Abstract
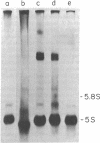
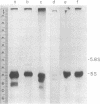
Single and multiple repeating units of three types of Xenopus 5S DNA recombined with the plasmid pMB9 serve as templates for the accurate synthesis of 5S RNA after their injection into Xenopus laevis oocyte nuclei. All 15 cloned single repeating units of X. laevis oocyte 5S DNA that were tested supported 5S RNA synthesis. Three cloned fragments of X. borealis oocyte 5S DNA and one cloned single repeating unit of X. borealis somatic 5S DNA were templates for 5S RNA synthesis. We conclude that the majority of repeating units of 5S DNA in these multigene families contain the information for accurate initiation and termination of 5S RNA synthesis. The ability of this system to detect sequence changes that affect transcription is demonstrated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Chow L. T., Dugaiczyk A., Hedgpeth J., Goodman H. M. DNA substrate site for the EcoRII restriction endonuclease and modification methylase. Nat New Biol. 1973 Jul 11;244(132):40–43. doi: 10.1038/newbio244040a0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Sugimoto K. 5 S DNAs of Xenopus laevis and Xenopus mulleri: evolution of a gene family. J Mol Biol. 1973 Aug 15;78(3):397–415. doi: 10.1016/0022-2836(73)90464-6. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Wensink P. C., Jordan E. Purification and some characteristics of 5S DNA from Xenopus laevis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3175–3179. doi: 10.1073/pnas.68.12.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. D., Brown D. D. The nucleotide sequence adjoining the 3' end of the genes coding for oocyte-type 5 S ribosomal RNA in Xenopus. J Mol Biol. 1976 Mar 25;102(1):1–14. doi: 10.1016/0022-2836(76)90070-x. [DOI] [PubMed] [Google Scholar]
- Carroll D., Brown D. D. Repeating units of Xenopus laevis oocyte-type 5S DNA are heterogeneous in length. Cell. 1976 Apr;7(4):467–475. doi: 10.1016/0092-8674(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis H., Wegnez M. Recherches biochimiques sur l'oogenése. 7. Synthése et maturation du RNA 5S dans les petitis oocytes de Xenopus laevis. Biochimie. 1973;55(9):1137–1151. doi: 10.1016/s0300-9084(73)80453-5. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. I. The AT-rich spacer. Cell. 1978 Apr;13(4):701–716. doi: 10.1016/0092-8674(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Mertz J. E., Gurdon J. B. Purified DNAs are transcribed after microinjection into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1502–1506. doi: 10.1073/pnas.74.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R., Cartwright E. M., Brownlee G. G., Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. II. The GC-rich region. Cell. 1978 Apr;13(4):717–725. doi: 10.1016/0092-8674(78)90221-0. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Brown D. D. Transcription of the ribosomal RNA genes of an amphibian by the RNA polymerase of a bacterium. J Mol Biol. 1970 Jul 28;51(2):361–377. doi: 10.1016/0022-2836(70)90148-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Laskey R. A., Finch J., Gurdon J. B. Selective DNA conservation and chromatin assembly after injection of SV40 DNA into Xenopus oocytes. Dev Biol. 1978 May;64(1):178–188. doi: 10.1016/0012-1606(78)90069-6. [DOI] [PubMed] [Google Scholar]