Abstract
Chlorpyrifos (CPF) is applied seasonally in Egypt by adolescent agricultural workers and the extent of occupational exposure and the potential for environmental CPF exposure in this population is poorly understood. Adolescent pesticide applicators (n=57; 12–21 years of age) and age matched non-applicators (n=38) from the same villages were followed for 10 months in 2010, spanning pre-application through post-application. Eight urine and 5 blood samples were collected from participants within this time period. Blood acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) (exposure/effect biomarker) and urine 3,5,6-trichloro-2-pyridinol (TCPy) (exposure biomarker) were used to assess occupational CPF exposures in pesticide applicators and environmental exposures in non-applicators. Applicators demonstrated significantly higher TCPy concentration and BChE depression than non-applicators throughout CPF application. This difference persisted for 4–7 weeks after the cessation of agricultural spraying. However, both groups exhibited significantly elevated TCPy and depressed BChE, compared to their respective baseline. The peak TCPy levels during the spray season (95% confidence interval) for non-applicators and applicators reached 16.8 (9.87–28.5) and 137 (57.4–329) ug/g creatinine, respectively. BChE levels (95% confidence intervals) during the spray were 1.47 (1.28–1.68) for non-applicators and 0.47 (0.24–0.94) U/ml for applicators. The longitudinal assessment of CPF biomarkers provided robust measures of exposure and effect throughout CPF application in adolescents and revealed significant exposures in both applicators and non-applicators. Biomarker data in the non-applicators, which mirrored that of the applicators, indicated that non-applicators received environmental CPF exposures. This suggests that similar exposures may occur in other residents of this region during periods of pesticide application.
Keywords: chlorpyrifos, acetylcholinesterase, butyrylcholinesterase, TCPy, occupational exposure, environmental exposure
Introduction
Chlorpyrifos (CPF) is one of the most commonly applied OP pesticides and is of public health concern both in the US as well as worldwide where it is often handled by workers with limited personal protective equipment. Recently, adult Egyptian agricultural workers were reported to have some of the highest occupational CPF exposures in the world and exhibited neurobehavioral deficits when compared with control populations (1–3).
Both animal and human models of chronic CPF exposure suggest that neurotoxicity is the primary endpoint of concern (4–8) with deficits found consistently in areas of motor speed and coordination, information processing speed, executive functioning, attention, and memory (6). These studies have been conducted in adult populations. Recent investigations in children have focused on the consequences of prenatal and immediate postnatal environmental OP pesticide exposures, however, whether children and adolescents are at increased risk of toxicity compared to exposed adults is an area of active research (9–12). In Egypt, adolescents are employed seasonally by the Ministry of Agriculture to apply pesticides to the national cotton crop and the schedule of application of CPF and other pesticides is strictly regulated by the Ministry. Preliminary research in this population of Egyptian adolescent applicators found that adolescent workers have increased incidence of neurological symptoms (blurred vision, dizziness, depression, etc.), greater cholinesterase inhibition, and more neurobehavioral deficits when compared to age-matched controls (13). However, no longitudinal analysis has been conducted in an exposed adolescent population, limiting the ability to determine whether these neurobehavioral deficits occur in a dose-dependent fashion, are progressive over time of application, or resolve post-application. In addition, only a few studies have been successful in demonstrating a link between neurobehavioral toxicity and commonly measured biomarkers or metabolites of CPF in adult or adolescent populations (13–14). This may be due in part to insufficient characterization of these exposure and effect biomarkers and the dearth of longitudinal analyses available in the current literature (6).
The classic mechanism of CPF and other OP pesticide toxicity is through the inhibition of the beta cholinesterases, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). In general, the parent compound is relatively non-toxic, however, the bioactive metabolite, chlorpyrifos oxon (CPF-O), is a potent cholinesterase inhibitor (15). CPF can be bioactivated to CPF-O or detoxified to TCPy in the liver by cytochrome P450 (CYP) enzymes (16). TCPy is excreted in the urine and is often used as a specific biomarker of exposure to CPF while blood AChE and BChE activity are used as biomarkers of effect with BChE being more susceptible to CPF inhibition and thus a more sensitive biomarker.
The current Egyptian adolescent applicator cohort is unique not only because of the young age of the participants (12–21 years) but because of the unique opportunity to characterize potential environmental exposures in age-matched adolescent non-applicators residing in the same villages in the Nile delta. A ten month study was conducted to characterize both occupational and environmental exposure to CPF using urine and blood biomarkers throughout the pesticide application season. These data will establish a clear exposure history for occupationally exposed male adolescent applicators and environmentally exposed adolescent non-applicators which will support a longitudinal investigation of the relationship between biomarkers and neurobehavioral outcomes in this cohort. Furthermore, the finding will be relevant for other residents of this agricultural region during periods of agricultural pesticide application.
Methods
Study Population & Setting
This study was conducted over ten months from April 2010 to January 2011. Adolescents are hired seasonally by the Ministry of Agriculture to spray pesticides in the cotton fields with backpack sprayers working in teams of 3–4 with adult supervisors. The typical workday was from 8am–12pm and from 3pm–7pm, six days per week. The applicators meet at the field station in the morning and load equipment and supplies into vehicles to transport to the cotton fields. Once in the fields they would be involved in the mixing and filling of the backpack sprayers which were then applied to the fields.
For the current study male adolescent pesticide applicators aged 12–21 were enrolled from two field stations in the Menoufia governorate (similar to a state) in Egypt. The applicators (n=57) were recruited from the agricultural field stations in two nearby villages, El-Shohada and Berket El-Sabe, these are designated “field station 1” and “field station 2”, respectively. Data from both field stations were combined. Non-applicators (n=38) of the same age and background were chosen from residents of the same villages. Non-applicators have never worked as pesticide applicators for the cotton crop. One adolescent was excluded from final analysis due to lack of participation and two for questionable sample collection integrity. Written consent was obtained from all participants and their legal guardian. The study was approved by the Oregon Health & Science University IRB in June 2009, and by the Medical Ethics committee of the Faculty of Medicine, Menoufia University in July 2009.
Demographic Data Collection
Both applicators and non-applicators completed a questionnaire at baseline addressing their socio-demographic status such as education and housing information, household and occupational use of pesticides and lifestyle activities.
Chlorpyrifos Application
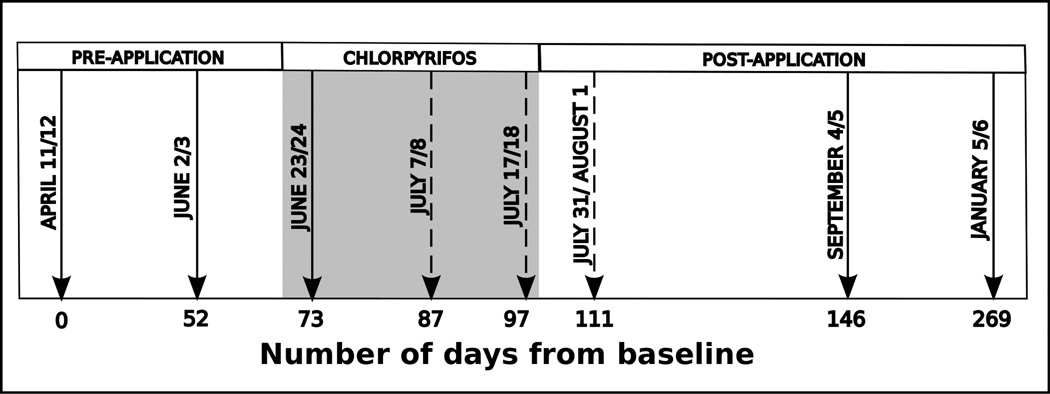
Chlorpyrifos application to the cotton crop is government regulated and thus follows the same general schedule year after year. However, exact dates of application vary between fields depending on local insect infestations in the fields. For field station 1, CPF was applied from June 23rd–July 4th (Study Day 73–84), July 7th–17th (Day 87–97), and from August 1st– 8th 2010 (Day 112–119). The field station 2 schedule was June 17th – July 18th, 2010 (Day 67–98).
TCPy analysis
Spot urine samples were collected at eight time points (Day 0, 52, 73, 87, 97, 111, 146 and 269) between April 11, 2010 (pre-spray baseline, Day 0) to January (post-spray, Day 269). Samples were collected from both applicators and non-applicators at the field stations during the lunch break. All field station 2 samples were collected one day after field station 1 samples. Urine samples were placed on wet ice in a cooler and transported to Menoufia University (Shebin El-Kom, Egypt), where they were stored at −20°C until being shipped to the University at Buffalo (Buffalo, NY, USA) on dry ice for analysis. Urine samples were analyzed for TCPy, the primary metabolite of CPF, by negative-ion chemical ionization gas chromatography–mass spectrometry, using 13C-15N-3,5,6-TCPy as an internal standard, as described previously (2). Creatinine concentrations were measured using the Jaffe reaction (17), and urine TCPy concentrations are expressed as micrograms TCPy per gram creatinine. The within-run imprecision of this assay is very low, as shown by a < 2% coefficient of variation and an intraclass correlation coefficient of 0.997.
ChE analysis
To establish the baseline ChE activity for each subject, pre-application blood draws occurred on study Days 0 and 52, prior to the start of the government-regulated CPF application period so that there were two total baseline blood samples drawn (Figure 1). If two blood samples were collected during the pre-spray season, the first (Day 0) sample was considered the baseline, otherwise Day 52 was considered the baseline. All dates refer to the field station 1 schedule. As with urine collection, field station 2 blood draws were consistently performed one day later. Additional draws were taken during CPF application on study Day 73 and after application on study Days 146 and 269. Blood samples were collected by venipuncture into 10-mL lavender top (EDTA) vacutainer tubes and immediately placed on wet ice and transported to Menoufia University, where they were analyzed in duplicate for AChE and BChE activity using an EQM Test-Mate kit (EQM Research Inc., Cincinnati, OH, USA) as described previously (3).
Figure 1.
Schedule of CPF application and sampling across the ten-month longitudinal study. The shaded area represents the CPF application period for field station 2. CPF was applied in field station 1 from Day 73–84, Day87–97and Day 112–119. All field station 2 collection dates were one day after field station 1 collection dates. Solid arrows represent combined blood and urine sample collection dates; dashed arrows represent urine-only collection dates relevant to the current study.
Statistics
Demographic Data
Demographic information was compared between applicators and non-applicators by a t-test for parametric data and by the Mann-Whitney U test for non-parametric data, with significance set at p<0.05.
Biomarker Analysis
Generalized estimating equations (GEE; 18–19) were used to individually model concentrations of TCPy, AChE and BChE over the course of time for applicators and non-applicators. GEE are sometimes described as estimating “population average” effects in that a given response is first averaged over subjects (either applicators or non-applicators) at each time point, and the model fitted to the time-specific averages. In this way, the model can accommodate incomplete data from participants since averages at each time point can still be estimated even if not all averages over time are based on the same number or specific subset of subjects. Data from participants from both field stations were combined and subsequently analyzed. Prior to model construction, each biomarker of exposure was log-transformed to improve symmetry and stabilize variability of the response. Each GEE (for a given biomarker) was constructed assuming Gaussian distribution of the (log-transformed) response with an identity link function and exchangeable correlation structure for repeated measures drawn from the same subject; standard errors were computed using a robust sandwich estimator of variance to guard against potential misspecification of the correlation structure. Patterns over time were complex enough to justify a “cell-means” model in which a separate (log-transformed) mean response was estimated at each time point for each of the two groups. Upon back transformation, these cell means from the log scale become geometric means on the original scale and these serve to estimate the median response in the underlying population (20). Changes over time within a group as well as differences between groups at a given point in time were found by forming the appropriate linear contrast of log-transformed cell means and then back-transforming the difference. Upon back transformation, these changes are expressed as a multiplicative effect between the medians in question. A Bonferroni adjustment was applied to all p-values so each family of comparisons (over time within group, between groups within time, or changes over time between groups) maintained an overall error rate of 5%; confidence intervals were similarly adjusted so the collection within a family had 95% coverage. The relationship between log peak TCPy concentration and each of AChE or BChE was investigated using a piecewise linear model in which the point of transition between the two linear segments was estimated from the data (21). If no point of transition was found over the range of observed log peak TCPy concentration then the model was simplified to just a single continuous linear trend.
Results
Study Population
The age of the 95 participants ranged from 12 to 21 with a mean age of 16.2 for applicator and 16.6 for non-applicators (Table 1). Only 3 participants were over the age of 19. The two populations had similar mean height, years of education, and smoking status. 3.5% of applicators and 10.5% of non-applicators reported cigarette use. This difference was not significant. Applicators had significantly lower weight and BMI and self-reported an average of a year more of lifetime home pesticide use than non-applicators (2.5 years vs. 1.6 years). Applicators report working approximately 4 hours per day, 4 days per week during the application season and have worked for the Ministry of Agriculture an average of 3.1 years. More than 70% of applicators and non-applicators had TCPy data for at least three of the eight collection periods and the estimated TCPy concentration at any given time point was always based on at least 29/57 applicators and 16/38 non-applicators. For cholinesterase activity, the number of applicators was reduced to 55 for AChE activity and 54 for BChE activity; the number of non-applicators was reduced to 37 (same for each response). Models involving A(B)ChE activity had estimates based on at least 50% of the applicators and at least 46% of the non-applicators at any given time point of interest.
Table 1.
Demographic information for participants
| Non-Applicator (n=38) |
Applicator (n=57) |
|
|---|---|---|
| Age (yrs) | 16.6 ± 2.4 | 16.2 ± 1.6 |
| Height (cm) | 166 ± 12 | 163 ± 9.2 |
| Weight (kg) | 62 ± 15 | 55 ± 8.8* |
| BMI (kg/m2) | 22 ± 3.6 | 20 ± 2.4* |
| Education (yrs) | 9.8 ± 1.8 | 9.9 ± 1.8 |
| Home Pesticide Use (yrs) | 1.6 ± 1.9 | 2.5 ± 1.9* |
| Current Smoker % (N) | 10.5 (4) | 3.5 (2) |
Data shown is Mean ± SD unless otherwise indicated
Sample size for height, weight, and BMI was 54 for applicators and 25 for non-applicators due to missing values
p< 0.05 compared to non-applicators, determined by t-test
Urinary TCPy Concentration
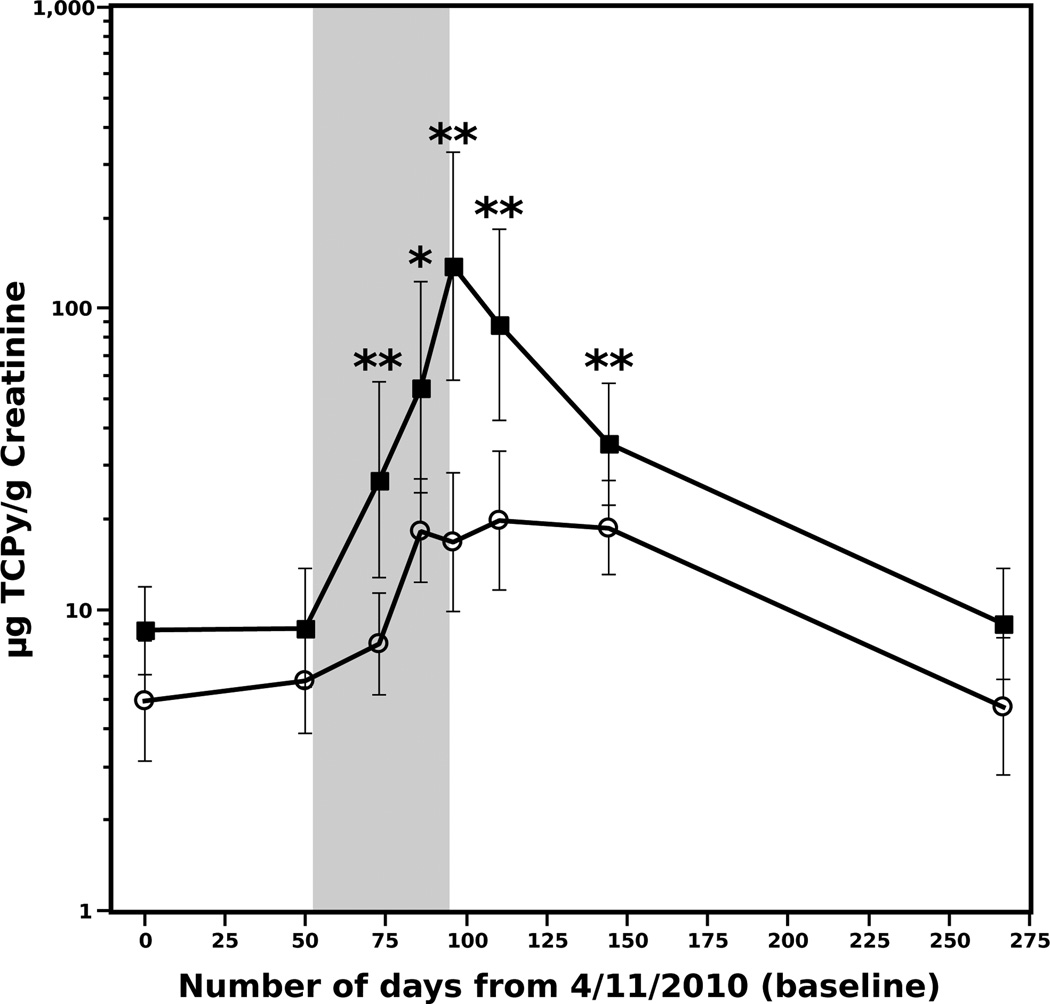
Median urinary TCPy concentrations for applicators and non-applicators are shown in Figure 2. At baseline, urinary TCPy concentration was higher in the applicators than the non-applicators, though this difference was not significant. At the beginning of the CPF application (Day 73), the applicator group had urinary TCPy concentrations ranging from 5.08 to 1760µg/g creatinine, significantly higher than the non-applicator group (2.03 to 54.1µg/g creatinine, Figure 2). Applicator urinary TCPy levels remained significantly higher than non-applicator levels through Day 146. Among applicators, peak TCPy levels occurred on Day 97 at the end of the CPF application period, followed by a steady decline thereafter and the temporal pattern observed for each group mirrored each other throughout the 10 month study (Figure 2). For both applicators and non-applicators, TCPy concentrations measured during the CPF application period were higher than baseline (Table 2). In addition, both groups experienced a similar magnitude of change in TCPy concentration relative to their respective baseline over time, with the exception of Day 97, on which applicators experienced a significantly greater increase in urinary TCPy concentration over baseline by a factor of approximately five (applicator vs. non-applicator, Supplemental Table 1).
Figure 2.
Estimated median urinary concentrations of urinary TCPy. Urinary TCPy concentration is shown in log scale. Squares represent applicators and circles represent non-applicators from both field stations; the shaded area represents the CPF application period for field station 2. Time points showing significant differences between applicators and non-applicators are marked with asterisks (*p< 0.05; **p< 0.001). Individual confidence intervals have been adjusted for multiple comparisons so that simultaneous coverage is 95% (Bonferroni method); p-values were similarly adjusted.
Table 2.
Longitudinal assessment of estimated median blood AChE and BChE and urine TCPy for CPF applicators and non-applicators relative to the respective within group baseline values (Day 0)
| Study Day | TCPy (µg/g creatinine) |
AChE (U/g Hgb) |
BChE (U/mL) |
|||
|---|---|---|---|---|---|---|
| Non Applicator |
Applicator | Non Applicator |
Applicator | Non Applicator |
Applicator | |
| Day 0 | 4.96 (3.13 –7.87) | 8.51 (6.09 –11.9) | 27.3 (25.9–28.9) | 27.1 (25.9–28.3) | 1.87 (1.74–2.01) | 1.64 (1.48–1.82) |
| Day 52 | 5.81 (3.86–8.74) | 8.69 (5.55–13.6) | 27.3 (25.6–29.1) | 26.5* (25.2–27.8) | 1.69* (1.51–1.91) | 1.56 (1.36–1.78) |
| Day 73 | 7.67 (5.18–11.4) | 27.0* (12.8–56.8) | 26.5* (24.7–28.4) | 25.4* (24.4–26.5) | 1.47* (1.28–1.68) | 0.47* (0.24–0.94) |
| Day 87 | 18.3* (12.3–27.1) | 54.6* (24.3–123) | - | - | - | - |
| Day 97 | 16.8* (9.87–28.5) | 137* (57.4–329) | - | - | - | - |
| Day 111 | 19.7* (11.6–33.6) | 88.3* (42.7–183) | - | - | - | - |
| Day 146 | 18.6* (13.0–26.7) | 35.5* (22.3–56.3) | 26.4* (25.0–27.9) | 24.6* (23.3–25.9) | 1.63* (1.43–1.85) | 1.24* (1.04–1.47) |
| Day 269 | 4.76 (2.82–8.04) | 9.0 (5.89–13.7) | 26.3* (24.7–28.0) | 26.1* (24.9–27.3) | 1.79 (1.59–2.02) | 1.68 (1.51–1.88) |
Estimated median values for TCPy, AChE, and BChE (95% CI).
significant difference (p<0.05) from the within group baseline (Day 0, April 11th, 2010)
Time points that are shaded are during the CPF application period for field station 2. Blood samples were not collected on Days 87, 97, and 111. Individual confidence intervals have been adjusted for multiple comparisons so that simultaneous coverage is 95% (Bonferroni method); p-values were similarly adjusted.
Cholinesterase Activity
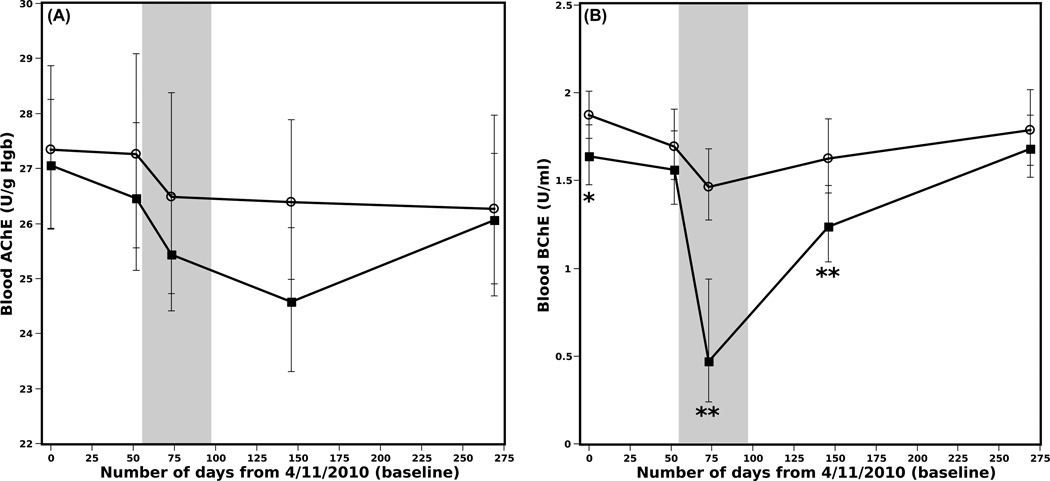
While there were no significant differences between applicators and non-applicators in blood AChE activity (Figure 3A), both groups experienced a slight but significant depression from baseline over the application period which persisted through Day 269 (Table 2). Additionally, on Day 146 applicators had a small but significantly more depressed AChE activity relative to baseline compared to non-applicators (applicator vs. non-applicator, Supplemental Table 1). Blood BChE showed a more dramatic decrease, both between non-applicators and applicators (Figure 3B) and within each group over time (Table 2). Applicator BChE activity was depressed compared to non-applicators even at baseline, though it was not significantly different at the second pre-spray time point (Day 52). The difference between the two groups persisted during the CPF application period and through Day 146 (Figure 3B). BChE was significantly depressed relative to baseline (Day 0) in non-applicators during the CPF application period and through Day 146, with recovery occurring by Day 269 (Table 2). Similarly, applicators had significantly depressed BChE activity relative to baseline during CPF application and at the first post-spray point (Day 146), with recovery occurring by Day 269 (Table 2). Additionally, on Day 73 applicators had a significantly more depressed BChE activity relative to baseline compared to non-applicators (applicator vs. non-applicator, Supplemental Table 1).
Figure 3.
Estimated median blood AChE activity (A) and BChE activity (B) for applicators (squares) and non-applicators (circles) from field stations 1 and 2 in 2010; the shaded area represents the CPF application period for field station 2. Time points showing significant differences between applicators and non-applicators are marked with asterisks (*p< 0.05; **p< 0.001). Individual confidence intervals have been adjusted for multiple comparisons so that simultaneous coverage is 95% (Bonferroni method); p-values were similarly adjusted.
Relationship between TCPy and blood Cholinesterase Activity
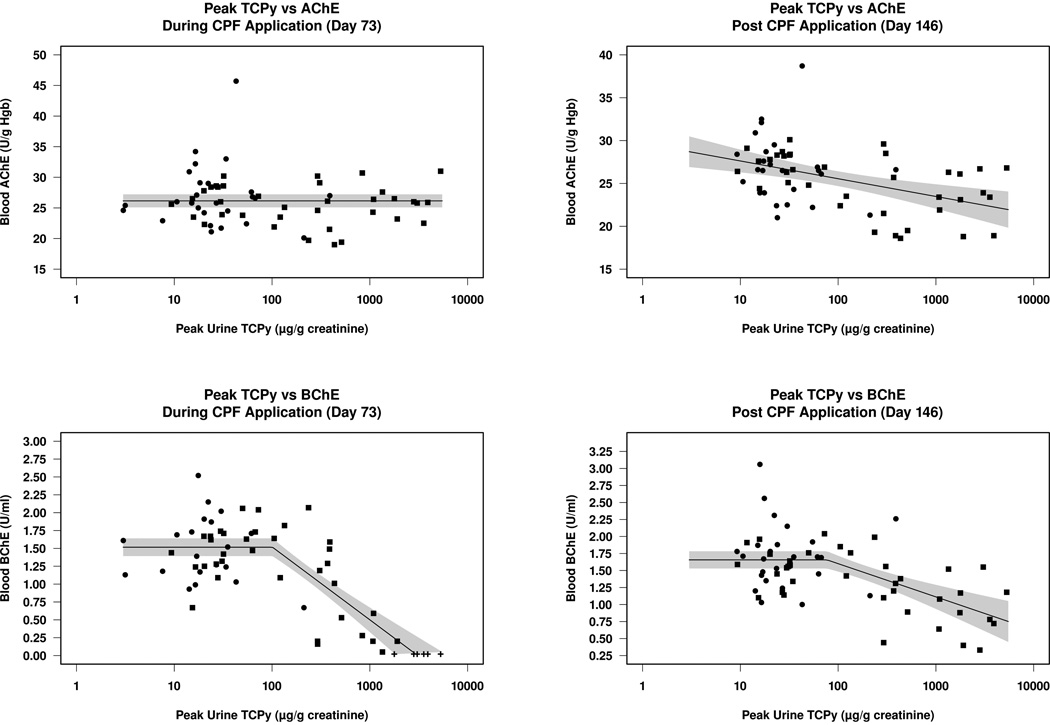
On Day 73, blood AChE was not correlated with the levels of CPF exposure represented by the peak urine TCPy, although at Day 146 (27–48 days following CPF application, depending on field station), AChE activity decreased slightly in individuals with higher peak TCPy levels (Figure 4 A&B). At this time, each ten-fold increase in peak urinary TCPy concentration was associated with a decrease of 2.1 (95% CI: 1–3.14) U/g Hgb. In contrast, blood BChE was markedly depressed on Day 73 during the CPF application period in those with higher peak TCPy levels (Figure 4C). The inflection point for this decrease occurred at a TCPy level of 101 (95% CI: 40.3–253) µg/g creatinine and after this breakpoint each ten-fold increase in peak urinary TCPy concentration was associated with a 1.02 (95% CI: 0.82–1.22) U/mL decrease in BChE activity. Less inhibition or partial recovery of BChE was observed at Day 146, however the inflection point of 77.0 (95%CI: 13.6–436) µg TCPy/g creatinine was similar to that at Day 73 (Figure 4D). After this breakpoint, each ten-fold increase in peak urinary TCPy concentration was associated with a 0.49 (95%CI: 0.30–0.68) U/mL decrease in BChE activity. When CPF exposure from baseline through the summer application period (Days 0–146) was estimated by a cumulative TCPy dosimetric calculated by determining the area under the curve for each individual’s plotted TCPy concentration over time, the relationship was very similar to that observed in Figures 4B and 4D (data not shown). Furthermore, peak TCPy was found to be highly correlated to an estimate of cumulative TCPy from Day 0–146 (R2 = 0.85), suggesting that both methods are appropriate for estimating CPF exposure during this longitudinal study.
Figure 4.
Relationship between peak urinary TCPy levels and blood AChE (A&B) and BChE (C&D) for each subject during the beginning of the CPF application period on Day 73 (A&C) and after CPF application on Day 146 (B&D). Applicators are represented by squares and non-applicators by circles. Reported BChE activity of “0” (below the limit of detection) is shown by a + sign. A piecewise-linear model, constrained to be constant (horizontal) prior to the transition point, was fitted to data combined from applicators and non-applicators; the shaded region represents the 95% confidence interval for the mean response at any given peak TCPy concentration.
Discussion
As expected, adolescents hired by the Egyptian Ministry of Agriculture to apply CPF to cotton fields had a wide range of urinary TCPy concentrations, peaking during the CPF application period, indicating variability in the magnitude of occupational CPF exposure for each participant. Somewhat surprisingly, the longitudinal study design clearly shows that age-matched non-applicator residents of the same villages are receiving environmental exposures that correlate with the CPF application schedule in the fields, mirroring the higher occupational exposures received by the applicators. Peak urine TCPy levels for applicators, ranging from 9.50–5302 µg/g creatinine, were reached at the end of the CPF application period (Day 97). For non-applicators, a plateau was reached towards the end of the spray season lasting from Day 87–146. The greatest range of urinary TCPy concentrations reached for non-applicators was 7.06–390 µg/g creatinine. As opposed to applicators, TCPy concentrations in non-applicators did not seem to reach an obvious maximum at the end of the spray season, although urinary concentrations were still clearly correlated to the CPF spray schedule in the fields. . Additionally, the magnitude of change from respective baseline values for applicators and non-applicators was not significantly different for the two groups throughout the study except at the peak concentration for applicators on Day 97 (Supplemental Table 1). At this point, at the end of the CPF application period, the TCPy level in the applicator group was peaking while non-applicators seemed to have reached a plateau. The persistence of elevated urinary TCPy levels in both groups, which finally return to baseline at Day 269, was unexpected considering the approximately 24 hours reported half-life of TCPy upon oral or dermal exposure to CPF (22). This finding points to possible continual environmental exposures and/or home contamination persisting for several weeks following the CPF application period. CPF may persist for extended periods of time indoors, has a half-life of several months in soil and may last from days to weeks on plants (23–24).
This is the first report of urinary TCPy concentrations for adolescents exposed occupationally to CPF. Urinary TCPy levels in adolescent applicators on Day 97, the peak time point during the study, (mean 719, estimated median 137 µg /g creatinine) did not reach the exceedingly high levels seen in exposed populations of adult Egyptian cotton field applicators previously reported by Farahat et al, in 2010 and 2011 (mean 2,471µg /g creatinine and 6,437g creatinine, respectively). However, the environmentally exposed adolescent non-applicators had peak TCPy levels (mean 44.9, estimated median 19.7 µg/g creatinine) well in excess of the unexposed adult controls (mean 6.25 µg /g creatinine) (2). Compared to US adolescent populations, the CPF exposure experienced by these young Egyptians is extremely high. In 2001–2002, the National Health and Nutrition Examination Survey reported a geometric mean urine TCPy of 2.09 µg /g creatinine in the general US adolescent population (24). Though a possible contributor to urinary TCPy levels may be ingestion of TCPy itself which is more persistent in the environment than CPF (25), the cholinesterase depression exhibited by the non-applicators argues against the exposure to TCPy in the environment as a major cause of their elevated urinary TCPy levels, since TCPy does not inhibit ChE activity (23–24).
One of the potential limitations of our study was the collection of spot urine samples from the participants, which in an earlier study was found to be susceptible to intraindividual variability, particularly at relatively low levels of TCPy (26). However, in previous studies conducted in adult Egyptian agriculture workers in 2007 and 2008, we found that daily urinary TCPy levels for a given worker were very similar in spot urine specimens collected at the beginning (2–3 pm) and end (7–8 pm) of a given work day (2–3). Therefore, in the present study, TCPy levels in a single spot urine specimen collected during the work day should provide a valid measure of exposure.
Blood AChE depression was slight but significant in both populations at each time point and did not return to baseline by the end of the study on Day 269. The observation that AChE activity never returned to baseline is not surprising in light of the long half-life of AChE (approximately 50 days) and the prolonged recovery seen in TCPy concentration and BChE activity seen after the cessation of OP pesticide spraying. However, given the normal variability of AChE, this slight inhibition is likely to be within the normal fluctuations in enzyme activity (27). BChE was more sensitive to CPF exposure, with median activity reduced by 37% from baseline in applicators and 13% in non-applicators during the CPF application period. For comparison, according to the Washington state cholinesterase monitoring guidelines in the US, worker habits and work environment should be examined and modified when AChE or BChE drops below 20% from baseline and workers are removed from sources of exposure with AChE depression greater than 30% or BChE depression greater than 40% (28). BChE activity for 14 of 57 adolescent applicators fell below 20% of their baseline, while 5 of 38 non-applicators fell below this threshold. This further emphasizes the relatively high exposures of this population, even in non-applicators, and the need for strategies for intervention. For both groups, BChE remained depressed at Day 146 of the study and returned to baseline by the end of the study (Day 269). The half-life of BChE is approximately 11 days in human plasma (29). Following the CPF application period, there is an 8–13 days spraying period of another OP pesticide, profenofos which concluded on Day 129 for both fields and may have contributed to the prolonged depression of BChE from baseline. No other OP pesticides are applied. Of note is the fact that baseline measure of TCPy and BChE suggest a small amount of CPF exposure before the beginning of the application period. Similar findings were apparent in adult Egyptian applicators and may be at least partially explained by residential use of the pesticide off-season (3). In our population, we asked the participants whether they had applied pesticides at homes prior to the study and subsequently documented the number of years of home pesticide application by themselves. Applicators reported an average of 2.5 years of home pesticide use while non-applicators reported an average of 1.6 years, lending evidence to episodic pesticide use outside of agricultural work (Table 1). This finding stresses the importance of obtaining baseline measurements particularly in populations that are likely to have continual residual exposures to pesticides year round.
Regarding the relationship between cumulative urinary TCPy and blood AChE and BChE in this population, both peak TCPy and cumulative TCPy were found to be good dosimetric options for CPF exposure in this longitudinal study design and equivalent to each other. As urine samples could not be collected from some participants at all eight time-points, peak TCPy was chosen for preserving a greater number of subjects without the loss of accuracy in the analysis of the relationship between TCPy and ChE activity. This has important implications for future similarly designed studies and supports the validity of either approach. During the spray season, on Day 73, there was essentially no relationship found between AChE activity and peak urinary TCPy concentration while after the application period (Day 146) there was a slight negative relationship. Both the longer period of CPF exposure on Day 146 and the long half-life of AChE (about 100 days) are consistent with this finding. For BChE, there was a strong negative relationship in activity with peak urinary TCPy concentration at both Day 73 and Day 146 with a similar break point of 101 and 77.0 µg/g creatinine, respectively. This no-effect level found in the current adolescent population is similar to that found by Garabrant et al (30) for CPF manufacturing workers (110 µg/g creatinine) and Farahat et al (3) for adult Egyptian workers (114 µg/g creatinine) though these previous studies used same day paired samples instead of peak TCPy. This suggests that adolescent workers respond similarly to adults for this response, however, the association of these biomarkers to neurotoxic responses remains unknown at this time. Unlike AChE, BChE depression was more closely correlated with peak TCPy concentration during the application period on Day 73 on which each ten-fold increase in peak urinary TCPy correlated with a 1.02 U/mL decrease in BChE compared to a smaller decrease of 0.49 U/mL on Day 146. This is consistent with the much shorter half-life of BChE compared to AChE and reflects possible partial recovery of BChE at this time point.
This is the first longitudinal study to our knowledge to examine biomarkers of CPF in an occupationally exposed adolescent population. Adolescents have smaller body size than adults making the biological doses of pesticides (levels of pesticides per unit of body weight) higher than adults (31). In addition, evidence from animal and human studies found that paraoxonase (PON 1),an organophosphate detoxifying enzyme, is less active in younger populations (32–33). A previous longitudinal study conducted in children (aged 2–5) also found a relationship between the period of agricultural OP pesticide use and levels of nonspecific OP metabolites in the participant’s urine, serving as a biomarker of environmental exposure (34). Only a few studies have fully characterized specific metabolites of OP pesticides in a longitudinal manner, thus the relationship between commonly assayed biomarkers of CPF exposure and detrimental outcomes such as neurobehavioral deficits remains unclear. A recent study by Quandt et al (35) characterized specific metabolites of OP and carbamate pesticides across the spray season in adult migrant farmworkers. However, there were no controls for comparison and no baseline was collected in the Quandt study due to the migrant nature of the workers. This characteristic also resulted in a complex pesticide exposure history. Recent longitudinal analyses focusing exclusively on CPF include Farahat et al (3) in Egyptian agricultural workers and Garabrant et al (30) in CPF manufacturers. Both of these studies were conducted with adult worker populations and only Garabrant reported data from controls. The current study is novel for being the first to examine an adolescent population. We had a geographically stable population including age-matched controls, a baseline measurement for each biomarker, a CPF-specific biomarker of exposure (TCPy), a well-defined period of CPF exposure, and a thoroughly characterized longitudinal analysis of urine TCPy and blood AChE and BChE. The pre-season baseline enables us to interpret seasonal changes in these biomarkers within an individual. Potential limitations of this study include that lack of an unexposed control group, limited information on non-occupational sources of CPF exposure, and a limited collection of biological specimens for biomarker analysis. Although small sample size is another potential limitation of the study the relative non-mobility of this population means that the same cohort can be expanded to increase power in future studies to examine neurobehavioral outcomes. The well-defined exposure to CPF allows a more accurate analysis of the cause-effect relationship between CPF and AChE and BChE depression. These factors will be helpful in establishing a relationship between these biomarkers and neurobehavioral deficits, which has been historically difficult. Finally, the extent of CPF exposure and BChE depression seen in both groups suggest that preventative measures need to be taken to minimize exposure of not only these adolescent workers but also the adolescent residents of these agricultural communities.
Supplementary Material
Acknowledgements
We thank the Egyptian Ministry of Agriculture and the adolescents and their parents for their participation, Steve Hutton (Dow Agrosciences, Indianapolis, IN) for providing 13C–15N– 3,5,6-TCP, Barbara McGarrigle for the urinary TCPy analytical work and the Research Team at Menoufia University.
Grant Information: The work was supported by the National Institute of Environmental Health Sciences (NIEHS, grants #ES017223 and NIEHS F30 ES020655). The content is solely the authors’ responsibility and does not necessarily represent official views of NIEHS.
Footnotes
Competing Financial Interest: The authors declare no conflict of interest.
Supplementary information is available at the Journal of Exposure Science and Environmental Epidemiology’s website.
References
- 1.Farahat TM, Abdel Rasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med. 2003;60:279–286. doi: 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farahat FM, Fenske RA, Olson JR, Galvin K, Bonner MR, Rohlman DS, et al. Chlorpyrifos exposures in Egyptian cotton field workers. Neurotoxicology. 2010;31:297–304. doi: 10.1016/j.neuro.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, et al. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect. 2011;119:801–806. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci. 2010;115:183–193. doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middlemore-Risher ML, Buccafusco JJ, Terry AV., Jr Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicol Teratol. 2010;32:415–424. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology. 2011;32:268–276. doi: 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol Sci. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- 8.Speed HE, Blaiss CA, Kim A, Haws ME, Melvin NR, Jennings M, et al. Delayed reduction of hippocampal synaptic transmission and spines following exposure to repeated, subclinical doses of organophosphorus pesticide in adult mice. Toxicol Sci. 2011;125:196–208. doi: 10.1093/toxsci/kfr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huen K, Harley L, Bradman A, Eskenazi B, Holland N. Longitudinal changes in PON1 enzymatic activities in Mexican–American mothers and children with different genotypes and haplotypes. Toxicol Appl Pharmacol. 2010;244:181–189. doi: 10.1016/j.taap.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandjean P, Harari R, Barr DB, Debes F. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics. 2006;117:e546–e556. doi: 10.1542/peds.2005-1781. [DOI] [PubMed] [Google Scholar]
- 11.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorling-Poulsen M, Andersen HR, Grandjean P. Potential developmental neurotoxicity of pesticides used in Europe. Environ Health. 2008;7:50. doi: 10.1186/1476-069X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel Rasoul GM, Abou Salem ME, Mechael AA, Hendy OM, Rohlman DS, Ismail AA. Effects of occupational pesticide exposure on children applying pesticides. Neurotoxicology. 2008;29:833–838. doi: 10.1016/j.neuro.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Rothlein J, Rohlman D, Lasarev M, Phillips J, Muniz J, McCauley L. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and non-agricultural Hispanic workers. Environ Health Perspect. 2006;114:691–696. doi: 10.1289/ehp.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amitai G, Moorad D, Adani R, Doctor BP. Inhibition of acetylcholinesterase and butyrylcholinesterase by chlorpyrifos-oxon. Biochem Pharmacol. 1998;56:293–299. doi: 10.1016/s0006-2952(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 16.Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR. Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos. Drug Metab Dispos. 2007;35:189–193. doi: 10.1124/dmd.106.012427. [DOI] [PubMed] [Google Scholar]
- 17.Fabiny DL, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clinical chemistry. 1971;17:696–700. [PubMed] [Google Scholar]
- 18.Lang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 19.Hardin JW, Hilbe JM. Generalized Estimating Equations. New York, NY: Chapman and Hall; 2003. [Google Scholar]
- 20.Aitkin M, Anderson D, Francis B, Hinde J. Statistical Modelling in GLIM. New York, NY: Oxford University Press; 1989. [Google Scholar]
- 21.Bacon DW, Watts DG. Estimating the transition between two intersecting straight lines. Biometrika. 1971;58:525–534. [Google Scholar]
- 22.Nolan RJ, Rick DL, Freshour NL, Saunders JH. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol Appl Pharmacol. 1984;73:8–15. doi: 10.1016/0041-008x(84)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- 24.CDC (Centers for Disease Control and Prevention) National Report on Human Exposure to Environmental Chemicals. [accessed 18 February 2012];2009 Available: http://www.cdc.gov/exposurereport.
- 25.US EPA. Chlorpyrifos Revised Risk Assessment and Agreement with Registrants. Washington, DC: U.S. Environmental Protection Agency; 2000. [Google Scholar]
- 26.Whyatt RM, Garfinkel R, Hoepner LA, Andrews H, Holmes D, Williams MK, et al. A biomarker validation study of prenatal chlorpyrifos exposure within an inner-city cohort during pregnancy. Environ Health Perspect. 2009;117:559–567. doi: 10.1289/ehp.0800041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigg HN, Knaak JB. Blood cholinesterases as human biomarkers of organophosphorus pesticide exposure. Rev Environ Contam Toxicol. 2000;163:29–111. doi: 10.1007/978-1-4757-6429-1_2. [DOI] [PubMed] [Google Scholar]
- 28.Cholinesterase Monitoring. Part J-1. Chapter 296–307. Washington Administrative Code; 2004. [Google Scholar]
- 29.Ostergaard D, Viby-Mogensen J, Hanel HK, Skovgaard LT. Half-life of plasma cholinesterase. Acta Anaesthesiol Scand. 1988;32:266–269. doi: 10.1111/j.1399-6576.1988.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 30.Garabrant DH, Aylward LL, Berent S, Chen Q, Timchalk C, Burns CJ, et al. Cholinesterase inhibition in chlorpyrifos workers: Characterization of biomarkers of exposure and response in relation to urinary TCPy. J Expo Sci Environ Epidemiol. 2009;19:634–642. doi: 10.1038/jes.2008.51. [DOI] [PubMed] [Google Scholar]
- 31.London L, Beseler C, Bouchard MF, Bellinger DC, Colosio C, Grandjean P, et al. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology. 2012;33:887–896. doi: 10.1016/j.neuro.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa LG, Li WF, Richter RJ, Shih DM, Lusis A, Furlong CE. The role of paraoxonase (PON1) in the detoxication of organophosphates and its human polymorphism. Chem Biol Interact. 1999;119–120:429–438. doi: 10.1016/s0009-2797(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 33.Costa LG, Cole TB, Vitalone A, Furlong CE. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin Chim Acta. 2005;352:37–47. doi: 10.1016/j.cccn.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Koch D, Lu C, Fisker-Andersen J, Jolley L, Fenske RA. Temporal association of children's pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study. Environ Health Perspect. 2002;110:829–833. doi: 10.1289/ehp.02110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quandt SA, Chen H, Grzywacz JG, Vallejos QM, Galvan L, Arcury TA. Cholinesterase depression and its association with pesticide exposure across the agricultural season among Latino farmworkers in North Carolina. Environ Health Perspect. 2010;118:635–639. doi: 10.1289/ehp.0901492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.