Abstract
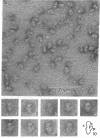
The structure of eukaryotic Artemia salina and prokaryotic Escherichia coli ribosomes has been compared by electron microscopy. Despite the established differences in size and in the amount and proportion of the protein and RNA moieties, both types of ribosomes appear to have substantial similarity in the overall shape and in the mutual orientation of the subunits on the monosome. The small subunit is located in the "crown" region of the large subunit lengthwise between the two side crests. However, high-resolution electron microscopy reveals distinct differences in the fine structure of both small and large subunits. The 40S A. salina subunit with three structural domains is more complex than the corresponding E. coli subunit. The 60S A. salina subunit has a less expressed "crown" region and shows a knob-like protrusion in the base. Structural asymmetry is a characteristic feature common to subunits and monosomes from both A. salina and E. coli.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boublik M., Brot N., Weissbach H. The conformational stability of ribosomal proteins L7 and L12 from E. coli. I. Circular dichroism study of the changes induced by ionic strength and solvents. Biopolymers. 1973;12(9):2083–2092. doi: 10.1002/bip.1973.360120914. [DOI] [PubMed] [Google Scholar]
- Boublik M., Hellmann W., Roth H. E. Localization of ribosomal proteins L7L12 in the 50 S subunit of Escherichia coli Ribosomes by electron microscopy. J Mol Biol. 1976 Nov 15;107(4):479–490. doi: 10.1016/s0022-2836(76)80079-4. [DOI] [PubMed] [Google Scholar]
- Brot N., Yamasaki E., Redfield B., Weissbach H. The binding of aminoacyl-tRNA and poly U to a soluble factor (S) extracted from ribosomes. Biochem Biophys Res Commun. 1970 Aug 11;40(3):698–707. doi: 10.1016/0006-291x(70)90960-5. [DOI] [PubMed] [Google Scholar]
- Deusser E., Weber J., Maschler R., Stöffler G., Wittmann H. G. Structural and functional evidence for a repeated 50S subunit ribosomal protein. Nat New Biol. 1973 Mar 14;242(115):47–49. doi: 10.1038/newbio242047a0. [DOI] [PubMed] [Google Scholar]
- Dzionara M. Ribosomal proteins. Secondary structure of individual ribosomal proteins of E. coli studied by circular dichroism. FEBS Lett. 1970 Jun 8;8(4):197–200. doi: 10.1016/0014-5793(70)80262-9. [DOI] [PubMed] [Google Scholar]
- Hardy S. J. The stoichiometry of the ribosomal proteins of Escherichia coli. Mol Gen Genet. 1975 Oct 3;140(3):253–274. doi: 10.1007/BF00334270. [DOI] [PubMed] [Google Scholar]
- Kurland C. G. Structure and function of the bacterial ribosome. Annu Rev Biochem. 1977;46:173–200. doi: 10.1146/annurev.bi.46.070177.001133. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Ribosome structure determined by electron microscopy of Escherichia coli small subunits, large subunits and monomeric ribosomes. J Mol Biol. 1976 Jul 25;105(1):131–139. doi: 10.1016/0022-2836(76)90200-x. [DOI] [PubMed] [Google Scholar]
- Möller W., Slobin L. I., Amons R., Richter D. Isolation and characterization of two acidic proteins of 60s ribosomes from Artemia salina cysts. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4744–4748. doi: 10.1073/pnas.72.12.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura Y., Blobel G., Sabatini D. Structure of liver ribosomes studied by negative staining. J Mol Biol. 1971 Sep 14;60(2):303–323. doi: 10.1016/0022-2836(71)90296-8. [DOI] [PubMed] [Google Scholar]
- Ochoa S. Initiation of protein synthesis. Naturwissenschaften. 1976 Aug;63(8):347–355. doi: 10.1007/BF00607927. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Sjöberg B. Small-angle X-ray scattering and crosslinking study of the proteins L7/L12 from Escherichia coli ribosomes. FEBS Lett. 1976 Jul 1;66(1):48–51. doi: 10.1016/0014-5793(76)80582-0. [DOI] [PubMed] [Google Scholar]
- Pestka S., Nirenberg M. Regulatory mechanisms and protein synthesis. X. Codon recognition on 30 S ribosomes. J Mol Biol. 1966 Oct 28;21(1):145–171. doi: 10.1016/0022-2836(66)90085-4. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Architecture of the Escherichia coli ribosome as determined by immune electron microscopy. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4820–4824. doi: 10.1073/pnas.72.12.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Determination of the location of proteins L14, L17, L18, L19, L22, L23 on the surface of the 5oS ribosomal subunit of Escherichia coli by immune electron microscopy. Mol Gen Genet. 1974;134(3):187–208. doi: 10.1007/BF00267715. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ochoa S. A supernatant factor involved in initiation complex formation with eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3059–3063. doi: 10.1073/pnas.68.12.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]