Abstract
HIV infection is frequently comorbid with methamphetamine (METH) dependence. Both factors are associated with impairment in inhibitory function that continues even after abstinence from the drug. Deficits in prepulse inhibition (PPI), a measure of sensorimotor gating, are induced by acute stimulant administration, but the combined effect of HIV and chronic METH exposure on PPI is not well characterized. We quantified baseline acoustic startle and PPI in mice expressing the HIV-1 gp120 envelope protein (gp120tg) and in wild-type (WT) littermates; thereafter, we administered a chronic regimen of METH or vehicle and tested startle and PPI after 7 days of drug withdrawal. We hypothesized that METH-treated gp120tg mice would exhibit PPI deficits compared with vehicle-treated WT or gp120tg animals. Before METH administration, drug-naive female gp120tg mice exhibited decreased PPI compared with female WT mice, whereas male gp120tg mice exhibited increased startle compared with other groups. After drug withdrawal, no consistent genotype effect was observed, but METH-treated mice exhibited increased PPI compared with vehicle, in contrast to previous reports of acute METH-induced PPI deficits. In summary, PPI impairment in HIV could depend on factors such as sex, whereas changes in PPI following METH withdrawal may depend on the quantity and duration of drug exposure.
Keywords: gp120, HIV, methamphetamine, mouse, prepulse inhibition, sensorimotor gating
Introduction
HIV-1 virus infection is frequently characterized by impairment in executive function, memory and inhibition linked to abnormalities in the basal ganglia and frontal cortex, collectively described as HIV-associated neurocognitive disorders (HAND; Antinori et al., 2007). Inhibitory deficits, defined as the inability to attenuate an action or thought (Goodwin and Jamison, 1990), remain common in the era of combined antiretroviral therapy and are associated with poor everyday functioning and high-risk behaviors, including the use of drugs such as methamphetamine (METH; Semple et al., 2006). Similar to HIV-infected individuals, METH-dependent individuals exhibit impaired inhibition, as demonstrated by poor performance on neurocognitive tests such as the Stroop and Wisconsin Card Sorting Task, elevated rates of self-reported disinhibition on the Frontal Systems Behavioral Scale, and the propensity to engage in risky sexual activities (Salo et al., 2002; Monterosso et al., 2005; Woods et al., 2005; Cattie et al., 2012; Meade et al., 2012). Comorbid METH dependence also increases neurocognitive deficits observed in HIV infection and augments HIV-associated neuropathology linked to poor inhibitory function, including neuronal loss and glial activation in the frontal cortex (Chang et al., 2002, 2005; Rippeth et al., 2004; Carey et al., 2006; Chana et al., 2006). Given the failure of combined antiretroviral therapy to effectively address inhibitory deficits associated with HIV and concurrent drug use, more investigation is required to elucidate the biological mechanisms and factors that underlie this phenomenon.
Although the manifestation of neurocognitive impairment in HIV-infected individuals is typically assessed by traditional neuropsychological assessment and/or self-report, some of the earliest neurological abnormalities in this disorder may be distinguished by more subtle disruption of inhibition related to sensorimotor gating (Polich et al., 2000). Gating represents a process by which excess or trivial sensory information is filtered out of awareness, enabling an individual to focus attention on the most salient or relevant stimuli (Braff and Geyer, 1990). One of the most common measures of sensorimotor gating is prepulse inhibition (PPI), in which the magnitude of a startle response to tactile or acoustic stimuli is reduced by the prior presentation of a nonstartling prepulse (Geyer and Swerdlow, 2001). PPI is regulated by a network of neural structures, including a cortico-striato-pallido-thalamic loop that involves regions implicated in inhibitory function in both HIV and METH (Chang et al., 2002; Chung et al., 2007). PPI deficits are observed across a wide variety of neuro-psychiatric populations (Braff et al., 2001; Perry et al. 2001) and have been induced by cross-species pharmacological manipulations in rodents, primates, and humans (Geyer et al., 2001). Acute administration of both direct and indirect dopamine (DA) agonists, including amphetamine and METH, disrupts PPI and has been used to model dopaminergic abnormalities and preattentive deficits associated with disorders such as schizophrenia. Although HIV infection is characterized by DA abnormalities and pathology in the neurocircuitry that regulates PPI, effects that may be exacerbated by comorbid METH use (Cadet and Krasnova, 2007), few studies have examined sensorimotor gating in this disorder (Minassian et al., 2013). In contrast to self-report and neuropsychological tasks that are impacted by factors such as motivation and fatigue, PPI quantifies an involuntary response that may also serve as a more specific indicator of altered inhibitory function (Feifel et al., 2009). In addition, PPI can be tested in both rodents and humans, enabling cross-species comparisons that explicate HIV-related neuropathology (Fitting et al., 2006c, 2007). Sensorimotor gating has not been assessed extensively in models of HIV, but one recent study observed that acute METH administration induced greater PPI deficits in HIV-1 transgenic (tg) rats compared with wild-type (WT) animals (Moran et al., 2012). This experimental design, however, is incongruent with the typical course of METH use in humans, in which the negative effects of the drug are associated with chronic exposure. In summary, the consequence of extended METH administration on PPI in an animal model of HIV has not been previously examined.
The objective of this study was to examine the effect of a chronic METH regimen on inhibitory deficits quantified by PPI in transgenic mice that constitutively express the gp120 protein (Toggas et al., 1994). Prior studies indicate that the combination of HIV infection and METH dependence is associated with impairment in inhibitory performance even after several months of abstinence from the drug (Rippeth et al., 2004). To parallel the human data, we chose to assess sensorimotor gating in mice after 1 week of METH withdrawal, similar to previous work (Henry et al., 2013). We initially hypothesized that drug-naive gp120 transgenic mice (gp120tg) would exhibit impaired PPI relative to WT animals during baseline testing before METH administration. Second, we proposed that METH-treated gp120tg mice would exhibit lower PPI after METH withdrawal compared with vehicle-treated gp120tg mice and WT animals given either METH or vehicle treatment.
Methods
Subjects
This study was part of a larger examination of the individual and combined effects of HIV and METH conducted by the Translational Methamphetamine AIDS Research Center (TMARC). Male and female transgenic mice expressing the HIV-1 envelope glycoprotein gp120 were obtained from the lab of Dr Eliezer Masliah at the University of California, San Diego. Gp120 is expressed in astrocytes under the control of a modified murine glial fibrillary acidic protein (GFAP) in animals generated from a mixed C57BL/6 × Sv129 (SJL/BL6/129) background (Toggas et al., 1994) and previously crossed with WT BDF1 mice from Charles River. Mice from the F6 BL6/129 × BDF1 generation (8–9 months old, n = 12–13/group) were tested in the current study and their nontransgenic littermates were used as controls. The genotype was confirmed by PCR analysis of tail DNA.
Mice were separated by sex and group and were housed in a climate-controlled environment with a reversed day/night cycle (lights on at 20:00 h, off at 08:00 h). Behavioral testing was conducted between 09:00 and 18:00 h. The animals were given free access to food (Haran Teklad, Madison, Wisconsin, USA) and water for the duration of the testing. All procedures were approved by the UCSD Institutional Animal Care and Use Committee and conformed to NIH guidelines.
Drug regimen
METH (Sigma, St. Louis, Missouri, USA) was dissolved in saline and administered subcutaneously at a 5 ml/kg injection volume (freebase weight). Stock solutions of the drug were prepared every 3–4 days and diluted as needed during the drug regimen. We administered an escalating dose-multiple binge METH regimen that was first tested in rats (Kuczenski et al., 2007) and was subsequently demonstrated to increase exploratory behavior in mice (Henry et al., 2013). This treatment schedule was originally developed to mimic the gradual dose progression in human METH addicts (Segal and Kuczenski, 1997). Most previous studies used subchronic regimens (5–10 drug injections) and/or relatively short periods of exposure (1 week) to examine the effect of repeated METH or amphetamine on PPI (Druhan et al., 1998; Russig et al., 2003; Arai et al., 2008; Nakato et al., 2010). In the present study, we utilized this drug schedule to represent the escalation/binge behaviors that typically characterize METH dependence and produce neurodegenerative effects associated with METH use (Kuczenski et al., 2007).
In this study, gp120tg and WT mice were treated three times per day (10:00; 13:15; 17:30 h) for 14 days with vehicle (saline) or escalating doses of METH, starting with 0.1 mg/kg and increasing to 4.0 mg/kg, with a stepwise increase of 0.1 mg/kg per injection. After this 14-day period, animals received four daily injections of 6.0 mg/kg METH or vehicle at 2-h intervals (10:00, 12:00, 14:00, and 16:00 h) during an 11-day ‘binge’ period (Fig. 1).
Fig. 1.
Schematic representation of the experimental timeline. Mice were tested for baseline prepulse inhibition (PPI) before the commencement of a chronic 25-day methamphetamine (METH) regimen, including an escalation period of 14 days, during which the dose was increased from 0.1 to 4.0 mg/kg (freebase), and an 11-day ‘binge’ interval of 6 mg/kg injections. PPI was measured again after 7 days of withdrawal from METH.
Fresh syringes were used for every injection given to each mouse.
Apparatus
Behavioral testing was performed as described previously (Geyer and Dulawa, 2003; Powell et al., 2008). The startle response was assessed in eight startle chambers (SR-LAB; San Diego Instruments, San Diego, California, USA). Each chamber contained a clear nonrestrictive Plexiglas cylinder resting on a platform below high-frequency speakers that produced a constant background noise of 65 dB(A) and emitted the acoustic stimuli during the test. Mouse startle responses produce cylinder vibrations, which were converted to analog signals by an attached piezoelectric unit and stored as digitized data on a computer. At each stimulus onset, 65 consecutive 1 ms readings were obtained to determine the average amplitude of the acoustic startle response. SR-LAB equipment was calibrated regularly to ensure consistently accurate measurement.
Prepulse inhibition session
The test session was designed to assess variations in both the prepulse intensity and the interstimulus interval (ISI) on the basis of previously published protocols (Varty et al., 2006; Young et al., 2010b, 2011). Each session was initiated with a 5-min acclimation period during which the animals were habituated to the 65 dB(A) background noise. Startle pulses were presented for 40 ms, prepulse stimuli were presented for 20 ms, and the average intertrial interval between stimulus presentations was 15 s (range 7–23 s). Every other trial was a no stimulus (NOSTIM) trial, in which no acoustic stimulus was presented. The startle session was divided into five blocks. Blocks 1 and 5 each included five pulse-only trials, in which a 120 dB(A) pulse was presented alone. Block 2 assessed PPI and included four trial types (10 of each), including 120 dB(A) startle pulse intensities presented alone or preceded by 69, 73, or 81 dB(A) prepulse stimuli. Prepulses were administered 100 ms before the pulse stimulus. Block 3 assessed the startle response to different pulse intensities [80, 90, 100, 110, 120 dB(A)], but did not include any prepulse trials. In block 4, the ISI between prepulse and pulse was varied; mice were presented with 120 dB(A) pulses alone or preceded by a 73 dB(A) prepulse separated by a 25, 50, 100, 200, or 500-ms interval (four trials for each interval).
Experimental design
Baseline startle response and PPI were assessed in drug-naive 8–9-month-old male and female gp120tg and WT mice (n = 25–26/group). This age group was initially selected on the basis of prior work indicating the presence of behavioral deficits in 9–12-month-old gp120tg mice (D’Hooge et al., 1999; Maung et al., 2012). After the first test, animals were baseline-matched to receive either the chronic METH regimen or saline treatment, on the basis of PPI response during the 81 dB(A) trials. Five days after baseline testing, METH or vehicle administration was initiated in the four groups (male WT, male gp120tg, female WT, female gp120tg) with 12–13 mice under each treatment condition. Mice were weighed every 3–4 days during METH treatment to assess the effect of the chronic drug exposure on body weight. After 7 days of withdrawal from the chronic METH schedule, acoustic startle and PPI were quantified. This 7-day withdrawal period was selected on the basis of evidence of impaired cognitive performance in the Morris water maze at this time point in METH-treated gp120tg mice (Dr Eliezer Masliah, personal communication).
Dependent measures and statistical analyses
The amplitude of the startle response was quantified as the average startle magnitude during the 65-ms recording window. Habituation to the startle response was assessed as the percentage decrease in startle amplitude in pulse-alone 120-dB(A) trials from block 1 to blocks 2, 3, 4, and 5. The percentage of PPI for each type of prepulse intensity was calculated as [100 − (prepulse amplitude/pulse amplitude) × 100].
Statistical analyses were carried out using SPSS. Startle responding and PPI were assessed separately for blocks 2, 3, and 4 using mixed analysis of variance (ANOVA; drug × genotype × sex) with prepulse intensity (block 2), pulse intensity (block 3), and ISI (block 4) as within-subjects factors. Further assessments used analysis of covariance (ANCOVA) with startle reactivity as a covariate to determine whether group differences in PPI may have been impacted by alterations in startle responding. Post-hoc differences were assessed using Tukey’s honestly significant difference with an α-level of 0.05.
Results
Baseline session
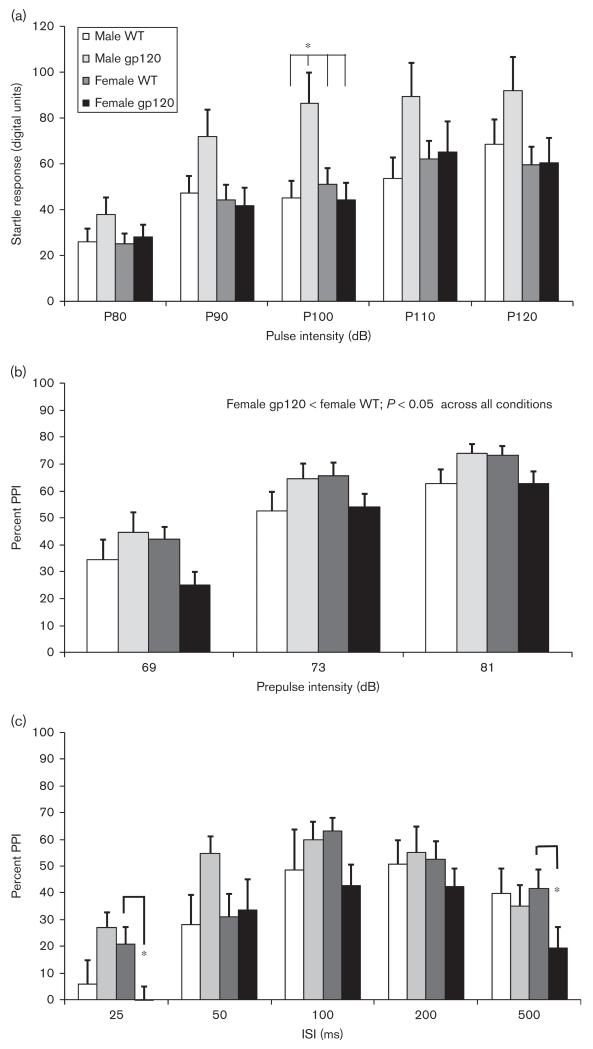
We observed a significant interaction between pulse intensity, sex, and genotype for acoustic startle responding [F(4,96) = 2.6, P < 0.05; Fig. 2a]. Male gp120tg mice tended to show increased startle relative to the other groups across all pulse intensities, with a significant sex by genotype interaction for the 100-dB pulse [F(1,99) = 6.6, P < 0.05]. Post-hoc tests indicated that male gp120tg animals exhibited increased startle relative to the other three conditions (P < 0.05) for the 100-dB stimulus. Male mice demonstrated a slight but significant increase in movement compared with female mice during the NOSTIM trials [F(1,99) = 6.7, P < 0.05], but there was no effect of genotype.
Fig. 2.
Baseline acoustic startle and prepulse inhibition (PPI) were assessed in 9-month-old wild-type (WT) and gp120 transgenic mice (gp120) before administration of the drug regimen (n = 25–26 per group). (a) Male gp120 transgenic mice exhibited increased startle response relative to other groups during P100 dB pulse-only trials. Genotype did not significantly affect PPI in male mice, but female gp120 transgenic mice showed a significant main effect of reduced sensorimotor gating compared with WT mice across (b) prepulse intensities in block 2 and (c) interstimulus intervals (ISI) in block 4, including 25 and 500 ms. Data are shown as means ± SEM. *P < 0.05.
In block 2, PPI was significantly increased at higher prepulse intensities [F(1,99) = 89.6, P < 0.001] and there was a significant interaction between genotype and sex [F(1,99) = 6.4, P < 0.05]; however, there was no main effect of either factor or interaction with PPI intensity. Subsequent analyses carried out separately for each sex revealed that female gp120tg mice exhibited significantly reduced PPI compared with female WT mice [F(1,50) = 5.4, P < 0.05], but genotype differences in male mice did not reach significance [F(1,49) = 2.1, NS; Fig. 2b]. To determine whether PPI differences were affected by changes in startle reactivity, these ANOVAs were repeated with block 2 pulse-alone startle responding included as a covariate. PPI in female gp120tg mice remained significantly lower than that in female WT mice [F(1,49) = 4.6, P < 0.05], whereas genotype differences in male mice were not observed [F(1,48) = 0.6, NS].
In block 4, when PPI was assessed across varying ISIs, we also observed a significant sex by genotype interaction [F(1,99) = 5.4, P < 0.05], as well as an interaction between genotype and ISI level [F(4,396) = 2.6, P < 0.05; Fig. 2c]. In female mice, there was a trend toward reduced PPI in gp120tg mice relative to WT mice across all ISI levels [F(1,50) = 3.0, P = 0.09], in addition to a significant interaction between ISI and genotype [F(1,47) = 3.0, P < 0.05]; no significant differences were observed in male mice. When startle reactivity was included as a covariate, female gp120tg mice again exhibited a trend toward reduced PPI compared with female WT mice [F(1,49) = 3.5, P = 0.07], but the male groups still did not differ [F(1,49) = 1.2, NS]. Post-hoc tests indicated that female gp120tg mice exhibited lower PPI relative to female WT mice with an ISI of 500 ms (P < 0.05) and 25 ms (P < 0.05; Fig. 2c). We did observe a trend toward increased PPI in gp120tg mice compared with WT mice at the 50-ms ISI (P = 0.08), a result driven primarily by higher PPI in the male gp120tg animals; however, this was attenuated when startle reactivity was included as a covariate (P = 0.15).
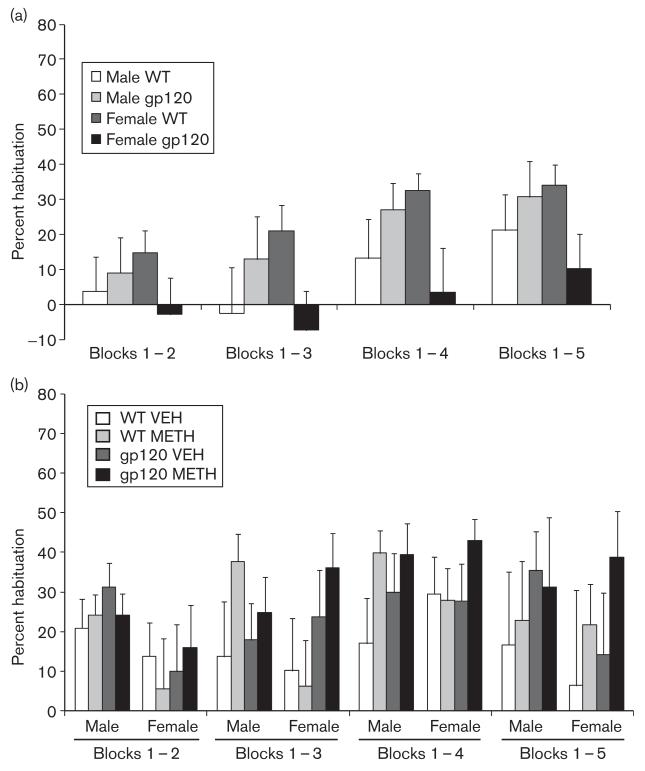
We detected either a trend or a significant interaction between genotype and sex for the percent habituation to startle between the first block and blocks 3 [F(3,99) = 3.9, P = 0.05], 4 [F(3,99) = 5.0, P < 0.05], and 5 [F(3,99) = 3.3, P = 0.07]. This pattern was characterized by a reduction in habituation in female gp120tg mice compared with female WT mice (Fig. 4a), although group differences did not reach significance with the Tukey post-hoc test.
Fig. 4.
Percent habituation to the pulse-only startle response, from block 1 to blocks 2 through 5, (a) during baseline testing (n = 25–26/group) and (b) after 7 days of METH withdrawal (n = 10–13 per group). A significant genotype by sex interaction was noted for block 1–4 habituation at (a) baseline (P < 0.05), although individual group differences did not reach significance with the Tukey post-hoc test. gp120, gp120 transgenic mice; METH, methamphetamine; VEH, vehicle; WT, wild type.
Seven-day methamphetamine withdrawal
The METH regimen was tolerated well by the animals, and all mice completed the drug treatment. We did not observe any significant main effect of drug or drug by genotype interaction on mouse body weight during METH treatment; however, gp120tg mice did exhibit lower weight overall compared with WT mice [F(1,47) = 4.4, P < 0.05].
No significant group or interaction effects were observed for the startle response after drug withdrawal (Fig. 3a). In the NOSTIM trials, there were trends toward decreased movement in METH-treated mice compared with vehicle-treated animals [F(1,88) = 3.9, P = 0.052], as well as greater movement in male mice relative to female mice [F(1,88) = 3.9, P = 0.052].
Fig. 3.
Acoustic startle and prepulse inhibition (PPI) in the eight treatment groups after 7 days of methamphetamine (METH) withdrawal (n = 10–13/group). There were no significant group effects on (a) startle response or any effect of genotype on PPI in block 2; however, METH treatment increased overall PPI relative to vehicle (VEH). (b) Male gp120 transgenic mice exhibited greater PPI compared with male wild-type (WT) mice only under the 50-ms interstimulus interval (ISI) condition in block 4, whereas female METH-treated mice exhibited higher PPI relative to vehicle-treated female mice at (c) 100-ms ISI; no genotype differences were observed in female mice. Overall, METH-treated mice exhibited greater PPI compared with vehicle-treated mice in (b) block 2 and under the (c) 100-ms ISI condition in block 4. *P < 0.05.
We observed no significant effect or interaction for sex or genotype with a repeated-measures ANOVA for PPI in block 2, but METH-treated mice exhibited significantly higher PPI relative to vehicle-treated mice [F(1,88) = 6.2, P < 0.05; Fig. 3b]. This effect remained significant even when block 2 responding to pulse-alone trials was included as a covariate [F(1,88) = 6.5, P < 0.05]. To further examine these findings, we assessed PPI separately for male and female mice at each prepulse intensity level. There were no significant main effects of genotype or genotype-drug interactions for either sex. METH treatment significantly increased PPI relative to vehicle treatment in male mice at the 69 dB [F(1,43) = 5.6, P < 0.05] and 81 dB [F(1,43) = 4.2, P < 0.05] prepulse levels, with a trend toward higher PPI at the 73 dB prepulse [F(1,43) = 4.0, P = 0.05]. METH-treated female mice also exhibited a trend toward higher PPI compared with vehicle-treated female mice, but drug treatment effects did not reach significance [69 dB: F(1,43) = 2.7, P = 0.11; 73 dB: F(1,43) = 0.78, NS; 81 dB: F(1,43) = 1.25, NS].
When the ISI was varied for PPI trials in block 4, we observed significant interactions between genotype and ISI level [F(4,85) = 6.2, P < 0.001] and between sex and ISI level [F(4,85) = 3.4, P < 0.05; Fig. 3c]. Similar to the findings in block 2, METH-treated mice exhibited a trend toward higher PPI compared with vehicle-treated mice with a 100-ms ISI [F(1,88) = 3.1, P = 0.08]; this effect reached significance when startle reactivity was included as a covariate [F(1,87) = 6.1, P < 0.05]. When the data were analyzed separately by sex, we observed that female METH-treated mice exhibited significantly higher PPI compared with female vehicle-treated mice with a 100-ms ISI [F(1,44) = 4.5, P < 0.05]. We also observed trends toward a genotype by drug interaction for male mice in the 50-ms ISI [F(1,44) = 4.5, P < 0.05] and 100-ms ISI [F(1,44) = 4.5, P < 0.05] trials, with a significant interaction for the 200-ms ISI [F(1,44) = 4.5, P < 0.05]. However, the genotype by drug trends and interaction in male mice were not maintained when startle reactivity was included as a covariate. Male mice tended to show higher PPI compared with female mice with a 500-ms ISI [F(1,88) = 3.9, P = 0.05], whereas male gp120tg mice showed greater PPI relative to male WT mice with a 50-ms ISI [F(1,44) = 8.0, P < 0.01], a result driven by higher PPI in the METH-treated animals.
Female mice showed reduced percent habituation to startle between block 1 and block 2 compared with male mice [F(1,88) = 4.7, P = 0.05] (Fig. 4b); no other effects or interactions as regards genotype, drug treatment, or sex were observed for habituation across the session.
Discussion
Neuropsychiatric disorders are frequently characterized by deficits in PPI, but relatively little is known about gating impairment in neuroviral disease, especially in the context of concurrent substance use. Our results indicated that female but not male gp120tg mice exhibited PPI deficits compared with WT mice before drug administration. This effect was not augmented in METH-treated mice after withdrawal, contrary to our hypothesis; in contrast, after 7 days of drug withdrawal, PPI was higher in male and female METH-treated animals relative to vehicle-treated animals. When startle reactivity was included as a covariate, the effect of genotype on baseline PPI was maintained in female mice and the effect of METH treatment remained significant after withdrawal; however, there were no significant genotype by drug interactions for either sex. Interestingly, recent work shows that administration of this METH regimen induced greater exploratory behavior (hole investigations) in female mice after 1 week of withdrawal compared with vehicle-treated female mice, but no differences were observed in male animals (Henry et al., 2013). In that study, METH-treated female gp120tg mice also showed the highest level of exploration relative to the other female groups, indicating a combined effect of drug treatment and HIV protein expression (Henry et al., 2013). It is not clear why METH withdrawal appears to increase exploration but reverse PPI deficits in female gp120tg mice, but these phenomena may be mediated by altered dopaminergic and noradrenergic function (Yamashita et al., 2006; Young et al., 2010a). Although PPI has not been examined extensively in individuals with HIV, one recent study has reported that HIV+ participants as a group did not demonstrate PPI deficits compared with healthy individuals; however, HIV+ individuals with HAND exhibited impaired PPI relative to cognitively intact HIV+ individuals (Minassian et al., 2013). This report, along with our current data, suggests that abnormalities in inhibitory function assessed by sensorimotor gating do not occur as a global phenomenon in HIV, but may emerge in association with higher-order cognitive deficits or biological variations affected by sex. It is worth noting that the human study included primarily male HIV+ participants (86%); hence, it is not yet clear whether the sex differences observed in gp120tg mice will translate to the human population. Greater PPI deficits in HIV+ individuals were also associated with worse performance in working memory, but were unrelated to other cognitive domains (Minassian et al., 2013). Whereas the relationship between PPI and cognitive function is inconsistent (Young et al., 2009), PPI is reported to be correlated with working memory in C57BL/6 mice (Singer et al., 2013). Future studies could examine the relationship between PPI and other cognitive domains in gp120tg animals to determine whether there is an association similar to the link between PPI impairment and HAND in HIV+ individuals (Minassian et al., 2013).
Our findings contribute to the limited literature describing PPI and acoustic startle in rodent models of HIV. Previous reports show that hippocampal injection of gp120 reduced PPI and increased startle responding in adult but not neonatal Sprague–Dawley rats (Fitting et al., 2007); in addition, hippocampal injection of the HIV transactivator of transcription protein decreased percent PPI in 1–3-month-old male but not female Sprague–Dawley rats (Fitting et al., 2006a, 2006b). Moran et al. (2012, 2013) reported sensorimotor gating alterations in HIV-1 transgenic female rats (maximal PPI at a shorter ISI interval) and HIV-1 transgenic male rats (lower PPI with acute METH exposure) compared with WTrats, but neither study tested both male and female rats; hence, potential sex differences in this model are not clear. Female subjects across several species (human, rat, and mouse) tend to exhibit lower PPI, especially during a certain period of the estrus cycle (Koch, 1998; Ison and Allen, 2007), although some reports indicate that sex differences in mice are rare (Willott et al., 2003). Elevated levels of estrogen may impact mesoaccumbal DA, consequently affecting sensorimotor gating. METH treatment may also differentially affect rodents on the basis of sex, as female rodents are reported to show greater DA transporter density and metabolize the drug more slowly than male rodents (Bhatt and Dluzen, 2005; Milesi-Halle et al., 2005). The mechanisms that account for the sex differences in our data are unclear, but might be elucidated by determining whether male and female gp120tg mice show marked differences in DA function.
The present study, to our knowledge, is the first report indicating that withdrawal from chronic METH treatment leads to an improvement in PPI. This finding differs from those of numerous papers indicating that both acute and chronic amphetamine and METH treatment impair sensorimotor gating during and after drug exposure (Ralph et al., 1999; Peleg-Raibstein et al., 2006; Chao et al., 2012). One key feature of the current method is the amount and intensity of METH administration. Previous papers reporting stimulant-induced impairment in PPI during drug withdrawal typically use subchronic protocols that involve 5–10 drug injections and/or drug exposure for 1 week (Murphy et al., 2001; Tenn et al., 2003; Arai et al., 2008; Nakato et al., 2010). In the current report, we utilized a procedure explicitly designed to mimic escalation/binge behaviors associated with METH dependence, administering a total of 86 injections of drug or vehicle over a 25-day regimen before assessing sensorimotor gating (Kuczenski et al., 2007). Although this protocol differs from prior work, several reasons justify its use, including: (i) an improved representation of human METH exposure; (ii) inducing tolerance to the drug that reportedly minimizes the hyperthermic effect of higher METH doses and; (iii) evidence of neuropathology in the neocortex and limbic system, regions that modulate PPI (Segal and Kuczenski, 1997; Segal et al., 2003; Kuczenski et al., 2007). Preliminary replication studies with our current regimen indicate that METH exposure decreases PPI near the end of the chronic treatment (unpublished observations); pretreatment versus post-treatment alterations in PPI will be described in a subsequent report. A systematic comparison of effects of chronic METH administration on sensorimotor gating over various doses and time points will help elucidate the extent to which drug exposure could have differential effects on PPI function. The neurobiological mechanisms underlying the current findings with METH remain highly speculative, but could potentially involve alterations in neurotransmitter systems that modulate PPI, including DA, norepinephrine, and γ-aminobutyric acid (Segal et al., 2005; Groman et al., 2012).
There are several limitations to consider in this study. Expression of the gp120 protein, although inducing neurotoxic effects to the rodent brain that closely mirror the neuropathology of HIV, is not a perfect representation of the disease. One difference is that the animals express the HIV-1 envelope protein in astrocytes, which are not a site of productive viral infection in the human brain. These transgenic mice also express the protein constitutively since birth, which may induce neurotransmitter alterations that do not accurately reflect biological processes that occur with adult HIV infection. The chronic METH regimen was selected to mimic a gradual dose of METH progression and is reported to minimize hyperthermic responses to the higher METH doses (Segal et al., 2003; Kuczenski et al., 2007); however, we did not quantify temperature changes in this mouse cohort and cannot exclude hyperthermia as a contributing factor. We assessed PPI after a week of withdrawal on the basis of earlier reports of METH effects on inhibitory functioning (Dalley et al., 2007) and pilot data indicating gp120tg cognitive impairment at this interval (Dr Eliezer Masliah, personal communication). However, the behavioral effects of the drug may vary over different time points, as suggested by both rodent and human studies on chronic METH use (Cattie et al., 2012). Subsequent studies will quantify PPI both during and after this METH regimen and assess sensorimotor gating in other HIV models, such as expression of the transactivator of transcription protein.
Conclusion
Sensorimotor gating was impaired in female mice expressing the HIV gp120 protein, whereas acoustic startle was increased in male gp120tg mice. Contrary to expectations, chronic METH administration did not reduce PPI after a week of drug withdrawal, but instead increased gating. These findings highlight clinical work indicating that the cognitive effects of METH in HIV+ individuals may largely depend upon variable factors such as the length of abstinence, total quantity of use, age, and sex (Iudicello et al., 2010). Future cross-species assessment of sensorimotor gating in HIV+ patients and preclinical disease models will clarify relevant HIV-related neuropathology and interactions with comorbid substance use.
Acknowledgements
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI). The TMARC is comprised of: Director – Igor Grant, MD; Co-Directors – Ronald J. Ellis, MD, PhD, Scott L. Letendre, MD, and Cristian L. Achim, MD, PhD; Center Manager – Steven Paul Woods, Psy.D; Assistant Center Manager – Aaron M. Carr, BA; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, MD (Core Director), Ronald J. Ellis, MD, PhD, Rachel Schrier, PhD; Neuropsychiatric (NP) Core: Robert K. Heaton, PhD (Core Director), J. Hampton Atkinson, MD, Mariana Cherner, PhD, Thomas D. Marcotte, PhD, Erin E. Morgan, PhD; Neuroimaging (NI) Core: Gregory Brown, PhD (Core Director), Terry Jernigan, PhD, Anders Dale, PhD, Thomas Liu, PhD, Miriam Scadeng, PhD, Christine Fennema-Notestine, PhD, Sarah L. Archibald, MA; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, MD, PhD (Core Director), Eliezer Masliah, MD, Stuart Lipton, MD, PhD, Virawudh Soontornniyomkij, MD; Administrative Coordinating Core (ACC) – Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, PhD (Unit Chief), Clint Cushman, BA (Unit Manager); ACC – Statistics Unit: Ian Abramson, PhD (Unit Chief), Florin Vaida, PhD, Reena Deutsch, PhD, Anya Umlauf, MS; ACC – Participant Unit: J. Hampton Atkinson, MD (Unit Chief), Jennifer Marquie-Beck, MPH (Unit Manager); Project 1: Arpi Minassian, PhD (Project Director), William Perry, PhD, Mark Geyer, PhD, Brook Henry, PhD; Project 2: Amanda B. Grethe, PhD (Project Director), Martin Paulus, MD, Ronald J. Ellis, MD, PhD; Project 3: Sheldon Morris, MD, MPH (Project Director), David M. Smith, MD, MAS, Igor Grant, MD; Project 4: Svetlana Semenova, PhD (Project Director), Athina Markou, PhD, James Kesby, PhD; Project 5: Marcus Kaul, PhD (Project Director).
The manuscript was supported by the Translational Methamphetamine AIDS Research Center (TMARC; P50DA026306) funded by the National Institute On Drug Abuse (NIDA), a grant from the National Institute of Mental Health (NIMH; (R01-MH071916), and support from the US Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Conflicts of interest
M.A. Geyer holds an equity interest in San Diego Instruments. For the remaining authors, there are no conflicts of interest.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Takahashi K, et al. Involvement of pallidotegmental neurons in methamphetamine- and MK-801-induced impairment of prepulse inhibition of the acoustic startle reflex in mice: reversal by GABAB receptor agonist baclofen. Neuropsychopharmacol. 2008;33:3164–3175. doi: 10.1038/npp.2008.41. [DOI] [PubMed] [Google Scholar]
- Bhatt SD, Dluzen DE. Dopamine transporter function differences between male and female CD-1 mice. Brain Res. 2005;1035:188–195. doi: 10.1016/j.brainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiat. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotox Res. 2007;12:181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immuno-suppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10:185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Woods SP, Iudicello JE, Posada C, Grant I. Elevated neurobehavioral symptoms are associated with everyday functioning problems in chronic methamphetamine users. J Neuropsych Clin N. 2012;24:331–339. doi: 10.1176/appi.neuropsych.11080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, et al. Cognitive deficits and degeneration of interneurons in HIV + methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiat. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao YL, Chen HH, Chen CH. Effects of repeated electroconvulsive shock on methamphetamine-induced behavioral abnormalities in mice. Brain Stimul. 2012;5:393–401. doi: 10.1016/j.brs.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, Franck F, Mucke L, De Deyn PP. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–4402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, et al. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacol. 2007;32:1195–1206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- Druhan JP, Geyer MA, Valentino RJ. Lack of sensitization to the effects of d-amphetamine and apomorphine on sensorimotor gating in rats. Psychopharmacology (Berl) 1998;135:296–304. doi: 10.1007/s002130050513. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minassian A, Perry W. Prepulse inhibition of startle in adults with ADHD. J Psychiatr Res. 2009;43:484–489. doi: 10.1016/j.jpsychires.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Intrahippocampal injections of Tat: effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol Biochem Behav. 2006a;84:189–196. doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006b;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006c;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal gp120 injection: an examination early in development. Neurotoxicology. 2007;28:101–107. doi: 10.1016/j.neuro.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. In: Crawley JN, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley & Sons; New York: 2001. pp. 8.17.1–8.17.15. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. In: Crawley JN, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley & Sons; New York: 2003. pp. 8.17.1–8.17.15. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness. Oxford UP; New York: 1990. [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, et al. Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32:5843–5852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell M, Perry W, Young JW, Minassian A. Behavioral effects of chronic methamphetamine treatment in HIV-1 gp120 transgenic mice. Behav Brain Res. 2013;236:210–220. doi: 10.1016/j.bbr.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD. Pre- but not post-menopausal female CBA/CaJ mice show less prepulse inhibition than male mice of the same age. Behav Brain Res. 2007;185:76–81. doi: 10.1016/j.bbr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Vigil O, Scott JC, Cherner M, Heaton RK, et al. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol. 2010;32:704–718. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiol Behav. 1998;64:625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maung R, Medders KE, Sejbuk NE, Desai MK, Russo R, Kaul M. Genetic knockouts suggest a critical role for HIV co-receptors in models of HIV gp120-induced brain injury. J Neuroimmune Pharmacol. 2012;7:306–318. doi: 10.1007/s11481-011-9328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Watt MH, Sikkema KJ, Deng LX, Ranby KW, Skinner D, et al. Methamphetamine use is associated with childhood sexual abuse and HIV sexual risk behaviors among patrons of alcohol-serving venues in Cape Town, South Africa. Drug Alcohol Depend. 2012;126:232–239. doi: 10.1016/j.drugalcdep.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milesi-Halle A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–213. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, et al. Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc. 2013;19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10:415–424. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, Fend M, Russig H, Feldon J. Latent inhibition, but not prepulse inhibition, is reduced during withdrawal from an escalating dosage schedule of amphetamine. Behav Neurosci. 2001;115:1247–1256. doi: 10.1037//0735-7044.115.6.1247. [DOI] [PubMed] [Google Scholar]
- Nakato Y, Abekawa T, Ito K, Inoue T, Koyama T. Lamotrigine blocks the initiation and expression of repeated high-dose methamphetamine-induced prepulse inhibition deficit in rats. Neurosci Lett. 2010;481:183–187. doi: 10.1016/j.neulet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Sydekum E, Russig H, Feldon J. Withdrawal from repeated amphetamine administration leads to disruption of prepulse inhibition but not to disruption of latent inhibition. J Neural Transm. 2006;113:1323–1336. doi: 10.1007/s00702-005-0390-5. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiat. 2001;50:418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Polich J, Ilan A, Poceta JS, Mitler MM, Darko DF. Neuroelectric assessment of HIV: EEG, ERP, and viral load. Int J Psychophysiol. 2000;38:97–108. doi: 10.1016/s0167-8760(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Powell SB, Young JW, Ong JC, Caron MG, Geyer MA. Atypical antipsychotics clozapine and quetiapine attenuate prepulse inhibition deficits in dopamine transporter knockout mice. Behav Pharmacol. 2008;19:562–565. doi: 10.1097/FBP.0b013e32830dc110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Russig H, Murphy CA, Feldon J. Prepulse inhibition during withdrawal from an escalating dosage schedule of amphetamine. Psychopharmacology (Berl) 2003;169:340–353. doi: 10.1007/s00213-002-1254-4. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiat Res. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. An escalating dose ‘binge’ model of amphetamine psychosis: behavioral and neurochemical characteristics. J Neurosci. 1997;17:2551–2566. doi: 10.1523/JNEUROSCI.17-07-02551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacol. 2003;28:1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK. Prolonged exposure of rats to intravenous methamphetamine: behavioral and neurochemical characterization. Psychopharmacology (Berl) 2005;180:501–512. doi: 10.1007/s00213-005-2188-4. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Zians J, Grant I, Patterson TL. Methamphetamine use, impulsivity, and sexual risk behavior among HIV-positive men who have sex with men. J Addict Dis. 2006;25:105–114. doi: 10.1300/J069v25n04_10. [DOI] [PubMed] [Google Scholar]
- Singer P, Hauser J, Llano Lopez L, Peleg-Raibstein D, Feldon J, Gargiulo PA, et al. Prepulse inhibition predicts working memory performance whilst startle habituation predicts spatial reference memory retention in C57BL/6 mice. Behav Brain Res. 2013;242:166–177. doi: 10.1016/j.bbr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Tenn CC, Fletcher PJ, Kapur S. Amphetamine-sensitized animals show a sensorimotor gating and neurochemical abnormality similar to that of schizophrenia. Schizophr Res. 2003;64:103–114. doi: 10.1016/s0920-9964(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Varty GB, Powell SB, Lehmann-Masten V, Buell MR, Geyer MA. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behav Brain Res. 2006;169:162–167. doi: 10.1016/j.bbr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Willott JF, Tanner L, O’Steen J, Johnson KR, Bogue MA, Gagnon L. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav Neurosci. 2003;117:716–727. doi: 10.1037/0735-7044.117.4.716. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, et al. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19:35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Fukushima S, Shen HW, Hall FS, Uhl GR, Numachi Y, et al. Norepinephrine transporter blockade can normalize the prepulse inhibition deficits found in dopamine transporter knockout mice. Neuropsychopharmacol. 2006;31:2132–2139. doi: 10.1038/sj.npp.1301009. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010a;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Wallace CK, Geyer MA, Risbrough VB. Age-associated improvements in cross-modal prepulse inhibition in mice. Behav Neurosci. 2010b;124:133–140. doi: 10.1037/a0018462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011;222:183–192. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]