Abstract
Background
Primary prevention of Alzheimer disease and other types of dementia (all-cause dementia) is an important public health goal. Evidence to date is insufficient to recommend any lifestyle change to prevent or delay the onset of dementia.
Objective
To assess the association between objectively measured midlife cardiorespiratory fitness (“fitness”) levels and development of all-cause dementia in advanced age.
Design
Prospective, observational cohort study.
Setting
Preventive medicine clinic.
Patients
19 458 community-dwelling, nonelderly adults who had a baseline fitness examination.
Measurements
Fitness levels, assessed using the modified Balke treadmill protocol between 1971 and 2009, and incident all-cause dementia using Medicare Parts A and B claims data from 1999 to 2009.
Results
1659 cases of incident all-cause dementia occurred during 125 700 person-years of Medicare follow-up (median follow-up, 25 years [interquartile range, 19 to 30 years]). After multivariable adjustment, participants in the highest quintile of fitness level had lower hazard of all-cause dementia than those in the lowest quintile (hazard ratio, 0.64 [95% CI, 0.54 to 0.77]). Higher fitness levels were associated with lower hazard of all-cause dementia with previous stroke (hazard ratio, 0.74 [CI, 0.53 to 1.04]) or without previous stroke (hazard ratio, 0.74 [CI, 0.61 to 0.90]).
Limitations
Dementia diagnoses were based on Medicare claims, and participants generally were non-Hispanic white, healthy, and well-educated and had access to preventive health care. This study evaluated fitness levels, so a specific exercise prescription cannot be generated from results and the findings may not be causal.
Conclusion
Higher midlife fitness levels seem to be associated with lower hazards of developing all-cause dementia later in life. The magnitude and direction of the association were similar with or without previous stroke, suggesting that higher fitness levels earlier in life may lower risk for dementia later in life, independent of cerebrovascular disease.
Alzheimer disease and other types of dementia (all-cause dementia) are important public health problems, particularly in light of the aging population (1). One in 8 persons aged 65 years or older has Alzheimer disease (2). Aggregate payments for health care, long-term care, and hospice for 5.4 million Americans with dementia are $200 billion currently and projected to increase to $1.1 trillion by 2050 (2). Because of the effect of dementia on quality of life and functional status, identifying preventable causes of dementia is critical. The most cost-effective measures to prevent dementia would be those mediated through lifestyle changes requiring minimal medical intervention. However, a recent consensus statement on dementia prevention concluded that there was insufficient evidence to promote any lifestyle change as an effective preventive measure, including increasing self-reported physical activity (3) or improving measured cardiorespiratory fitness (“fitness”) levels. Hence, there are no specific public health recommendations for the prevention of cognitive disability other than maintaining good general health. Limitations of existing research, including small sample size, inadequate quality of exposure measurements, lack of consistent definitions of Alzheimer disease or other dementia-causing conditions, and short duration of follow-up have hampered the development of more specific preventive guidelines (3). Thus, large-scale, long-term studies are needed to evaluate effectiveness of modifiable risk factors for both cognitive decline and dementia in elderly persons.
We evaluated the association between objectively measured midlife fitness levels and the development of all-cause dementia in a large cohort of men and women with a long duration of follow-up. We hypothesized that persons with greater midlife fitness levels would have lower risk for dementia in later life and that this association would be, at least partially, independent of antecedent cerebrovascular disease.
METHODS
Study Population
The Cooper Center Longitudinal Study (CCLS) is an observational database of patient visits to the Cooper Clinic in Dallas, Texas, a preventive medicine practice that began in 1970. Analysis of data from this cohort has demonstrated that high fitness levels protect against all-cause mortality (4) and several illnesses, such as stroke (5) and diabetes mellitus (6, 7). Participants are community-dwelling, generally healthy persons who are either self-referred or referred by their employers for preventive health examinations, which include a standardized medical examination by a physician, anthropometric measurements, fasting laboratory studies, and a maximal treadmill exercise test for objectively measured fitness levels. Data acquired at the Cooper Clinic for the CCLS are not based on a systematic research protocol but rather on the previously mentioned preventive health evaluations and patient-specific clinical recommendations, resulting in variable follow-up intervals. Participants provided informed consent for the use of their data for research. The CCLS database is maintained by The Cooper Institute, a nonprofit, independent research institute with the overarching research goal of assessing the effect of lifestyle behaviors on health outcomes. Privacy precautions are maintained through The Cooper Institute policies. The data collection and informed consent processes are reviewed and approved annually by the Institutional Review Board at The Cooper Institute.
The study cohort initially comprised 28 968 persons in the CCLS who had an exercise treadmill test between 1971 and 2009 who could be matched with Medicare administrative claims data between 1999 and 2009, the available years of outcome data at the time of this study (8). We excluded participants lacking traditional fee-for-service Medicare for whom individual claims data were not available (n = 2973), those without a complete set of baseline variables (n = 4152), those with myocardial infarction or stroke at their midlife examination visit (n = 472) or chronic illness leading to early disability or renal dialysis on Medicare when younger than 65 years (n = 368), and those who had their first CCLS visit at age 65 years or older (n = 821). We also excluded participants who entered the data set with a prevalent diagnosis of all-cause dementia in or before 1999 or at age 67 years or younger and those with stroke in or before 1999 or at age 65 years or younger (n = 724), thresholds based on Centers for Medicare & Medicaid Services algorithms for definition of all-cause dementia and stroke (8). Thus, the final cohort included 19 458 men and women at their midlife examination, greater than 95% of whom had their baseline examination more than 9 years before Medicare entry.
Midlife Exposures
Measurement of Midlife Health Status
Study participants gave a comprehensive medical history, including self-reported medical, family, and social history confirmed by the clinic physician (4). Self-reported educational history was available only for 4220 participants (21.7%) because it was voluntary for patients to answer this question. They had physical examinations, including height and weight measured using a standard clinical stadiometer and scale to calculate body mass index and seated resting blood pressure measured with a calibrated sphygmomanometer. Laboratory studies, done after a 12-hour fast, included fasting blood glucose and cholesterol profile and were measured in accordance with standard procedures.
Cardiorespiratory Fitness Level Measurement
Exercise testing is a component of the Cooper Clinic basic examination and is done on all persons without a medical or orthopedic contraindication. Fitness level was measured as maximal time on a treadmill test using the modified Balke protocol (9); time on treadmill with this protocol is highly correlated (r = 0.92) with measured maximal oxygen uptake (VO2max) in both men and women (10, 11). Maximal metabolic equivalents (METs) (1 MET = 3.5 mL × O2·kg−1 × min−1) were estimated by regression from the final treadmill speed and grade (12). Treadmill times were compared with age- and sex-specific normative data on treadmill performance within the CCLS so that treadmill time could be classified into age- and sex-specific quintiles of fitness level for each participant. Although no uniform consensus for the precise range of low fitness level exists, quintile 1 has been considered “low fit” in previous work from The Cooper Institute (13). Historically, the low-fit category was most highly associated with increased morbidity and mortality rates (4–7).
Outcome Variables
Medicare data were obtained from the Centers for Medicare & Medicaid Services for all CCLS participants who were aged 65 years or older by 31 December 2009 and eligible for Medicare between 1 January 1999 and 31 December 2009. The diagnoses used in this study were obtained from the Chronic Condition Data Warehouse (8), which contains data on chronic disease diagnoses derived from well-established algorithms to identify chronic conditions among Medicare beneficiaries for research purposes (8).
Incidence of all-cause dementia during the surveillance period was the primary outcome of interest. All-cause dementia diagnoses were determined by standard algorithms using administrative data that have been previously validated (8). Specifically, a diagnosis of all-cause dementia is defined among persons having at least 3 years of Medicare coverage with at least 1 inpatient, skilled-nursing facility, home health agency, hospital outpatient, or physician or supplier claim with any diagnosis identified by the following International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM), codes: 331.0, 331.1, 331.11, 331.19, 331.2, 331.7, 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 294.0, 294.1, 294.10, 294.11, 294.8, and 797. These codes primarily include Alzheimer disease, senile dementia, and presenile dementia, in addition to only 4 codes for vascular dementia (290.40, 290.41, 290.42, and 290.43). A diagnosis of stroke was defined when ICD-9 codes 430, 431, 434.00, 434.01, 434.10, 434.11, 434.90, 434.91, 435.0, 435.1, 435.3, 435.8, 435.9, 436, or 997.02 were listed as a diagnosis on at least 1 inpatient or 2 outpatient claims during a 1-year period (8).
We determined the overall prevalence of all-cause dementia at attained ages 70, 75, 80, and 85 years by classifying all participants alive at these ages according to the presence or absence of dementia at the corresponding age. Attained age in the Medicare data block was defined as the age of participants before dementia diagnosis, death, or censoring at 2009.
Statistical Analysis
We constructed Kaplan–Meier curves to illustrate the unadjusted association between midlife fitness level and incident dementia, treating deaths as censoring events. We developed a Cox proportional hazards model with attained age as the time scale, adjusting for midlife fitness level modeled as a 5-level categorical covariate corresponding to age- and sex-adjusted quintiles of fitness level, demographic and study variables (sex, examination age, examination year, and examination age × follow-up time interaction term), and clinical variables (hypertension, fasting glucose level, current tobacco use, body mass index, total cholesterol level, hyperlipidemia, systolic blood pressure, and diabetes). We repeated the analysis with midlife fitness level modeled as a continuous variable. To test the proportional hazards assumption, we tested covariate × attained age effects and retained significant terms to address residual nonproportional hazards. We confirmed that hazards were proportional in the model. Main effects were estimated at the mean attained age. Sensitivity analyses were done among 4220 participants with self-reported education.
To assess the association between fitness level and the development of dementia with or without intervening stroke, we used a failure time model to investigate the multivariate outcomes: incident all-cause dementia without previous stroke and incident all-cause dementia after stroke (14). This model fits marginal Cox proportional hazards models to each outcome and uses the robust sandwich estimate of the covariance matrix to account for correlation of score residuals of the same participant in different marginal models (15). A 2-sided P value less than 0.050 was considered statistically significant. All statistical analyses were done using SAS, version 9.2 (SAS Institute, Cary, North Carolina).
Role of Funding Source
The study was funded by the Cooper Institute, University of Texas Southwestern Medical Center; the National Heart, Lung, and Blood Institute; and the American Heart Association. The funding source had no role in the design of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
RESULTS
Table 1 provides the characteristics of the study sample in midlife by fitness level category. As expected, lower fitness levels at midlife examination were associated with a higher prevalence of traditional cardiovascular risk factors, including increased body mass index, hypertension, diabetes, hyperlipidemia, and smoking. Mean follow-up from CCLS examination was 24.0 years (SD, 8.3) (median, 25.0 years [interquartile range, 19 to 30 years]) with a mean of 7.2 years of observation in the Medicare data.
Table 1.
Characteristics of Participants at Midlife Visit, by Quintile of Fitness Level
| Variable | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | All Quintiles | P Value* |
|---|---|---|---|---|---|---|---|
| Participants, n | 3322 | 3882 | 4085 | 4213 | 3956 | 19 458 | – |
| Female, n (%) | 552 (16.6) | 774 (19.9) | 746 (18.3) | 1020 (24.2) | 1008 (25.5) | 4100 (21.1) | <0.001 |
| Mean baseline age (SD), y | 46.8 (8.6) | 48.5 (8.8) | 50.0 (8.4) | 51.2 (8.7) | 51.7 (8.2) | 49.8 (8.7) | <0.001 |
| Mean education (SD), y† | 15.2 (2.7) | 15.5 (2.5) | 15.6 (2.6) | 15.8 (2.6) | 16.0 (2.5) | 15.7 (2.6) | <0.001 |
| Hypertension, n (%) | 835 (25.1) | 741 (19.1) | 692 (16.9) | 669 (15.9) | 505 (12.8) | 3442 (17.7) | <0.001 |
| Diabetes, n (%) | 168 (5.1) | 112 (2.9) | 95 (2.3) | 75 (1.8) | 45 (1.1) | 495 (2.5) | <0.001 |
| Smoker, n (%) | 961 (28.9) | 783 (20.2) | 614 (15.0) | 403 (9.6) | 235 (5.9) | 2996 (15.4) | <0.001 |
| Mean alcohol use (SD), drinks/wk‡ | 8.5 (10.9) | 8.6 (10.9) | 8.9 (11.9) | 8.8 (11.0) | 8.7 (10.8) | 8.7 (11.1) | 0.002 |
| Mean body mass index (SD), kg/m2 | 28.1 (4.9) | 26.4 (3.7) | 25.8 (3.3) | 25.0 (3.0) | 23.8 (2.6) | 25.7 (3.8) | <0.001 |
| Mean systolic blood pressure (SD), mm Hg | 123.7 (14.8) | 121.6 (14.3) | 121.1 (14.7) | 120.9 (14.7) | 120.5 (14.9) | 121.5 (14.7) | <0.001 |
| Mean diastolic blood pressure (SD), mm Hg | 82.6 (10.1) | 81.5 (9.8) | 80.8 (9.7) | 80.2 (9.4) | 79.2 (9.1) | 80.8 (9.7) | <0.001 |
| Mean glucose level (SD) mmol/L mg/dL |
5.8 (1.3) 103.7 (22.9) |
5.6 (1.0) 101.7 (18.2) |
5.6 (0.8) 100.0 (14.1) |
5.5 (0.7) 99.1 (12.5) |
5.5 (0.7) 98.2 (12.8) |
5.6 (0.9) 100.4 (16.4) |
<0.001 |
| Mean total cholesterol level (SD) mmol/L mg/dL |
5.7 (1.1) 220.3 (41.2) |
5.6 (1.0) 216.1 (39.7) |
5.6 (1.0) 215.2 (39.0) |
5.5 (1.0) 211.5 (38.2) |
5.4 (1.0) 207.7 (36.7) |
5.5 (1.0) 213.9 (39.1) |
<0.001 |
| Mean cardiorespiratory fitness level (SD), MET | 8.1 (1.4) | 9.4 (1.4) | 10.4 (1.4) | 11.3 (1.6) | 13.3 (2.2) | 10.6 (2.4) | <0.001 |
MET = metabolic equivalent.
P value for trend by the Jonckheere–Terpstra test.
Data available for 4220 participants.
Data available for 16 849 participants.
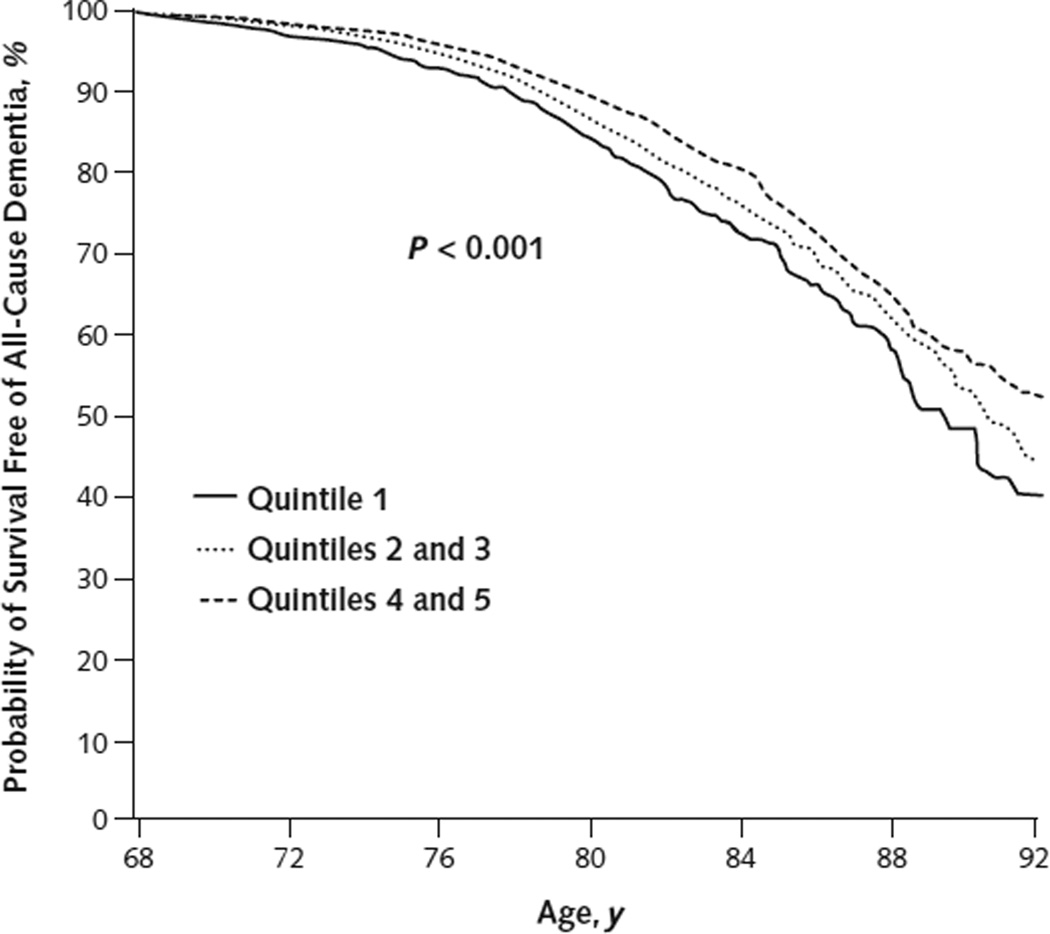
We saw 1659 cases of incident all-cause dementia during 125 700 total person-years of Medicare follow-up, with a strong association between dementia and attained age. In cross-sectional analyses, the prevalence of dementia by age was 0.8%, 2.9%, 8.3%, and 14.8% at ages 70, 75, 80, and 85 years, respectively. Higher fitness levels in midlife were associated with a lower risk for incident dementia (Figure) and a lower adjusted hazard of dementia (hazard ratio [HR] for quintile 5 vs. quintile 1, 0.64 [95% CI, 0.54 to 0.77]) (Table 2). Findings were similar when fitness level was modeled as a continuous covariate (HR per MET, 0.93 [CI, 0.90 to 0.95]).
Figure.
Dementia-free survival, according to midlife fitness level.
Table 2.
Cox Proportional Hazards Model for Incident Alzheimer Disease and Related Types of Dementia
| Effect, by Model Adjustment | Hazard Ratio (95% CI) |
PValue |
|---|---|---|
| Adjustment 1 (n = 19 458)* | ||
| Quintile 1 (reference) | 1.00 | – |
| Quintile 2 | 0.87 (0.75–1.01) | 0.069 |
| Quintile 3 | 0.78 (0.67–0.91) | 0.001 |
| Quintile 4 | 0.70 (0.60–0.81) | <0.001 |
| Quintile 5 | 0.64 (0.54–0.76) | <0.001 |
| Adjustment 2 (n = 19 458)† | ||
| Quintile 1 (reference) | 1.00 | – |
| Quintile 2 | 0.88 (0.76–1.02) | 0.089 |
| Quintile 3 | 0.79 (0.67–0.92) | 0.002 |
| Quintile 4 | 0.70 (0.60–0.83) | <0.001 |
| Quintile 5 | 0.64 (0.54–0.77) | <0.001 |
| Hypertension‡ | 1.15 (1.01–1.31) | 0.041 |
| Fasting glucose level (per 1 mg/dL [0.06 mmol/L]) |
1.00 (1.00–1.01) | 0.077 |
| Current tobacco use | 1.11 (0.97–1.28) | 0.126 |
| Body mass index (per 3 kg/m2) | 0.97 (0.92–1.02) | 0.184 |
| Total cholesterol level (per 40 mg/dL [1.04 mmol/L]) |
0.96 (0.89–1.02) | 0.193 |
| Hyperlipidemia§ | 1.07 (0.92–1.24) | 0.37 |
| Systolic blood pressure (per 20 mm Hg) | 0.97 (0.90–1.06) | 0.50 |
| Diabetes║ | 1.06 (0.86–1.30) | 0.56 |
Model adjusted for sex, examination age, examination year, and examination age × follow-up time.
Model adjusted for sex, examination age, examination year, examination age × follow-up time, and listed risk factors.
Self-reported or measured systolic blood pressure ≥140 mm Hg.
Self-reported or measured total cholesterol level ≥200 mg/dL (≥5.18 mmol/L).
Self-reported or measured fasting glucose level ≥126 mg/dL (≥6.99 mmol/L).
In the subpopulation of 4220 persons with self-reported educational level, 212 developed dementia over 21 696 person-years, with no statistically significant association between education and dementia (unadjusted HR, 0.96 [CI, 0.91 to 1.01]), probably reflecting the high level of educational attainment in the study sample (>15 years). The point estimate for the association between fitness level and dementia in this subgroup after adjustment for other clinical covariates was similar to that for the full population but with wider CIs (HR for quintile 5 vs. quintile 1, 0.77 [CI, 0.43 to 1.36]).
In a sensitivity analysis that included participants with missing baseline variables, hazard ratios were similar to the primary analysis (HR for quintile 5 vs. quintile 1, 0.67 [CI, 0.58 to 0.78]).
Finally, the association between higher midlife fitness level and risk for all-cause dementia was similar among 1216 participants without previous stroke (HR for quintile 5 vs. quintile 1, 0.74 [CI, 0.61 to 0.90]) and 443 participants with previous stroke (HR, 0.74 [CI, 0.53 to 1.04]), suggesting that the association between higher fitness level and risk for dementia is independent of intervening cerebrovascular disease.
DISCUSSION
In this cohort of generally healthy, community-dwelling persons having preventive health evaluations, we saw an association between midlife fitness levels, measured with treadmill testing, and lower risk for dementia in later life independent of other cardiovascular risk factors. The association seemed to be present with or without intervening cerebrovascular events, suggesting that the effect of midlife fitness levels on the prevention of dementia in later life may have both vascular and nonvascular mechanisms.
A recent National Institutes of Health Consensus Statement identified evidence suggesting that physical activity may prevent dementia (3). However, the literature identified in that review primarily addressed the effect of self-reported physical activity, not objectively measured fitness levels, on dementia. The review also included studies with shorter follow-up, less clearly defined exposures, or inconsistent definitions of the dementia outcome variable (3). One of the largest studies, the Canadian Study of Health and Aging, reported 5-year follow-up of 4615 participants showing that physical activity (vs. no physical activity) was associated with a lower risk for cognitive impairment and Alzheimer disease (16). Several studies confirmed the association of higher doses of physical activity with decreased risk for Alzheimer disease (17–20). Other studies showed risk modification only with certain physical activities, such as dancing (21); only with vascular dementia (22); or not at all (23). In addition, several studies have addressed the effect of some midlife behaviors, including physical activity, on dementia with conflicting outcomes (23–27).
The few studies that have examined the effect of fitness related to dementia are limited to intermediate outcomes (28–31), such as brief cognitive testing or brain volume, or death. One study in community-dwelling persons demonstrated that lower baseline fitness levels were associated with greater cognitive decline by the Mini-Mental State Examination over 6 years (28). From an anatomical perspective, high fitness levels have been associated with attenuation in the brain atrophy of normal aging (29), as well as greater total brain (30) and medial temporal lobe (31) volume. Finally, previous work in the CCLS cohort found that fitness levels were inversely related to dementia mortality risk in men and women (32). Thus, these studies suggest that fitness levels seem to be inversely correlated to intermediate outcomes as well as dementia mortality. In contrast, our study demonstrates an inverse association between fitness levels and dementia itself.
Greater fitness levels may be associated with lower dementia risk through several mechanisms. First, enhanced fitness levels are associated with lower risk for diabetes (33) and hypertension (34, 35), which are established risk factors for dementia. Physical activity and fitness levels may also have direct effects on the brain that lower dementia risk. Higher fitness levels have been associated with greater brain volume, which may be associated with enhanced cognitive function (29–31). In 1 very small study evaluating the effect of exercise on cerebral vasculature, exercising participants showed an increased number of small-caliber vessels and less tortuosity of cerebral vessels (36), potentially improving cerebral blood flow. In addition, in TgCRND8 mice voluntary exercise decreased the presence of amyloid in the frontal cortex and hippocampus, likely related to change in the processing of this neurotoxic protein (37). Other potential mechanisms include enhanced neuroplasticity and production of growth factors, such as brain-derived neurotrophic factor (38).
This study has several strengths, including the large size of the study cohort, the long duration of follow-up, and a detailed medical history obtained at the midlife examination that enabled us to exclude persons with preexisting cerebrovascular and cardiovascular diseases. It also has limitations. First, the dementia diagnosis was based on Medicare claims data and not on clinically detailed data sources, but these data have been reported to be largely concordant with clinical diagnoses of dementia in an Alzheimer disease registry (39) and have a sensitivity of 85% and specificity of 89% for dementia when compared with clinical dementia assessments in the Health and Retirement Study (40). In addition, Medicare data have been used to study dementia prevalence (41) and health care costs (42). The cross-sectional prevalence of dementia is somewhat lower than expected (43), due in part to the underestimation of disease by Medicare data and the lower burden of risk factors in this cohort, but it is associated appropriately with increasing age. Second, CCLS participants are generally healthy, non-Hispanic white (95%), and of middle to upper socioeconomic strata; therefore, they may not be representative of other populations. However, there is still great variability in the fitness level measurements. Third, unmeasured confounders, such as lifestyle factors associated with fitness levels, could account for the observed association between low fitness levels and dementia. Fourth, we excluded 4152 participants because of missing covariates at study entry. Nevertheless, sensitivity analysis showed similar effects of fitness levels on dementia development.
Finally, educational level was available for only 22% of the cohort and, therefore, we could not adjust for education in our primary analysis. Nevertheless, we believe that our findings are unaffected by education. We saw only trivial differences in education across fitness level quintiles. Second, we conclude from our sensitivity analyses that education is not an important confounder in the pathway from low fitness levels to all-cause dementia in this data set. In this regard, we believe that the homogenous nature of the CCLS represents an important quality, strengthening the observed association between midlife fitness levels and all-cause dementia independent of the effects of education.
This study specifically addressed fitness levels, which is an objective measure, rather than self-reported behavior. Because cardiorespiratory fitness levels are results of habitual physical activity and gene–environment interactions for any given person, it is not possible to give a generalizable physical activity dose recommendation from this study. Nonetheless, fitness levels are modifiable through physical training. Previous reports have shown that physical activity, or exercise, of at least moderate intensity for at least 150 minutes per week for 5 to 6 months results in an improvement of 3 to 5 mL/kg per min VO2max (maximum oxygen consumption), or 1 to 2 METs, in fitness levels (44–46). Thus, physical activity changes in midlife may lead to improved fitness levels, resulting in less all-cause dementia with aging.
In summary, higher midlife fitness levels were associated with lower hazards of developing Alzheimer disease and other types of dementia in men and women older than 65 years followed in a large prospective cohort for an average of 24 years. Future studies should address the dose–response relationship with physical activity needed to modify fitness levels to inform public health recommendations for dementia prevention. In addition, studies on the effect of midlife physical activity and fitness levels on brain structure and function may further elucidate the mechanisms of the protective effect of fitness levels.
Context
Identification of modifiable risk factors is important for public health efforts to prevent dementia.
Contribution
In this observational study of a group of healthy community-dwelling adults, higher treadmill-measured levels of physical fitness in middle age were associated with lower hazards of developing all-cause dementia after age 65 years, independent of previous stroke.
Caution
The findings are not causal and do not suggest a target level of fitness or exercise program.
Implication
Higher physical fitness levels in middle age may be protective against dementia. If the findings are verified, a lower physical fitness level may be a modifiable risk factor for the disease.
—The Editors
Acknowledgment
The authors thank Kenneth H. Cooper, MD, MPH, for establishing the CCLS; the late Fred R. Meyer, former Chief Executive Officer of The Cooper Institute; the Cooper Clinic for collecting the data; and The Cooper Institute for data management.
Grant Support: By The Cooper Institute. Dr. Berry receives funding from the Dedman Family Scholar in Clinical Care endowment at the University of Texas Southwestern Medical Center; the National Heart, Lung, and Blood Institute; and the American Heart Association.
Primary Funding Source: The Cooper Institute; University of Texas Southwestern Medical Center; National Heart, Lung, and Blood Institute; and American Heart Association.
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M12-1302.
Reproducible Research Statement: Study protocol: Available from Dr. DeFina (ldefina@cooperinst.org). Statistical code and data set: Available from Dr. Willis (bwillis@cooperinst.org).
Author Contributions: Conception and design: L.F. DeFina, B.L. Willis, W.L. Haskell, J.D. Berry.
Analysis and interpretation of the data: L.F. DeFina, B.L. Willis, A. Gao, D. Leonard, M.F. Weiner, J.D. Berry.
Drafting of the article: L.F. DeFina, B.L. Willis, N.B. Radford, M.F. Weiner, J.D. Berry.
Critical revision of the article for important intellectual content: L.F. DeFina, B.L. Willis, N.B. Radford, D. Leonard, W.L. Haskell, M.F. Weiner, J.D. Berry.
Final approval of the article: L.F. DeFina, B.L. Willis, N.B. Radford, A. Gao, D. Leonard, W.L. Haskell, J.D. Berry.
Statistical expertise: B.L. Willis, A. Gao, D. Leonard.
Obtaining of funding: J.D. Berry.
Administrative, technical, or logistic support: L.F. DeFina, B.L. Willis, J.D. Berry.
Collection and assembly of data: L.F. DeFina, B.L. Willis.
References
- 1.Chapman DP, Williams SM, Strine TW, Anda RF, Moore MJ. Dementia and its implications for public health. Prev Chronic Dis. 2006;3:A34. [PMID: 16539775] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [PMID: 22404854] [DOI] [PubMed] [Google Scholar]
- 3.Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Cox NJ, et al. NIH State-of-the-Science Conference Statement: Preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010:27. [PMID: 20445638] [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [PMID: 2795824] [DOI] [PubMed] [Google Scholar]
- 5.Hooker SP, Sui X, Colabianchi N, Vena J, Laditka J, LaMonte MJ, et al. Cardiorespiratory fitness as a predictor of fatal and nonfatal stroke in asymptomatic women and men. Stroke. 2008;39:2950–2957. doi: 10.1161/STROKEAHA.107.495275. [PMID: 18688008] [DOI] [PubMed] [Google Scholar]
- 6.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130:89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [PMID: 10068380] [DOI] [PubMed] [Google Scholar]
- 7.Sui X, Hooker SP, Lee IM, Church TS, Colabianchi N, Lee CD, et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31:550–555. doi: 10.2337/dc07-1870. [PMID: 18070999] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chronic Condition Data Warehouse. 2012 Aug 1; Accessed at www.ccwdata.org/index.htm.
- 9.Willis BL, Morrow JR, Jr, Jackson AW, Defina LF, Cooper KH. Secular change in cardiorespiratory fitness of men: Cooper Center Longitudinal Study. Med Sci Sports Exerc. 2011;43:2134–2139. doi: 10.1249/MSS.0b013e31821c00a7. [PMID: 21448076] [DOI] [PubMed] [Google Scholar]
- 10.Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [PMID: 961576] [DOI] [PubMed] [Google Scholar]
- 11.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–373. doi: 10.1016/0002-8703(82)90275-7. [PMID: 7064770] [DOI] [PubMed] [Google Scholar]
- 12.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed. New York: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 13.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [PMID: 18056904] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 15.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 16.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [PMID: 11255456] [DOI] [PubMed] [Google Scholar]
- 17.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [PMID: 15383515] [DOI] [PubMed] [Google Scholar]
- 18.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [PMID: 16418406] [DOI] [PubMed] [Google Scholar]
- 19.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [PMID: 19671904] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [PMID: 19671905] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [PMID: 12815136] [DOI] [PubMed] [Google Scholar]
- 22.Ravaglia G, Forti P, Lucicesare A, Pisacane N, Rietti E, Bianchin M, et al. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology. 2008;70:1786–1794. doi: 10.1212/01.wnl.0000296276.50595.86. [PMID: 18094335] [DOI] [PubMed] [Google Scholar]
- 23.Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement. 2008;4:324–331. doi: 10.1016/j.jalz.2008.07.002. [PMID: 18790459] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [PMID: 12196314] [DOI] [PubMed] [Google Scholar]
- 25.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc. 2003;51:410–414. doi: 10.1046/j.1532-5415.2003.51117.x. [PMID: 12588587] [DOI] [PubMed] [Google Scholar]
- 26.Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [PMID: 16239176] [DOI] [PubMed] [Google Scholar]
- 27.Andel R, Crowe M, Pedersen NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci. 2008;63:62–66. doi: 10.1093/gerona/63.1.62. [PMID: 18245762] [DOI] [PubMed] [Google Scholar]
- 28.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [PMID: 12657064] [DOI] [PubMed] [Google Scholar]
- 29.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [PMID: 12586857] [DOI] [PubMed] [Google Scholar]
- 30.Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [PMID: 18625967] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, et al. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [PMID: 19812458] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu R, Sui X, Laditka JN, Church TS, Colabianchi N, Hussey J, et al. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med Sci Sports Exerc. 2012;44:253–259. doi: 10.1249/MSS.0b013e31822cf717. [PMID: 21796048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akomolafe A, Beiser A, Meigs JB, Au R, Green RC, Farrer LA, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63:1551–1555. doi: 10.1001/archneur.63.11.1551. [PMID: 17101823] [DOI] [PubMed] [Google Scholar]
- 34.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [PMID: 16033691] [DOI] [PubMed] [Google Scholar]
- 35.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [PMID: 15668425] [DOI] [PubMed] [Google Scholar]
- 36.Bullitt E, Rahman FN, Smith JK, Kim E, Zeng D, Katz LM, et al. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. AJNR Am J Neuroradiol. 2009;30:1857–1863. doi: 10.3174/ajnr.A1695. [PMID: 19589885] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [PMID: 15858047] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster PP, Rosenblatt KP, Kuljiš RO. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Front Neurol. 2011;2:28. doi: 10.3389/fneur.2011.00028. [PMID: 21602910] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor DH, Jr, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol. 2002;55:929–937. doi: 10.1016/s0895-4356(02)00452-3. [PMID: 12393082] [DOI] [PubMed] [Google Scholar]
- 40.Taylor DH, Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17:807–815. doi: 10.3233/JAD-2009-1099. [PMID: 19542620] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor DH, Jr, Sloan FA, Doraiswamy PM. Marked increase in Alzheimer’s disease identified in medicare claims records between 1991 and 1999. J Gerontol A Biol Sci Med Sci. 2004;59:762–766. doi: 10.1093/gerona/59.7.m762. [PMID: 15304542] [DOI] [PubMed] [Google Scholar]
- 42.Taylor DH, Jr, Sloan FA. How much do persons with Alzheimer’s disease cost Medicare? J Am Geriatr Soc. 2000;48:639–646. doi: 10.1111/j.1532-5415.2000.tb04721.x. [PMID: 10855599] [DOI] [PubMed] [Google Scholar]
- 43.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [PMID: 17975326] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duncan JJ, Gordon NF, Scott CB. Women walking for health and fitness. How much is enough? JAMA. 1991;266:3295–3299. [PMID: 1960829] [PubMed] [Google Scholar]
- 45.Skinner JS, Jaskólski A, Jaskólska A, Krasnoff J, Gagnon J, Leon AS, et al. HERITAGE Family Study. Age, sex, race, initial fitness, and response to training: the HERITAGE Family Study. J Appl Physiol. 2001;90:1770–1776. doi: 10.1152/jappl.2001.90.5.1770. [PMID: 11299267] [DOI] [PubMed] [Google Scholar]
- 46.Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169:269–278. doi: 10.1001/archinternmed.2008.545. [PMID: 19204218] [DOI] [PMC free article] [PubMed] [Google Scholar]