more strongly associated with reward-related activation in the striatum and mPFC for boys exposed to maternal depression in early childhood. We explored whether the strength of effects between maternal depression and warmth on reward function depended on their developmental timing.
Method
Participants were 120 boys from a longitudinal project on vulnerability in boys from low-income families.26 Families were recruited to the study when boys were between the ages of 7 and 17 months of age from Women, Infants, and Children (WIC) Nutritional Supplement centers in the greater Pittsburgh area. All participants were boys because of the project’s original focus on the developmental precursors of antisocial behavior. Of the mothers, 55% were European American, 41% African American, and 4% were of other races/ethnicities (e.g., biracial, Hispanic). At the 18-month assessment, mothers ranged from 17 to 43 years old (M=28.20 years). In reporting relationship status, 66% of mothers were married or cohabitating, 27% were single, and 7% were separated, divorced, or widowed. Average family income was $1,094 per month and the mean SES score was 23.4 using the Hollingshead Index, indicating working-class status.27 All procedures received Institutional Review Board approval at the University of Pittsburgh and all subjects provided consent for their participation in the study.
Boys and their mothers participated in several structured parent–child interaction tasks at 18/24 months and 10/11 years. Mothers were interviewed about their history of depression during the child’s lifetime using a semistructured interview (SCID)28 when boys were 42 months old. At age 20, boys participated in an fMRI assessment with a monetary reward task. At the time of the scan, boys were medically and neurologically healthy and were free of all substances and medications.
Originally, 310 boys and their families were recruited to participate in the longitudinal project. Of these 310 families, 307 families had data from the 18/24 month parent–child interaction tasks, 253 families had data from the 10/11 year parent–child interaction tasks, and 261 mothers reported on their history of depression at the SCID assessment when boys were 42 months. Of the 310 boys, 186 boys participated in fMRI scan at age 20, with 166 boys having usable fMRI data (n=12 removed due to difficulty with the task or misunderstanding the task; n=6 due to insufficient coverage; n=2 due to being on drugs or psychotic during the scan). Altogether, the number of participants with usable scan data, maternal depression data, and maternal warmth data during early childhood and early adolescence was 120.
There were no significant differences in mother’s education level or family income at 18 months, or maternal warmth at 18/24 months or 10/11 years for participants with fMRI data compared to those without this data or for participants with a maternal history of depression and those without this history.
Measures
History of Maternal Depression
Mothers completed the mood disorders module of the SCID28 with a clinically trained staff person trained to reliability by a licensed psychologist, when boys were 42 months old. Of the 120 dyads in the study, 20 percent of mothers (n=24) reported history of major depressive disorder (MDD) or dysthymia (DD) within the child’s lifetime (past 3.5 years). Mothers with a history of bipolar disorder were excluded from the study because of differences in neural circuitry function in bipolar and unipolar depression.29
Maternal Depressive Symptoms
Mothers reported on current depressive symptoms using the BDI when boys were 10 and 11, a widely used measure of depressive symptoms.30 The scale had strong reliability (α = .88). We used an average of maternal BDI score at 10 and 11 (r=.71, p<.001) for analyses evaluating whether maternal warmth and depression effects remained when considering maternal depressive symptoms at ages 10/11.
Maternal Warmth During Early Childhood
At 18 and 24 months, mothers and their toddlers participated in 2 semistructured tasks: a series of teaching tasks and a clean-up task. In the teaching tasks, mothers and sons spent 9 minutes working on three 3-minute interactive tasks that were designed to be slightly difficult for toddlers to complete on their own (e.g., a puzzle). In the clean up task, mothers were instructed to have their sons clean up the toys in the laboratory play room. Mothers’ global warmth was coded on intensity of maternal warmth using the Early Parenting Coding System31 on a 4-point Likert scale (1=none; 4=a lot) at both time points. This yielded four maternal warmth scores. Intraclass coefficients ranged from .67 to .94 for these tasks, with a mean of .81, indicating adequate reliablity.32 Maternal warmth during all four interactions were significantly correlated (rs=.16–.46, p’s<.01) and were averaged to create a maternal warmth during early childhood composite.
Maternal Warmth During Early Adolescence
Mothers and their sons participated in 2 similar discussion tasks when boys were 10 and 11 years old. At age 10, the topic of discussion was based on areas of current disagreement and selected by the primary caregiver (e.g., keeping room clean).33 At age 11, the target child was asked to select a topic for which he could use assistance (e.g., a friend is being mean).34 Maternal warmth was coded globally on the intensity of maternal warmth (e.g., smiles with her child) on a 9-point Likert scale (1=low, 9=high) during this task at both ages and was significantly correlated (r=.39, p <.01). Intraclass coefficients ranged from .67 to .84, with a mean of .74, indicating adequate reliability.32 The codes for ages 10 and 11 were averaged to create a composite maternal warmth during early adolescence.
Boys’ Psychopathology
At age 20, boys reported on their lifetime history of Axis I clinical disorders using the SCID28 with a clinically trained staff person trained to reliability with a licensed clinical psychologist. History of major depressive disorder (MDD) or dysthymia, substance dependence, and antisocial personality disorder (ASPD) were included in our models due to their relevance to reward function. Of the 120 boys, 13% of boys had a history of MDD or dysthymia, 14% had a history of substance dependence, and 8% had a history of ASPD. Rates of these disorders were comparable in boys not included in analyses (11% with MDD, 11% with substance dependence, and 10% with ASPD).
Neural Response to Reward
Boys completed a reward-related fMRI paradigm at age 20. The fMRI paradigm was an 8-minute slow event-related card-guessing game that evaluates neural response to the anticipation and receipt of monetary reward feedback.21 Participants received win, loss, or no-change (neutral) feedback for each trial. Participants were told that their performance would determine a monetary reward after the scan, with $1 for each win and 50 cents deducted for each loss. Trials were presented in pseudorandom order with predetermined outcomes. Earnings totaled $6 for all participants. Trials were presented in a single run, with 24 trials total and a balanced number of trial types within runs (i.e., 12 possible-win vs. no-change trials, 12 possible-loss vs. no-change trials). During each trial, participants guessed via button press whether a visually presented card, with a possible value of 1–9, was higher or lower than 5 (4s), learned the trial type (possible-win, possible-loss) to anticipate feedback (6s), and received feedback (won money, lost money, or no change; 1s plus 6s intertrial interval). Baseline was defined as the last 3s of each intertrial interval, as the hemodynamic response is likely to have resolved by this point in the trial. Participants were unaware of fixed outcome probabilities (i.e., all participants receive the same trials) (see Nusslock et al., 2012, for task figure)29.
fMRI Acquisition, Processing, and Analysis
Each participant was scanned using a Siemens 3T Trio scanner. BOLD functional images were acquired with a gradient echo planar imaging (EPI) sequence and covered 39 axial slices (3.1mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (repetition time [TR]/time to echo [TE]=2000/25ms, field of view [FOV]=20cm, matrix=64×64). All scanning parameters were selected to optimize the quality of the blood-oxygen-level–dependent (BOLD) signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired a reference EPI scan that we visually inspected for artifacts (e.g., ghosting) and for good signal across the entire volume of acquisition. The fMRI data from all included participants were cleared of such problems.
Preprocessing and whole-brain image analyses were completed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). For each scan, structural images for each participant were segmented, and functional images were realigned to correct for head motion, coregistered to the segmented structural data, spatially normalized into standard stereotaxic space (Montreal Neurological Institute template) using a 12-parameter affine model, and smoothed with a 6mm full-width at half-maximum Gaussian filter. Participants’ data were inspected for adequate coverage of the ventral striatum (>80%). All remaining participants had movement <2mm in each plane on average across all frames. Groups did not differ in movement.
Data Analytic Strategy
Anatomical regions of interest (ROI) were constructed using the WFU PickAtlas Tool (v1.04). The striatal ROI was defined as a 3,642-voxel size 20mm radius sphere, centered on the ventral striatum using Talairach coordinates x=0, y=0, and z=0 and encompassing dorsal and ventral striatum. The mPFC region of interest (ROI) was centered around coordinates x=0, y=42, and z=18, and defined as a 5,393-voxel size 25 mm-radius sphere including medial Brodmann Area (BA)10 and BA32.21
For the analyses of interest, we conducted multiple regression analyses in SPM8 in which activation in the striatum and mPFC during the contrasts of reward anticipation > baseline, reward outcome > baseline, loss anticipation > baseline, and loss outcome > baseline were the outcome variables. Simulations in the AlphaSim program in AFNI were used to estimate the minimum number of contiguous voxels in each cluster required to avoid Type 1 error at a corrected p<.05 for each ROI (89 for striatum; 136 for mPFC). We computed 4 separate regression models (one for each contrast of interest) because of the strong correlation between our conditions (rs = .69–.71, ps < .01). For each of the regression models, maternal warmth during early childhood, maternal warmth during early adolescence, maternal history of depression, and the two 2-way multiplicative interactions between maternal history of depression and maternal warmth (either during early childhood or during early adolescence) were entered as independent variables. Diagnosis of either substance dependence or ASPD (as a categorical variable) was entered as a control variable based on this sample’s high rates of those disorders (14% and 8%, respectively). For all significant interactions, simple slope analyses35 were conducted within SPM8 and restricted to clusters in which interactions were found. Post-hoc analyses in which maternal BDI at ages 10/11 was substituted for maternal history of depression (SCID) tested whether maternal warmth and depression effects remain when we consider maternal depressive symptoms during early adolescence (rather than during early childhood).
Results
Table 1 reports the means and standard deviations for maternal warmth. Maternal warmth during childhood and during early adolescence were positively correlated (r=.16, p<.02). Maternal warmth during childhood and early adolescence were unrelated to maternal depression. Mothers with a history of depression on the SCID had higher levels of depressive symptoms at 10/11 (F=7.84, p<.01). Our regression models revealed main effects for maternal warmth on reward function at age 20 (Table 2). Greater maternal warmth during childhood predicted less mPFC activation when anticipating or losing rewards. Greater maternal warmth during early adolescence was associated with less mPFC activation during reward outcome and greater caudate activation during loss outcome.
Table 1.
Sample Characteristics and Maternal Warmth by Group and Task
| Variable, m (SD) | History of Depression, n = 24 | Healthy, n = 96 |
|---|---|---|
| Socioeconomic Status | 23.50 (10.39) | 22.70 (8.61) |
| Maternal Education | 12.65 (1.61) | 12.54 (1.50) |
| Maternal Warmth (18 months) | ||
| Clean Up | 2.84 (.37) | 2.81 (.47) |
| Teaching | 2.85(.44) | 2.84 (.44) |
| Maternal Warmth (24 months) | ||
| Clean Up | 2.69 (.44) | 2.64 (.65) |
| Teaching | 2.85 (.44) | 2.87 (.37) |
| Maternal Warmth (10 years) | ||
| Discussion | 4.81 (1.64) | 4.87 (1.64) |
| Maternal Warmth (11 years) | ||
| Discussion | 4.02 (1.97) | 4.19 (1.96) |
Table 2.
Maternal Depression and Warmth and Reward Function
| Variable | Region | x y z | t | Cluster size |
|---|---|---|---|---|
| Reward Anticipation | Externalizing Disorder | |||
| Putamen (−) | −14, 13, −2 | 2.08 | 206 | |
| Maternal History of Depression | ||||
| Maternal Warmth during Early Childhood | ||||
| Maternal Warmth during Early Adolescence | ||||
| Maternal Warmth during Early Childhood × Maternal History | ||||
| Putamen (−) | −16, 10, 0 | 3.27 | 323 | |
| BA 9 (−) | 2, 44, 25 | 2.95 | 479 | |
| Post-Hoc (Boys with Maternal History) | ||||
| Putamen (−) | −16, 10, 0 | 3.19 | 158 | |
| BA 9 (−) | 0, 40, 27 | 3.06 | 374 | |
| Maternal Warmth during Early Adolescence × Maternal History | ||||
| Caudate Body | −2, −1, 13 | 3.45 | 319 | |
| Post-Hoc (Boys with Maternal History) | ||||
| Caudate Body (+) | −2, −1, 11 | 2.66 | 157 | |
| Reward Outcome | Externalizing Disorder | |||
| BA 9 (−) | 10,54,50 | 3.02 | 167 | |
| Maternal History of Depression | ||||
| Maternal Warmth during Early Childhood | ||||
| Maternal Warmth during Early Adolescence | ||||
| BA 10 (−) | 2, 63, 8 | 3.95 | 891 | |
| Maternal Warmth during Early Childhood × Maternal History | ||||
| Putamen (−) | −18, 8, −2 | 3.28 | 536 | |
| Post-Hoc (Boys with Maternal History) | ||||
| Putamen (−) | −18, 8, −2 | 2.82 | 252 | |
| Maternal Warmth during Early Adolescence × Maternal History | ||||
| Caudate Head (+) | 4, 18, 0 | 2.76 | 185 | |
| Post-Hoc | ||||
| Loss Anticipation | Externalizing Disorder | |||
| Putamen (−) | 16, 6, −5 | 3.34 | 179 | |
| Maternal History of Depression | ||||
| Maternal Warmth during Early Childhood | ||||
| BA 10 (−) | 2, 49, 7 | 2.61 | 409 | |
| Maternal Warmth during Early Adolescence | ||||
| Maternal Warmth during Early Childhood × Maternal History | ||||
| Putamen (−) | −16, 8, 0 | 3.02 | 185 | |
| Post-Hoc (Boys with Maternal History) | ||||
| Putamen (−) | −14, 8, 0 | 3.18 | 152 | |
| Maternal Warmth during Early Adolescence × Maternal History | ||||
| Caudate Body (+) | 0, 4, 5 | 2.35 | 273 | |
| Post-Hoc | ||||
| Loss Outcome | Externalizing Disorder | |||
| Maternal History of Depression | ||||
| Maternal Warmth during Early Childhood | ||||
| BA 9 (−) | 4, 44, 20 | 2.56 | 186 | |
| Maternal Warmth during Early Adolescence | ||||
| Caudate Head (+) | 8, 9, −6 | 3.20 | 175 | |
| Maternal Warmth during Early Childhood × Maternal History | ||||
| Maternal Warmth during Early Adolescence × Maternal History | ||||
| BA 10 (−) | 4, 55, 5 | 3.28 | 376 | |
| Post-Hoc |
Note: Talairach coordinates. Findings are significant at p < .05. Multiple comparisons are controlled for using AlphaSim. BA = Brodmann’s Area.
Maternal warmth during childhood interacted with maternal history of depression in predicting striatal and mPFC activation during reward anticipation and striatal activation during loss anticipation and reward outcome. Simple slopes revealed that greater maternal warmth was associated with less activation in mPFC when anticipating rewards in boys exposed to maternal depression. Unexpectedly, less maternal warmth during early childhood was associated with greater striatal activation when anticipating reward and loss and when winning reward, but only for boys exposed to maternal depression.
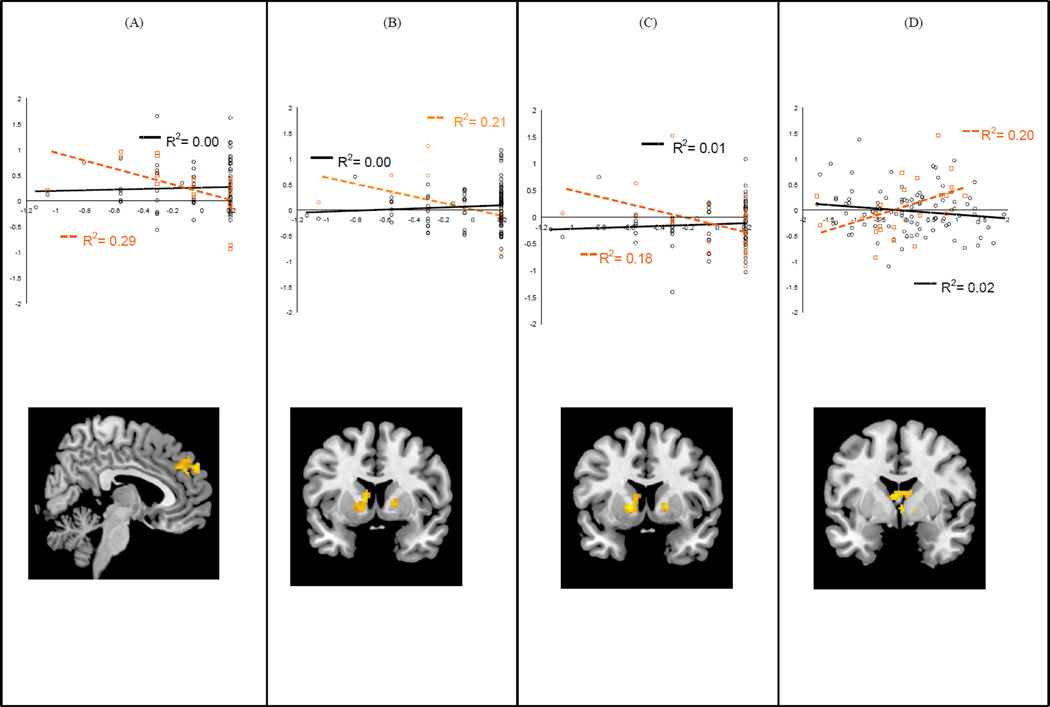
Maternal warmth during early adolescence interacted with maternal history of depression in predicting striatal activation during reward anticipation and reward outcome. Simple slopes were significant only for reward anticipation; greater maternal warmth was associated with greater caudate activation during reward anticipation, but, again, only for boys exposed to maternal depression (Figure 1).
Figure 1.
For boys exposed to maternal depression, greater maternal warmth during early childhood predicted (A) less medial prefrontal cortex (mPFC) activation during reward anticipation, (B) less striatal activation during reward anticipation, and (C) less striatal activation during loss anticipation; (D) Greater maternal warmth during adolescence predicted greater striatal activation during reward anticipation. Note: Maternal warmth values (x-axis) have been centered. Boys exposed to maternal depression are plotted in orange.
Main effects from models using maternal BDI at age 10/11 instead of maternal SCID were consistent with hypotheses (see Table S1, available online). Interactive effects using maternal BDI were substantively similar to interactive effects using maternal SCID but did not pass correction for multiple comparisons.
To address possible confounds, we tested whether history of depression or ADHD influenced our findings by conducting analyses of variance (ANOVAs) in SPSS using extracted mean response in clusters associated with interactive effects of maternal warmth and maternal depression. Neither lifetime history of depression (Fs=.02–.51) nor KSADS36 diagnosis of ADHD at age 10/11 (n=25; Fs=.00–1.77) was significantly related to reward response.
Discussion
Our large, longitudinal study provides evidence that maternal history of depression and maternal warmth during early childhood (18/24 months) and early adolescence (10/11 years) are associated with neural processing of reward in early adulthood. The focus of the study was whether the impact of maternal warmth on reward function differed depending on exposure to maternal depression. Consistent with hypotheses, maternal warmth during childhood was more strongly associated with reward function in early adulthood for boys exposed to maternal depression.
First, greater maternal warmth in early childhood was associated with less activation in the mPFC when anticipating rewards for boys exposed to maternal depression. Early childhood, particularly toddlerhood, is a period in which self-regulatory processes are forming rapidly12 and are developing largely through dyadic processes such as mimicking of maternal affect.7 Accordingly, greater maternal warmth during early childhood may influence neural regulatory processes that sustain positive affect (rather than dampen) when anticipating a rewarding experience (e.g., a mother’s smile).
Maternal warmth during both developmental periods interacted with maternal history of depression to predict reward function in the striatum; however, the direction of the effect appeared to be developmentally specific. In early childhood, lower levels of maternal warmth in boys exposed to maternal depression were associated with greater striatal response during reward anticipation, loss anticipation, and reward outcome. Less maternal warmth (a personally relevant social reward) may be particularly problematic during this developmental stage, in which maternal warmth is typically high and may influence the types of rewards that boys exposed to maternal depression value (i.e., heightened focus on monetary rewards vs. social rewards).
In early adolescence, higher levels of maternal warmth were associated with greater caudate activation during reward anticipation at age 20 for boys exposed to maternal depression. During the transition to adolescence when intensity of parent–child conflict increases, greater maternal warmth may be particularly important for boys exposed to maternal depression. This positive social experience during a socially challenging developmental period could promote adaptive striatal function and encourage pursuit of abstract social rewards.13,14
Our findings that maternal warmth was related to both hypo- and hyperactivation of the striatum in boys exposed to a depressed mother fits with developmental psychopathology literature on maternal depression, which indicates that having a depressed mother places youth at risk for a range of clinical disorders, including some associated with hypo-sensitivity and others associated with hypersensitivity to reward (e.g., depression and conduct problems).3,14,17 Maternal warmth appears to be important across development, although its influence on reward function may depend on developmental context and exposure to maternal depression and lead to different problems later in development.
Rather than hyper or hypoactivation of the striatum or mPFC in response to reward, healthy reward function is thought to be characterized by flexible elicitation of and communication within reward circuitry (i.e., positive connectivity between the striatum and mPFC).37 These flexible patterns likely indicate adaptive, regulated levels of pleasure and self-processing during the pursuit and receipt of rewards. In contrast, children who exposed to depressed and less warm parents may show reduced positive or greater negative connectivity in these regions, indicating that regions may not be acting in tandem or that one region may be over-regulating the other (e.g., heightened mPFC activation to dampen striatal response).37
Regardless of maternal depression history, greater maternal warmth in early childhood was associated with less mPFC when anticipating loss whereas greater maternal warmth in early adolescence was associated with less mPFC and greater striatal activation when winning rewards and experiencing loss, respectively. Greater maternal warmth may promote neural regulatory processes that sustain positive affect during early adolescence when planning abstract social rewards is more valued.14 Greater maternal warmth may prevent boys from overanalyzing their performance during disappointment. Instead, as indicated by heightened caudate activation when experiencing loss, boys who have experienced greater maternal warmth may find the pursuit of rewards pleasurable even when experiencing disappointment. These neural responses to reward and loss may promote healthy reward-seeking.
Our findings provide empirical evidence for what has been long suspected in the field of developmental psychopathology: maternal affective behavior is associated with brain function in regions relevant to psychopathology, particularly for youth exposed to maternal depression. This association is likely due to a combination of genetic factors influencing reward processing and learned differences in enjoyment of reward experiences due to maternal affective socialization.4 This socialization may be subtle, as simple as the ways in which mothers interact with their children. Boys exposed to maternal depression may be more malleable and develop neuroregulatory processes in close relation to their environment, thereby being susceptible not only to developing reward disruptions but also to forming adaptive reward function in the context of positive influences.
Maternal warmth during early childhood emerged as the most robust predictor of later reward function, particularly the anticipation of reward or loss. Likewise, maternal depression measured during early childhood interacted more strongly with maternal warmth to predict reward function relative to maternal depression measured during early adolescence. This may indicate that preventive interventions should start early in promoting warm maternal relationships in depressed mothers. Our findings may indicate that maternal factors may play a role in how boys form expectations about rewarding events, more so than in how they experience these events. Contrary to other studies8,10, maternal history of depression was not directly related to adolescent reward function.
The current study is the first, to our knowledge, to evaluate the longitudinal effect of early maternal affective factors on reward function during the transition from adolescence to adulthood. We used an ethnically diverse sample of boys with various clinical disorders, including MDD and substance dependence, which heightens our study’s external validity. Our study of maternal warmth is noteworthy as few studies have focused on how positive maternal experiences may play a role in brain function and this is a needed area of research for intervention purposes.
Despite these strengths, the study has several limitations. We were unable to evaluate other aspects of maternal depression (e.g., boys’ exposure to psychotropic drugs in utero, severity of maternal depression) that might have affected boys’ reward response. We focused on maternal depression only, but other forms of parental psychopathology may have been associated with boys’ reward function. Other intervening variables such as stressors closely linked to maternal mood (e.g., trauma) may have explained altered reward responding in these boys. We note that boys’ history of depression or attention-deficit/hyperactivity disorder (ADHD) were not associated with these reward responses. Additionally, the presence of a warm secondary caregiver (father, grandparent) may have significantly impacted boys’ reward function. Measurement of reward function across development would have allowed us to test how family relationships affect child brain development more directly. The use of a social reward paradigm would have allowed us to test whether maternal warmth is related to neural response to social rewards such as peer affiliation. However, our monetary reward paradigm reliably activates primary reward regions implicated in social processes21,29 and gives us a general picture of how social influences may affect reward processing. As all participants in our study were boys of low socioeconomic status, results from this study may not be generalizable to girls or boys from other sociodemographic backgrounds.
The current findings have clinical relevance and suggest the long-term impact of early parenting experiences on boys’ affective brain function. Our findings highlight exposure to maternal depression as a mechanism for susceptibility to psychopathology via vulnerable reward systems and provide a hopeful and constructive goal for clinicians—increasing warmth in the parent-child relationship. These findings are a step toward understanding how warm parent-child relationships create a healthy context for brain development.
Supplementary Material
Acknowledgments
This research was supported by grant R01 MH050907 from the National Institutes of Health to Dr. Shaw and R01 DA02622 to Drs. Shaw and Forbes.
The authors thank the staff and study families of the Pitt Mother and Child Project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Disclosure: Drs. Morgan, Shaw, and Forbes report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kochanska G, Aksan N. Mother-child mutually positive affect, the quality of child compliance to requests and prohibitions, and maternal control as correlates of early internalization. Child Dev. 1995;66:236–254. [Google Scholar]
- 2.Steelman LM, Assel MA, Swank PR, Smith KE, Landry SH. Early maternal warm responsiveness as a predictor of child social skills: Direct and indirect paths of influence over time. J Appl Dev Psychol. 2002;23:135–156. [Google Scholar]
- 3.Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clin Child Fam Psychol Rev. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- 4.Goodman SH, Gotlib IH. Risk for psychopathology in children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychol Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- 5.Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting: A meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- 6.Dietz LJ, Birmaher B, Williamson DE, et al. Mother-child interactions in depressed children and children at high risk and low risk for future depression. J Am Acad Child Adolesc Psychiatry. 2008;47:574–582. doi: 10.1097/CHI.0b013e3181676595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster CJ, Garber J, Durlak JA. Current and past maternal depression, maternal interaction behaviors and children’s externalizing and internalizing symptoms. J Abnorm Child Psychol. 2008;36:527–537. doi: 10.1007/s10802-007-9197-1. [DOI] [PubMed] [Google Scholar]
- 8.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expression in children and adolescents at risk for major depression. Am J Psychol. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 9.Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. J Abnormal Psychol. 2005;114:28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joorman J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–386. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson G, Ashman SB, Panagiotides H, et al. Preschool outcomes of children of depressed mothers: Role of maternal behavior, contextual risk, and children’s brain activity. Child Dev. 2003;74:1158–1175. doi: 10.1111/1467-8624.00599. [DOI] [PubMed] [Google Scholar]
- 12.Kopp CB. Antecedents of self-regulation: A developmental perspective. Dev Psychol. 1982;18:199–214. [Google Scholar]
- 13.Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR. The role of the family context in the development of emotion regulation. Social Dev. 2007;16:361–388. doi: 10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: Development of prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;38:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Phil Trans R Soc. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychol. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: A meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2012 doi: 10.1016/j.jad.2013.06.039. [published online July 12] [DOI] [PubMed] [Google Scholar]
- 23.Guyer AE, Kaufman J, Hogdon H, et al. Behavioral alterations in reward system function: The role of childhood maltreatment and psychopathology. J Am Acad Child Adolesc Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- 24.Shaw DS, Shelleby E. Early-onset conduct problems: Intersection of conduct problems and poverty. Ann Rev Clin Psychol. doi: 10.1146/annurev-clinpsy-032813-153650. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumais A, Lesage AD, Alda M, et al. Risk factors for suicide completion in major depression: A case-control study of impulsive and aggressive behaviors in men. Am J Psych. 2005;162:2116–2124. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- 26.Shaw DS, Hyde LW, Brennan LM. Early predictors of boys’ antisocial trajectories. Dev Psychopathol. 2012;24:871–888. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollingshead AB. Four factor index of social status. Yale University, Department of Sociology; 1975. Unpublished manuscript. [Google Scholar]
- 28.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R, Patient Edition/Non-patient Edition (SCID-P/SCID-NP) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- 29.Nusslock R, Almeida JRC, Forbes EE, et al. Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory. Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 31.Winslow EB, Shaw DS, Bruns H, Kiebler K. Proceedings of the Society for Research in Child Development. Indianapolis, IN: 1995. Parenting as a mediator of child behavioral problems and maternal stress, support, and adjustment. [Google Scholar]
- 32.Bakeman R, Gottman JM. Observing interaction: An introduction to sequential analysis. 2nd ed. New York: Cambridge University Press; 1997. Assessing observer agreement; pp. 56–80. [Google Scholar]
- 33.Hetherington EM, Clingempeel WG, Anderson ER, et al. Coping with marital transitions: A family systems perspective. Monogr Soc Res Child Dev. 1992;57:227. [Google Scholar]
- 34.Allen JP, Porter M, McFarland C, McElhaney KB, Marsh P. The relation of attachment security to adolescents’ paternal and peer relationships, depression, and externalizing behavior. Child Dev. 2007;78:1222–1239. doi: 10.1111/j.1467-8624.2007.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: 1991. [Google Scholar]
- 36.Orvaschel H, Puig-Antich J. Schedule for affective disorders and schizophrenia for school-age children: Epidemiologic 4th version. Ft. Lauderdale, FL: Nova University, Center for Psychological Study; 1987. [Google Scholar]
- 37.Heller AS, Johnstone T, Shackman AJ, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.