Abstract
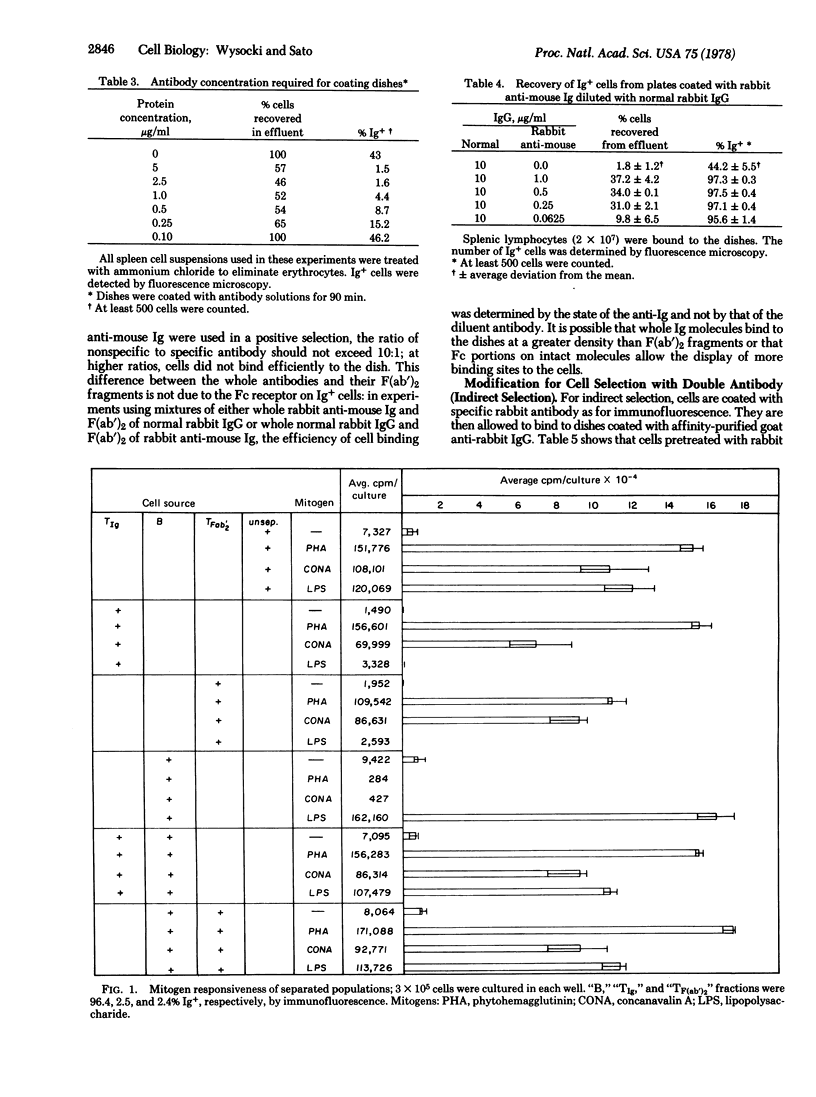
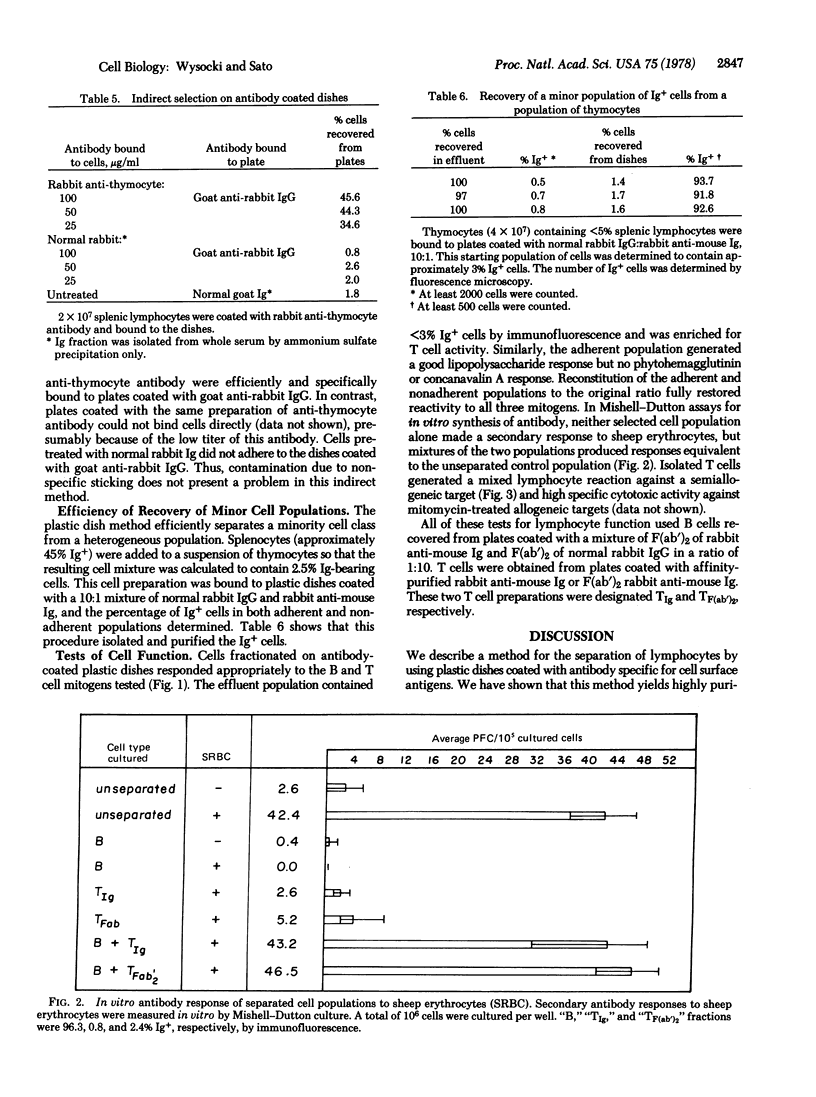
We have developed a simple method for the fractionation of T and B lymphocytes. Plastic dishes coated with antibodies specific for mouse Ig selectively bind splenic B lymphocytes. The adherent cells are easily removed by gentle pipetting; both adherent and nonadherent populations retain immunologic function. In a typical experiment, when 3 X 10(7) splenic lymphocytes were added to a 100 X 15 mm plastic dish coated with microgram quantities of anti-Ig, 98 % of the nonadherent cells were Ig negative and 97% of the adherent cells were Ig positive. The method is sufficiently sensitive to allow detection and separation of cell types comprising as little as 2% of the total population and can be modified to allow the selection of cells by a double-antibody procedure. We believe that the plastic dish method will be generally useful for fractionating cells on the basis of their cell surface antigens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catt K., Tregear G. W. Solid-phase radioimmunoassay in antibody-coated tubes. Science. 1967 Dec 22;158(3808):1570–1572. doi: 10.1126/science.158.3808.1570. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Masuda T., Herzenberg L. A. Demonstration that antigen-binding cells are precursors of antibody-producing cells after purification with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1934–1938. doi: 10.1073/pnas.69.7.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. J., Letarte-Muirhead M., Williams A. F. Analysis in deoxycholate of three antigenic specificities associated with the rat Thy-1 molecule. Eur J Immunol. 1975 Apr;5(4):282–285. doi: 10.1002/eji.1830050413. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Sato V. L., Waksal S. D., Herzenberg L. A. Identification and separation of pre T-cells from nu/nu mice: differentiation by preculture with thymic reticuloepithelial cells. Cell Immunol. 1976 Jun 1;24(1):173–185. doi: 10.1016/0008-8749(76)90142-8. [DOI] [PubMed] [Google Scholar]
- Schlossman S. F., Hudson L. Specific purification of lymphocyte populations on a digestible immunoabsorbent. J Immunol. 1973 Jan;110(1):313–319. [PubMed] [Google Scholar]


