Abstract
Background
Cardiorespiratory fitness (fitness) is associated with cardiovascular disease (CVD) mortality. However, the extent to which fitness improves risk classification when added to traditional risk factors is unclear.
Methods and Results
Fitness was measured by the Balke protocol in 66 371 subjects without prior CVD enrolled in the Cooper Center Longitudinal Study between 1970 and 2006; follow-up was extended through 2006. Cox proportional hazards models were used to estimate the risk of CVD mortality with a traditional risk factor model (age, sex, systolic blood pressure, diabetes mellitus, total cholesterol, and smoking) with and without the addition of fitness. The net reclassification improvement and integrated discrimination improvement were calculated at 10 and 25 years. Ten-year risk estimates for CVD mortality were categorized as <1%, 1% to <5%, and ≥5%, and 25-year risk estimates were categorized as <8%, 8% to 30%, and ≥30%. During a median follow-up period of 16 years, there were 1621 CVD deaths. The addition of fitness to the traditional risk factor model resulted in reclassification of 10.7% of the men, with significant net reclassification improvement at both 10 years (net reclassification improvement=0.121) and 25 years (net reclassification improvement=0.041) (P<0.001 for both). The integrated discrimination improvement was 0.010 at 10 years (P<0.001), and the relative integrated discrimination improvement was 29%. Similar findings were observed for women at 25 years.
Conclusions
A single measurement of fitness significantly improves classification of both short-term (10-year) and long-term (25-year) risk for CVD mortality when added to traditional risk factors.
Keywords: cardiovascular disease, classification, exercise capacity, mortality, risk
Physical activity and cardiorespiratory fitness (fitness) are known to be associated with enhanced health and quality of life,1 and even small improvements in fitness have been associated with reduced cardiovascular and all-cause mortality.2– 4 The current physical activity guidelines recognize the importance of fitness and activity, recommending that all adults engage in at least moderate-intensity exercise for 150 minutes each week or vigorous-intensity exercise for 75 minutes each week.1
In addition to being an important focus for lifestyle interventions, low fitness has also been proposed as a novel cardiovascular disease (CVD) risk factor that could be used clinically to improve risk stratification.5–7 Fitness has a well-documented inverse association with CVD mortality,8–14 and with the use of conventional statistical techniques, low fitness has been associated with a 2- to 3-fold increased risk of death, independent of traditional CVD risk factors.5,12,14–16 However, the association of a risk marker with CVD does not necessarily translate into diagnostic or prognostic utility.17–19 For example, the polymorphism in chromosome 9p21 is associated with a 1.25-fold increased hazard of incident CVD but results in negligible improvement in risk reclassification.20 Thus, in a recent scientific statement, the American Heart Association recommends that initial association studies be followed by a more thorough assessment of the contribution of a marker to clinical risk assessment with the use of advanced statistical tests such as net reclassification improvement (NRI), integrated discrimination improvement (IDI), and relative IDI.21
Currently, the extent to which knowledge of baseline fitness level provides clinically meaningful risk information beyond traditional risk factors in the short term or in the long term is unclear. Therefore, we sought to determine the contribution of fitness to traditional risk factors in CVD risk classification using data from the Cooper Center Longitudinal Study (CCLS) (formerly from the Aerobics Center Longitudinal Study), a well-described cohort8,9,15 with a large sample size and long-term follow-up.
Methods
Study Population
The CCLS is an ongoing prospective study enrolling 1000 to 4000 participants each year at the Cooper Clinic in Dallas, TX, details of which have been described previously.8,9,15 These individuals were either self-referred or were referred by their employer or personal physician. For this study, we included all participants between the ages of 20 and 90 years with a complete clinical visit who underwent clinical examination at the Cooper Clinic between 1970 and 2006 (n=67 110). We excluded 739 participants with a prior history of myocardial infarction for our primary analysis, resulting in a final study sample of 66 371. All participants with a prior medical history other than myocardial infarction were included in the analyses. The majority of study participants were white, well educated, and from middle to upper socioeconomic strata. The CCLS undergoes annual review by the institutional review board of the Cooper Institute. The requirement for written informed consent was waived by the institutional review board at the University of Texas Southwestern Medical Center.
Measurements
All participants underwent a comprehensive clinical examination including self-reported personal medical history, physical examination, fasting blood levels of glucose and total cholesterol, and a maximal symptom-limited treadmill exercise test. Details of anthropometric and laboratory measurements and other variable definitions have been described previously.8,9,15 Diabetes mellitus was defined by self-report or a fasting blood glucose ≥126 mg/dL. Smoking habits (current smoker or not) were obtained from a standardized questionnaire. Fitness was assessed by a maximal treadmill exercise test with the use of the Balke protocol.8,9 The treadmill speed was set initially at 88 m/min. In the first minute, the grade was set at 0%, followed by 2% in the second minute and increased by 1% every minute thereafter. The test was terminated by volitional exhaustion reported by the participant or by the physician for medical reasons. With this protocol, the exercise time correlates highly with directly measured maximal oxygen uptake (r=0.92) and allows estimation of fitness in metabolic equivalent tasks (METs) (1 MET=3.5 mL/kg per minute of oxygen consumption).22 Exercise time for each participant was compared with age- and sex-specific nomograms on treadmill performance within the CCLS, allowing each individual’s exercise time to be classified into an age- and sex-specific quintile of fitness as described previously, with quintile 1 (lowest level of fitness) as the referent group.8,9 No individual was excluded on the basis of their performance on the exercise treadmill portion of the examination.
End Point
Participants were followed from the date of initial examination until death or end of follow-up on December 31, 2006 (range of follow-up period, 0.01 to 36 years). With the use of data from the National Death Index,23 CVD as the primary cause of death (indicated by International Classification of Diseases, Ninth Revision codes 390.0 to 458.9 or equivalent codes from International Classification of Diseases, Eighth Revision or International Classification of Diseases, Tenth Revision) was used as the primary outcome variable. To assess the importance of competing risks, we performed sensitivity analyses using all-cause death as the outcome variable.
Statistical Analysis
After the proportional hazards assumption was satisfied with the use of Schoenfeld residuals, Cox regression analyses were used to estimate the risk of CVD mortality with a traditional factor model (age, systolic blood pressure, diabetes mellitus, total cholesterol, and smoking) and after the addition of fitness (expressed as age- and sex-specific quintiles with quintile 1 as the referent group and 4 dummy variables for the other fitness quintiles). Separate CVD mortality risk prediction models were used for men and women. Lack of model fit was tested with the Hosmer-Lemeshow test.
To assess model discrimination (the ability of a marker to differentiate between individuals who did and did not have CVD death), we constructed time-dependent receiver operating characteristic curves to calculate the Harrell’s C statistic.24,25 The C statistics for models with and without fitness were compared with bootstrapping. Using the models with and without fitness, we calculated the NRI.18,26 Net correct reclassification was calculated separately for those who developed a CVD death (events) and those who survived until the censored date (December 31, 2006) or had a non-CVD death (nonevents), and then the total NRI was calculated by taking their sum, as described previously.26 Because there are no well-established analytical approaches to estimate the NRI with time-to-event data with a variable follow-up period, we estimated the NRI for 10 years (NRI10) by restricting our analyses to the first 10 years of follow-up among participants enrolled on or before December 31, 1996 (n=43 041). Because we used CVD death rather than coronary heart disease as the outcome variable, we followed the European guidelines27 and not the Framingham Risk Score to determine clinically meaningful risk categories. The 10-year risk thresholds for CVD mortality were categorized as <1% (low risk), 1% to <5% (intermediate risk), and ≥5% (high risk). Sensitivity analyses were performed after adjustment for body mass index, substituting fitness quintiles with actual measured METs, excluding participants with diabetes mellitus (n=2603) or those with an abnormal exercise ECG (n=3585) and including those with prior history of myocardial infarction (n=739).
After assessing improvement in reclassification in the short term (10 years), we investigated whether fitness continues to improve classification of the risk of CVD mortality in the long term (25 years). To estimate the NRI for 25 years (NRI25), we restricted our analyses to the first 25 years of follow-up among participants enrolled on or before December 31, 1981 (n=16 533). To our knowledge, there are no standard thresholds for 25-year risks of CVD mortality. Therefore, we constructed 25-year CVD mortality thresholds by extrapolating the 10-year thresholds to 25 years: <8% (low risk), 8% to <30% (intermediate risk), and ≥30% (high risk). For example, low 10-year (<1%) and low 25-year (<8%) risk thresholds both correspond to the 83rd percentile of predicted 10-and 25-year risks in the CCLS, respectively. In sensitivity analysis, we created 25-year risk thresholds, assuming an exponential relationship of risk over time, resulting in 25-year risk thresholds of <2.7%, 2.7% to <12.2%, and ≥12.2%.
Finally, additional analytical approaches were used to account for censoring28 and to estimate the category-free NRI10 and NRI25.29 The IDI and relative IDI were also calculated according to the method of Pencina et al.26
Using a Cox proportional hazards model similar to that used to estimate the risk of CVD mortality, we repeated the analyses, using all-cause mortality as the outcome variable. All statistical analyses were performed with the use of SAS for Windows (release 9.2; SAS Institute, Inc., Cary, NC). Two-sided P values <0.05 were considered significant.
Results
During a median follow-up period of 16 years (1 048 344 person-years), there were 4749 all-cause deaths, including 1621 CVD deaths. As expected, the majority of participants had a low Framingham Risk Score, and only 5% had an exercise test that was interpreted as abnormal by the treating physician (Table 1).
Table 1.
Participant Characteristics in the Cooper Center Longitudinal Study
| Characteristic | Men (n=49 307) | Women (n=17 064) |
|---|---|---|
| Age, y | 44 (38, 51) | 44 (37, 52) |
| Systolic BP, mm Hg | 120 (112, 130) | 110 (102, 120) |
| Diastolic BP, mm Hg | 80 (76, 88) | 76 (70, 82) |
| Total cholesterol, mg/dL | 205 (180, 232) | 196 (173, 222) |
| Diabetes mellitus, % | 5 | 2 |
| Smoking, % | 18 | 9 |
| Fitness level in METs | 11.3 (9.9, 13.1) | 9.4 (8.1, 10.8) |
| Framingham risk score | ||
| <5%, % | 59 | 95 |
| <10%, % | 81 | 97 |
| 10–20%, % | 13 | 0 |
| >20% or diabetes mellitus, % | 6 | 2 |
| Abnormal exercise ECG, n (%) | 2686 (5) | 899 (5) |
| CVD deaths, n (%) | 1462 (3) | 159 (1) |
| All-cause deaths, n (%) | 4134 (8) | 615 (4) |
| Follow-up period, y | 17 (7, 25) | 12 (5, 22) |
Data are presented as median (25th, 75th percentile) or percentages. BP indicates blood pressure; METs, metabolic equivalent tasks; and CVD, cardiovascular disease.
Fitness and CVD Mortality
With the use of the Hosmer-Lemeshow test, all models had a P value >0.05, suggesting that the models with and without fitness were well calibrated. All traditional risk factors were significantly associated with an increased risk for CVD mortality with and without fitness. Adjusted for these risk factors, all quintiles of fitness were significantly associated with decreased risk of CVD mortality compared with the lowest quintile, with a stepwise decrease in risk across higher quintiles of fitness (Figure).
Figure.
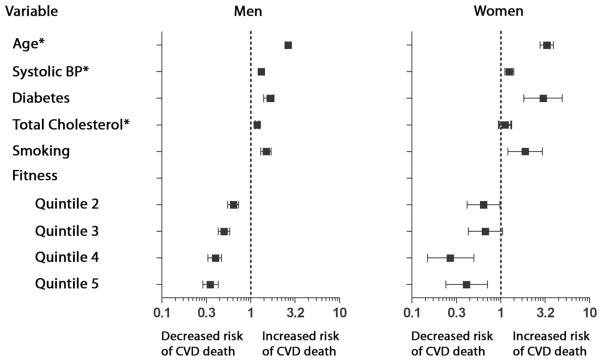
Association of traditional risk factors and fitness with cardiovascular disease (CVD) mortality. The hazard ratios were obtained with Cox proportional hazards models. Participants in the lowest age- and sex-specific fitness quintile were the reference category. *Hazard ratios are per 1 SD (per 10 years for age, per 14 mm Hg for systolic blood pressure [BP], and per 40 mg/dL for total cholesterol).
Among the 43 041 participants enrolled before 1996, there were 286 CVD deaths (265 men and 21 women) during the first 10 years of follow-up. Because there were too few deaths among women, analyses at 10 years were restricted to men. Adding fitness to traditional risk factors significantly improved discrimination, as reflected by an increase in the Harrell’s C statistic from 0.84 (95% confidence interval [CI], 0.82 to 0.86) to 0.86 (95% CI, 0.84 to 0.88) (P<0.001). Cross-tabulations of the 10-year estimated risk of CVD mortality with the use of the models with and without fitness are shown in Table 2. The addition of fitness to traditional risk factors resulted in reclassification of 10.7% of the men. The NRI10 for events was 0.113, and the NRI10 for nonevents was 0.008, achieving an overall NRI10 of 0.121 (P<0.001 for all). The IDI was 0.010 (P<0.001), and the relative IDI showed a 29% improvement in discrimination slope after the addition of fitness to traditional risk factors.
Table 2.
Reclassification of 10-Year Risk of CVD Mortality in Men Using Models With and Without Quintiles of Fitness (n=43 041)
| Model Without Fitness | Model With Fitness
|
||||||
|---|---|---|---|---|---|---|---|
| 0% to <1% | 1% to <5% | ≥5% | Overall | Reclassified as Higher Risk | Reclassified as Lower Risk | Net Correctly Reclassified | |
| Participants with CVD death | 49 | 19 | 0.113* | ||||
| 0% to <1% | 60 | 27 | 0 | 87 | |||
| 1% to <5% | 13 | 81 | 22 | 116 | |||
| ≥5% | 0 | 6 | 56 | 62 | |||
| Overall | 73 | 114 | 78 | 265 | |||
| Participants without CVD death | 1622 | 1882 | 0.008* | ||||
| 0% to <1% | 25 650 | 1298 | 0 | 26 948 | |||
| 1% to <5% | 1636 | 3719 | 324 | 5679 | |||
| ≥5% | 0 | 246 | 428 | 674 | |||
| Overall | 27 286 | 5263 | 752 | 33 301 | |||
| Net reclassification improvement | 0.121* | ||||||
| Net reclassification improvement (1% to <5% risk only) | 0.309* | ||||||
CVD indicates cardiovascular disease.
P<0.001.
In sensitivity analyses, we observed a similar pattern of results after accounting for censoring (NRI10=0.122), adjusting for body mass index (NRI10=0.118), excluding men with diabetes mellitus (NRI10=0.158), including those with prior myocardial infarction (NRI10=0.120), using continuous METs instead of quintiles of fitness (Table I in the online-only Data Supplement; NRI10=0.153), or excluding those with an abnormal exercise study (NRI10=0.102) (P<0.001 for all). The category-free NRI10 was 46% (P<0.001).
Among the 16 533 participants enrolled before 1981, 721 men and 60 women had a CVD death during 25 years of follow-up. Among men, the Harrell’s C statistic increased from 0.81 (95% CI, 0.79 to 0.82) to 0.82 (95% CI, 0.80 to 0.83), NRI25 was 0.041, the IDI was 0.012 (P<0.001 for all), and the relative IDI was 11.1% (Table 3). In women, the Harrell’s C statistic increased from 0.86 (95% CI, 0.81 to 0.91) to 0.88 (95% CI, 0.82 to 0.92) (P=0.21), the NRI25 was 0.131 (P=0.04), the IDI was 0.018 (P=0.07), and the relative IDI was 13.5%. Similar findings were observed with the category-free NRI after we accounted for censoring and at different thresholds for the NRI25 (data not shown).
Table 3.
Reclassification of 25-Year Risk of CVD Mortality Using Models With and Without Quintiles of Fitness Stratified by Sex (n=16 533)
| Model Without Fitness | Model With Fitness
|
||||||
|---|---|---|---|---|---|---|---|
| 0% to <8% | 8% to <30% | ≥30% | Overall | Reclassified as Higher Risk | Reclassified as Lower Risk | Net Correctly Reclassified | |
| Men with CVD death | 77 | 47 | 0.042* | ||||
| 0% to <8% | 227 | 49 | 0 | 276 | |||
| 8% to <30% | 29 | 276 | 28 | 333 | |||
| ≥30% | 0 | 18 | 94 | 112 | |||
| Overall | 256 | 343 | 122 | 721 | |||
| Men without CVD death | 486 | 473 | −0.001 | ||||
| 0% to <8% | 10 199 | 404 | 0 | 10 603 | |||
| 8% to <30% | 412 | 1592 | 82 | 2086 | |||
| ≥30% | 0 | 61 | 156 | 217 | |||
| Overall | 10 611 | 2057 | 238 | 12 906 | |||
| Net reclassification improvement | 0.041* | ||||||
| Net reclassification improvement (8% to <30% risk only) | 0.155‡ | ||||||
| Women with CVD death | 11 | 3 | 0.133† | ||||
| 0% to <8% | 25 | 7 | 0 | 32 | |||
| 8% to <30% | 1 | 13 | 4 | 18 | |||
| ≥30% | 0 | 2 | 8 | 10 | |||
| Overall | 26 | 22 | 12 | 60 | |||
| Women without CVD death | 41 | 33 | −0.003 | ||||
| 0% to <8% | 2676 | 35 | 0 | 2711 | |||
| 8% to <30% | 28 | 89 | 6 | 123 | |||
| ≥30% | 0 | 5 | 7 | 12 | |||
| Overall | 2704 | 129 | 13 | 2846 | |||
| Net reclassification improvement | 0.131† | ||||||
| Net reclassification improvement (8% to <30% risk only) | 0.346‡ | ||||||
CVD indicates cardiovascular disease.
P<0.01.
P<0.05.
P<0.001.
When all-cause death was used as the outcome variable, among men, the Harrell’s C statistic increased from 0.75 (95% CI, 0.74 to 0.76) to 0.76 (95% CI, 0.75 to 0.77), the IDI was 0.02, the relative IDI was 10%, and the category-free NRI was 23% (P<0.001 for all). In women, the Harrell’s C statistic increased from 0.75 (95% CI, 0.73 to 0.77) to 0.76 (95% CI, 0.74 to 0.79), the IDI was 0.02, the relative IDI was 12%, and the category-free NRI was 12% (P<0.001 for all).
Discussion
In >66 000 individuals with >1 million person-years of follow-up, a single measurement of fitness as measured by exercise treadmill time significantly improved measures of discrimination and reclassification of CVD mortality risk when added to traditional risk factors. Even after 25 years of follow-up, baseline fitness provided modest improvement in discrimination and reclassification, particularly in women. These data extend prior observations on the association of fitness with CVD mortality, suggesting the potential clinical utility of incorporating fitness estimates in CVD risk prediction algorithms.
Several prior studies have observed a consistent, inverse association between levels of fitness and the risk for CVD mortality, including the CCLS,8,9,15 the Veterans Affairs study,14 and the Lipid Research Clinics Study.10 In addition, a few recent studies have demonstrated that low fitness is associated with increased CVD and all-cause mortality, even after adjustment for global risk scores.5–7,12,16 In the present study, we assessed the performance of fitness in improving clinically relevant metrics of discrimination, calibration, and reclassification beyond traditional risk factors.
Although traditional risk factors alone provided excellent discrimination of CVD mortality in the CCLS cohort, the addition of fitness improved all measures of discrimination. Given the limitation of using discrimination as the sole risk-prediction performance criterion,17 the ability of a novel marker to reclassify subjects across clinically relevant thresholds of risk represents a new benchmark.21 The NRI represents clinically meaningful improvement in risk classification achieved with a new marker and is calculated by measuring the net change in risk categories among cases and controls after the addition of a new marker to the baseline model.18,26 Unlike the NRI, the IDI is a measure of improvement in discrimination that is independent of risk categories and represents the improvements in true-positive rates minus the worsening in false-positive rates with the new marker.18,26 The relative IDI represents proportional improvement in discrimination by the new model versus the traditional model.18,26
Of note, the addition of fitness to traditional risk factors correctly reclassified 18.5% of cases into a higher-risk category but incorrectly reclassified 7.2% of cases into a lower-risk category, for a net correct classification of 11.3% among men with CVD death. In contrast, the addition of fitness had a negligible effect on the reclassification of noncases, resulting in an overall significant NRI10 of 0.121 (Table 2). These findings suggest that an assessment of fitness may have significant impact on risk prediction algorithms by providing a meaningful improvement in the identification of those individuals at risk for future CVD mortality. In addition, these data might also be useful to practicing clinicians, facilitating more effective risk communication regarding the health benefits of fitness. Not only is fitness a marker for CVD that improves multiple clinically relevant risk-prediction performance metrics, but fitness itself represents an established treatment target with few associated risks.1
Risk prediction in the general population remains a challenge because the majority of the general population is low risk.21,30 –32 Prior efforts have emphasized the potential role of blood-based markers (eg, C-reactive protein and cystatin C) and imaging modalities (eg, brachial flow-mediated dilation and carotid intima-media thickness) in risk prediction algorithms.33–36 With the exception of coronary artery calcium, most of these novel risk markers have limited discrimination and reclassification ability.33–37 Although significant improvements in the NRI are achieved with coronary artery calcium,37 there are particular concerns about radiation exposure and cost with the widespread use of coronary artery calcium scanning.38 In contrast, significant NRI is achieved with the addition of fitness without any associated radiation exposure. At a minimum, the findings from the present study suggest that in the short term, a single measurement of fitness is at least as useful as most biomarkers and imaging modalities in stratifying CVD risk.
Current CVD risk prediction algorithms (Framingham Risk Score, European Systematic Coronary Risk Evaluation [SCORE]) assess only short-term CVD risk and classify >98% of women aged <60 years and 80% of men aged <50 years as low short-term risk for CVD39 despite the fact that >1 in 3 adults will develop CVD in their lifetime.38,40,41 In response, recent guidelines on primary prevention of CVD have emphasized long-term risk assessment in individuals at low short-term risk.32,40 By extending the follow-up time in this study, we were able to accrue enough events to assess the reclassification ability of fitness for long-term risk separately in men and women. We observed that the improvement in risk classification persisted even after 25 years of follow-up, particularly in women (Table 3). The NRI25 in the overall cohort was lower than the NRI10, which may be due to changes in fitness level over time, thus weakening the association of fitness with long-term CVD mortality. Nevertheless, these data suggest the potential role for fitness as a novel approach for long-term risk prediction, particularly in women.
Limitations
Several limitations should be noted. First, the CCLS represents a unique cohort of predominantly white participants with high socioeconomic status and lower risk factor burden compared with the general population.39 Despite these unique characteristics, levels of fitness and the effect of traditional risk factors in the CCLS are overall quite similar to those observed in the general population.41,42 Importantly, we believe that the healthy nature of the CCLS participants actually represents an important strength, providing an estimate of the contribution of fitness to reclassification of risk for CVD mortality among low-risk individuals not referred for exercise testing. Although further analyses regarding cost-effectiveness of exercise testing are needed, simpler methods of fitness assessment, such as the Rockport Walking Test, may make assessing fitness an efficient and effective CVD risk stratification tool.43
Second, the MET levels used in this study are estimated from treadmill testing and not measured by metabolic testing. Although participants were encouraged not to hold onto the railing and were given encouragement to exert maximal effort, the fitness level could be overestimated if subjects used the handrails on the treadmill for support.
Third, we did not have high-density lipoprotein cholesterol in our baseline model, and additional adjustment for high-density lipoprotein cholesterol may have attenuated the effect of fitness on the NRI. However, prior literature suggests a more limited contribution of high-density lipoprotein cholesterol to risk prediction for CVD mortality.44 Similarly, data on other laboratory measurements, such as hemoglobin A1C and high-sensitivity C-reactive protein, were also not available.
Fourth, using CVD death as the outcome variable based on the National Death Index has well-recognized limitations, with potential misclassification of the cause of death at older ages.45 In addition, competing risks increase with advancing age because of the higher levels of non-CVD death at older ages. Nevertheless, in the present study, we also performed sensitivity analyses using all-cause death as the outcome variable and observed a similar pattern of results. Furthermore, we have shown recently that the effect of fitness on long-term risk for CVD mortality remains even after adjustment for competing risks.41
Finally, we acknowledge that fitness levels and risk factor burden may have changed during the follow-up period. We believe that this observation actually represents an important strength of our findings, suggesting that a single, baseline measure of fitness retains its effect on the reclassification of risk for CVD mortality at 25 years of follow-up.
Conclusions
In a predominantly low-risk, asymptomatic cohort of individuals without known CVD, the addition of fitness to traditional risk factors significantly improves reclassification of the risk of CVD mortality across short-term and long-term follow-up.
CLINICAL PERSPECTIVE.
Cardiorespiratory fitness has a well-documented inverse association with cardiovascular disease mortality independent of traditional risk factors. However, the extent to which fitness provides clinically meaningful risk information beyond traditional risk factors is unclear. In the present study, we assessed the contribution of fitness to traditional risk factors in cardiovascular disease risk classification using data from >66 000 asymptomatic individuals enrolled in the Cooper Center Longitudinal Study. During a median follow-up period of 16 years, there were 1621 cardiovascular disease deaths. We found that the addition of fitness to the traditional risk factor model resulted in significant net reclassification improvement at both 10 years and 25 years. These findings suggest that an assessment of fitness may have significant impact on risk prediction algorithms by providing a meaningful improvement in the identification of those individuals at risk for future cardiovascular disease mortality. In addition, these data might also be useful to practicing clinicians, facilitating more effective risk communication regarding the health benefits of fitness.
Acknowledgments
We thank Dr Kenneth H. Cooper for establishing the CCLS, the Cooper Clinic staff for collecting the clinical data, and the Cooper Institute for maintaining the database.
Sources of Funding
Dr Berry receives funding from (1) the Dedman Family Scholar in Clinical Care endowment at University of Texas Southwestern Medical Center, (2) grant K23 HL092229 from the National Heart, Lung, and Blood Institute, and (3) grant 10BG1A4280091 from the American Heart Association. Dr Rohatgi is supported by National Institutes of Health Clinical and Translational Science Awards grant UL1 RR024982. The funding sources had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.110.003236/DC1.
Disclosures
Dr Berry reports receiving speaker’s fees from Merck/Schering-Plough. The other authors have no conflict of interest to disclose.
References
- 1.Physical Activity Guidelines for Americans. U.S. Department of Health and Human Services; 2008. [Accessed January 10, 2011]. http://www.health.gov/paguidelines. Last updated August 21, 2009. [Google Scholar]
- 2.Dorn J, Naughton J, Imamura D, Trevisan M. Results of a multicenter randomized clinical trial of exercise and long-term survival in myocardial infarction patients: the National Exercise and Heart Disease Project (NEHDP) Circulation. 1999;100:1764–1769. doi: 10.1161/01.cir.100.17.1764. [DOI] [PubMed] [Google Scholar]
- 3.Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 4.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 5.Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA. 2004;292:1462–1468. doi: 10.1001/jama.292.12.1462. [DOI] [PubMed] [Google Scholar]
- 6.Erikssen G, Bodegard J, Bjornholt JV, Liestol K, Thelle DS, Erikssen J. Exercise testing of healthy men in a new perspective: from diagnosis to prognosis. Eur Heart J. 2004;25:978–986. doi: 10.1016/j.ehj.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Laukkanen JA, Rauramaa R, Salonen JT, Kurl S. The predictive value of cardiorespiratory fitness combined with coronary risk evaluation and the risk of cardiovascular and all-cause death. J Intern Med. 2007;262:263–272. doi: 10.1111/j.1365-2796.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 8.Blair SN, Kohl HW, III, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 9.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 10.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men: the Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 11.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 13.Mora S, Redberg RF, Sharrett AR, Blumenthal RS. Enhanced risk assessment in asymptomatic individuals with exercise testing and Framingham Risk Scores. Circulation. 2005;112:1566–1572. doi: 10.1161/CIRCULATIONAHA.105.542993. [DOI] [PubMed] [Google Scholar]
- 14.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 15.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balady GJ, Larson MG, Vasan RS, Leip EP, O’Donnell CJ, Levy D. Usefulness of exercise testing in the prediction of coronary disease risk among asymptomatic persons as a function of the Framingham Risk Score. Circulation. 2004;110:1920–1925. doi: 10.1161/01.CIR.0000143226.40607.71. [DOI] [PubMed] [Google Scholar]
- 17.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 18.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 20.Paynter NP, Chasman DI, Buring JE, Shiffman D, Cook NR, Ridker PM. Cardiovascular disease risk prediction with and without knowledge of genetic variation at chromosome 9p21.3. Ann Intern Med. 2009;150:65–72. doi: 10.7326/0003-4819-150-2-200901200-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O’Donnell CJ, Smith SC, Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, Linnerud AC. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39– 46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 23.National Death Index User’s Manual: October, 2000. Hyattsville, MD: Department of Health and Human Services; 2000. [Google Scholar]
- 24.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25:3474–3486. doi: 10.1002/sim.2299. [DOI] [PubMed] [Google Scholar]
- 26.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 27.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Op Reimer WS, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Altiner A, Bonora E, Durrington PN, Fagard R, Giampaoli S, Hemingway H, Hakansson J, Kjeldsen SE, Larsen ML, Mancia G, Manolis AJ, Orth-Gomer K, Pedersen T, Rayner M, Ryden L, Sammut M, Schneiderman N, Stalenhoef AF, Tokgozoglu L, Wiklund O, Zampelas A. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14(suppl 2):E1–E40. doi: 10.1097/01.hjr.0000277984.31558.c4. [DOI] [PubMed] [Google Scholar]
- 28.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med. 2011;30:22–38. doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, Shea S, Sidney S, O’Leary DH, Chan C, Lloyd-Jones DM. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the Coronary Artery Risk Development in Young Adults Study and Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;119:382–389. doi: 10.1161/CIRCULATIONAHA.108.800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Cook N. Should age and time be eliminated from cardiovascular risk prediction models? Rationale for the creation of a new national risk detection program. Circulation. 2005;111:657–658. doi: 10.1161/01.CIR.0000154544.90488.52. [DOI] [PubMed] [Google Scholar]
- 32.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Jr, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 33.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R, Sr, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1:92–97. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten-year results from the Carotid Atherosclerosis Progression Study (CAPS) Eur Heart J. 2010;31:2041–2048. doi: 10.1093/eurheartj/ehq189. [DOI] [PubMed] [Google Scholar]
- 36.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KP, Einstein AJ, Berrington de Gonzalez A. Coronary artery calcification screening: estimated radiation dose and cancer risk. Arch Intern Med. 2009;169:1188–1194. doi: 10.1001/archinternmed.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford ES, Giles WH, Mokdad AH. The distribution of 10-year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43:1791–1796. doi: 10.1016/j.jacc.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 40.McPherson R, Frohlich J, Fodor G, Genest J Canadian Cardiovascular Society. Canadian Cardiovascular Society position statement: recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22:913–927. doi: 10.1016/s0828-282x(06)70310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berry JD, Willis B, Gupta S, Barlow CE, Lakoski SG, Khera A, Rohatgi A, deLemos JA, Haskell W, Lloyd-Jones DM. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at age 45-, 55-, and 65-years in men: the Cooper Center Longitudinal Study. J Am Coll Cardiol. doi: 10.1016/j.jacc.2010.10.056. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CY, Haskell WL, Farrell SW, Lamonte MJ, Blair SN, Curtin LR, Hughes JP, Burt VL. Cardiorespiratory fitness levels among US adults 20 – 49 years of age: findings from the 1999–2004 National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;171:426– 435. doi: 10.1093/aje/kwp412. [DOI] [PubMed] [Google Scholar]
- 43.Kline GM, Porcari JP, Hintermeister R, Freedson PS, Ward A, McCarron RF, Ross J, Rippe JM. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med Sci Sports Exerc. 1987;19:253–259. [PubMed] [Google Scholar]
- 44.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njolstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 45.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]


