Abstract
Mechanical properties of the extracellular matrix (ECM) play an essential role in cell fate determination. To study the role of mechanical properties of ECM in stem cell-mediated bone regeneration, we used a 3D in vivo ossicle model that recapitulates endochondral bone formation. Three-dimensional gelatin scaffolds with distinct stiffness were developed using 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) mediated zero-length crosslinking. The mechanical strength of the scaffolds was significantly increased by EDC treatment, while the microstructure of the scaffold was preserved. Cell behavior on the scaffolds with different mechanical properties was evaluated in vitro and in vivo. EDC-treated scaffolds promoted early chondrogenic differentiation, while it promoted both chondrogenic and osteogenic differentiation at later time points. Both micro-computed tomography and histologic data demonstrated that EDC-treatment significantly increased trabecular bone formation by transplanted cells transduced with AdBMP. Moreover, significantly increased chondrogenesis was observed in the EDC-treated scaffolds. Based on both in vitro and in vivo data, we conclude that the high mechanical strength of 3D scaffolds promoted stem cell mediated bone regeneration by promoting endochondral ossification. These data suggest a new method for harnessing stem cells for bone regeneration in vivo by tailoring the mechanical properties of 3D scaffolds.
Keywords: Mesenchymal stem cells, BMP2, Scaffold stiffness, Endochondral ossification, Bone regeneration, Biomaterials, Collagen
1. Introduction
At sites of tissue injury, both local and distant adult stem cells are recruited to the wound bed, subsequently differentiate to the specific progenitors, and when conditions are favorable, repair or regenerate the tissue. Stem cell fate in wound healing is determined by a combination of signals that includes growth factors, cytokines, hormones, biomechanical stress, interaction with cells at the site of injury, and the nature of the extracellular matrix (ECM) [1]. Recently, the mechanical properties of the ECM have attracted greater attention as the role of the ECM in stem cell differentiation has become better understood [2–5]. Previous studies indicated that soft matrices (~1kPa) favored mesenchymal stem cell (MSC) differentiation into neuronal-like cells, while moderately stiff matrices (~10kPa) promoted myogenic differentiation, and rigid matrices (~100kPa) stimulated osteogenic differentiation [2, 6, 7]. These findings suggest that the mechanical properties of biomaterial scaffolds used for stem cell delivery in tissue engineering could modify cell behavior and that designing materials to mimic the injured tissue may be an advantage. These principles have already been exploited to some extent. Therefore, aligning the mechanical property with the tissue of interest is one of the reasons that hydrogels (soft, <1kP) [8–11] and ceramics (stiff, >100kP) [12–15] are the most widely used biomaterials for pre-clinical cartilage and bone tissue engineering, respectively. Another example supporting this concept is the use of decellularized tissues (native ECM) that have been successfully used in tissue engineering and pre-clinical applications [16, 17].
Although decades of investigation have expanded our understanding of the ECM in vivo, much of our current knowledge regarding the mechanical properties of supporting matrices in stem cell fate determination is derived from in vitro cell culture models [2, 3, 5, 18–23]. However, typical two dimensional cell culture systems are not able to fully mimic the in vivo microenvironment that naturally modulates stem cell behavior [24, 25]. Therefore, in vivo studies will likely provide more instructive insights to understand the role of mechanical properties of ECM in stem cell-mediated tissue regeneration
One common strategy to improve the bone forming capacity of biomaterials is to add hydroxyapatite (HA) to polymer-based scaffolds because HA is not only able to increase the mechanical strength, but may also mimic the composition and structure of natural bone mineral [26–29]. However, it is often difficult to distinguish the contribution of mechanical properties from other modifications (e.g., chemical composition and structure). Previous observations suggest that very different mechanical strengths are required to support stem cells to differentiate to chondrocyte versus osteoblasts. However, many bone regeneration strategies, especially those induced by bone morphogenetic proteins (BMPs), are typically directed through an endochondral ossification process; that is, progenitor cells first differentiate to chondrocytes that subsequently undergo hypertrophy, are invaded by blood vessels and are subsequently replaced osetoblasts [30, 31]. To mimic endochondral bone formation, a strategy was developed in which stem cells were induced to chondrogenic differentiation in vitro prior to being transplanted in vivo [30, 32, 33]. Although chondrogenesis is a prerequisite for endochondral bone formation, osteogenesis and chondrogenesis may impede each other during bone development and regeneration [34, 35]. It is therefore essential to recognize that endochondral bone formation is a dynamic process that cannot be recapitulated in in vitro cell culture models. We hypothesized that the mechanical microenvironment required for osteogeneic differentiation by stem cells in vivo was different from that functioning in vitro systems. Therefore, to study the role of mechanical properties of ECM in stem cell-mediated bone regeneration, we used a BMP-induced, 3D in vivo ossicle model that represents an endochondral ossification process [36, 37]. Three-dimensional gelatin scaffolds with distinct elastic moduli were generated by crosslinking the material with 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC). EDC has been widely used in polymeric scaffold fabrication because it is a zero-length, non-toxic crosslinker that conjugates carboxylates (–COOH) to primary amines (–NH2) without the addition of linking molecules [38–40]. Moreover, we developed a technique to maintain the microstructure of gelatin scaffolds to prevent swelling during chemical crosslinking [41]. Therefore, the in vivo ossicle provided us a new and contrasting in vivo model to investigate the role of mechanical properties of matrices in stem cell-mediated bone regeneration.
2. Materials and Methods
2.1. Chemical crosslinking of scaffolds
Three-dimensional porous gelatin scaffolds (Pharmacia and Upjohn, Kalamazoo, MI), were crosslinked as previously described [41]. Briefly, the scaffolds were incubated in 50 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl (EDC) (Thermo Scientific, Rockford, IL) and 50 mM N-hydroxy-succinimide (NHS) (Sigma, St Louis, MO) and 50 mM (2-(N-morpholino) ethanesulfonic acid) hydrate (MES) buffer (pH 5.3,) (Sigma, St Louis, MO) at 4°C for 24 h. To maintain the microstructure of gelatin matrices, a 90/10 (v/v) acetone/water solvent mixture was used instead of water. Scaffolds treated with MES buffer/acetone/water served as the control groups. All scaffolds were then washed with distilled water 5×30 min and frozen at −80 °C for at least 12 h. The scaffolds were subsequently freeze-dried and stored in a desiccator.
2.2. Scaffold characterization
The surface morphology of the scaffolds was observed by scanning electron microscopy (SEM, Philips XL30 FEG) as previously described [41]. Briefly, the scaffolds were coated with gold particles using a sputter coater (DeskII, Denton vacuum Inc.) with gas pressure of 50 mtorr and 40 mA current for 200 s. Samples were analyzed at 30 kV. The elastic moduli of the 3D gelatin scaffolds were determined with an AR-G2 rheometer (TA Instruments, New Castle, DE) in the oscillatory mode with a fixed frequency of 1 Hz and an applied strain of 1% [42]. The scaffolds were soaked overnight in distilled H2O before undergoing mechanical testing. Four scaffolds were tested for each group (n=4).
2.3. Cell culture
Bone marrow mesenchymal stem cells (BMSCs) were harvested and cultured as described previously [15, 36]. The marrow content of 4–6 bones was plated into a T75 culture flask in BMSC growth medium comprised of α-MEM containing 10% fetal bovine serum (FBS) and 100U/ml penicillin, 100 mg/ml streptomycin sulfate (Gibco, Grand Island, NY). Non-adherent cells were removed and adherent BMSCs were cultured and expanded for further experiments. Primary cells prior to passage 4 were used in the experiments. The mouse mesenchymal stem cell line, C3H10T1/2, and pre-osteoblastic MC3T3-E1 cells were cultured in α-MEM containing 10% FBS, 100U/ml penicillin, 100 mg/ml streptomycin sulfate (Gibco, Grand Island, NY). C3H10T1/2 cells were transfected with adenovirus encoding BMP-2 (Ad-BMP2, University of Michigan Vector Core Laboratory, Ann Arbor, MI) at 500 MOI for cell differentiation in vitro. For osteoblastic differentiation in vitro, MC3T3-E1 cells were cultured in osteogenic medium containing α-MEM containing 10% FBS, 10 mM β-glycerophosphate and 50 mg/mL ascorbic acid-2-phosphate (Sigma-Aldrich, St. Louis, MO). Chondrocytes were isolated from fresh articular cartilage of adult bovine knee joints attained from a local slaughterhouse. Minced cartilage pieces (~1mm3) were digested overnight at 37°C in a 5% CO2 incubator with 0.25% collagenase (Sigma; St. Louis, MO) in high-glucose DMEM. The chondrocytes were collected by centrifugation at 500g for 5 min after filtering the suspension with a 70μm strainer. The cells were expanded in high-glucose DMEM containing 10% FBS, 100 U/mL penicillin and 100 mg/L streptomycin (Invitrogen; Carlsbad, CA).
2.4. Cell seeding on scaffolds
The EDC-treated and control gelatin scaffolds were prepared for cell seeding as previously described [43] with modifications. Briefly, the scaffolds were cut into defined dimensions (3.5×3.5×3.5mm) and soaked in 70% ethanol for 30 min and then exchanged with phosphate-buffered saline (PBS) for three times (30 min each). Subsequently, the scaffolds were dried to remove residual medium and air by compressing them between sterile gauze with moderate pressure. One million cells (C3H10T1/2/Ad-BMP2 or MC3T3-E1) were subsequently seeded into the scaffolds by capillary action [44] in a ultra-low attachment 96-well plate (Corning, Lowell, MA). All cell/scaffold constructs were transferred to a 24-well plate with 1mL α-MEM containing 10% FBS in each well after a 2h-attachment period and were cultured at 37 °C, 5% CO2. Cells on EDC-treated and non-treated control scaffolds were cultured for 1, 4 and 7 days and harvested for analysis. To estimate the number of the cells within each scaffold, total DNA was quantified using a DNeasy Blood & Tissue Kit (QIAGEN, Germantown, MD). The recombinant BMP2 concentration in the supernatant produced by C3H10T1/2 was quantified using an enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s protocol (R&D Systems, Minneapolis, MN).
2.5. Gene expression analysis
Total RNA was isolated using the RNeasy®Micro Kit (Qiagen, Germantow, MD). RNA concentration was determined at 260 nm and an equivalent amount of RNA (0.5 μg) was processed to generate cDNA using the High Capacity cDNA Reverse Transcript kit (Applied Biosystems, Forster City, CA). Quantitative PCR was performed with Taqman gene expression assays (Applied Biosystems, Forster City, CA) using the ABI PRISM 7500 sequence detection system (Applied Biosystems, Carlsbad, CA, USA). The primers/probes: GAPDH (Mm99999915), Runx2 (Mm00501584), OCN (Mm03413826), PPARγ (Mm01184322), FABP4 (Mm00445878), Sox9 (Mm00448840), COL2 (Mm01309555) and Aggrecan (Mm00545809) were all purchased from Applied Biosystems (Forster City, CA).
2.6. In vivo transplantation and analysis
For in vivo bone regeneration, mouse BMSCs were transduced with Ad-BMP2 at 500 MOI one day before implantation. One million BMSCs/Ad-BMP2 were then seeded on the gelatin scaffolds as described above. Inbred C57BJL/6 mice with an age range of 5–6 weeks (Charles River Laboratories) were used in the study. Animal surgeries were performed according to the guidelines approved by the University of Michigan Committee of Use and Care of Laboratory Animals. The dorsum of the animals was shaved and disinfected with povidoneiodine. One midsagittal incision was made on the dorsa and two subcutaneous pockets were created using blunt dissection. One scaffold was implanted subcutaneously into each pocket. Each animal received 2 cell-seeded constructs. After placement of scaffolds, the incisions were closed with staples. Five mice (n=5) were euthanized at 1 day, 4 days and 7 days after surgery, respectively. Each sample was split into two; one half was used for gene expression and one half for protein analysis. Five (n=5) and ten mice (n=10) were euthanized at 14 days and 42 days after surgery, respectively. Three and one transplants failed to form typical ossicles at 42 days after surgery in control and EDC treated group, respectively. The ossicles were fixed in Z-fix (Anatech Ltd.) for 2 days and then transferred to 70% ethanol until analyzed.
The ossicles were scanned at a voxel size of 18 μm using a microcomputed tomography (μCT) scanner (GE Healthcare Pre-Clinical Imaging). Micro View software (GE Healthcare Pre-Clinical Imaging) was used to generate a three-dimensional (3D) reconstruction. A fixed threshold (1000) was used to extract the mineralized bone phase and the bone morphometry was calculated as previously described [31].
Three million bovine chondrocytes were seeded into the control and EDC-treated scaffolds after ~4 passages in vitro. The cells/scaffolds were then subcutaneously implanted to nu/nu mice (5–6 weeks old, Charles River Laboratories) using the same protocol described above. The transplants were retrieved at 28 days after surgery and fixed for histological analysis (n=3).
2.7. Histological analysis
Histological analysis was carried out at the University of Michigan School of Dentistry histology core facility. Ten μm sections were cut from the middle of the scaffolds and stained with hematoxylin and eosin (H&E) or Safranin O, for light microscopic observation. For immunohistochemical detection, antigens in rehydrated sections were retrieved by pepsin treatment (Thermo Scientific, Rockford, IL) at 37 °C for 15 min, incubated with anti-collagen type 2 antibody (Thermo Scientific) at 1:50 dilutions at 25 °C for 2 h, and detected by a Cell & Tissue Staining Kits (R&D Systems, Inc. Minneapolis, MN) using the manufacturer’s instructions. Image J software (National Institute of Health) was used for bone area fraction (bone area/total area, %) and adipocyte number analysis from at least 7 random 4X fields of section.
2.8. Western Blot Analysis
Whole cell lysates were separated on 7.5% SDS–polyacrylamide gels and transferred to PVDF membranes. The membranes were incubated with 5% milk for 1 hour and incubated with primary antibodies overnight at 4 °C. Primary antibodies used were as follows: polyclonal anti-SOX9 (1:2,000, Abcam, Cambridge, MA), monoclonal anti-α Tubulin (TU-02) (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal anti-RUNX2 (1:250; Santa Cruz), and polyclonal anti-Osteocalcin (1:5,000; Santa Cruz). Blots were incubated with peroxidase-coupled secondary antibodies (Promega, Madison, WI) for 1 hour, and protein levels were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL). Membranes were reprobed with polyclonal anti-β-actin antibody (1:1,000; Cell Signaling) to control for equal protein loading.
2.9. Statistical Analysis and Image Editing
To determine statistical significance of observed differences between the study groups, a two-tailed homoscedastic t-test was applied. A value of p<0.05 was considered to be statistically significant while 0.05<p<0.10 was considered to represent a non-significant, but clear trend in cell or tissue response. Values are reported as the mean ± standard deviation (SD). Brightness and contrast were adjusted equally across all images for improved visibility.
3. Results
3.1. Scaffold Characterization after EDC-treatment
The macrostructure of EDC-treated (EDC) and non-treated (CON) scaffolds, as observed by SEM, were not appreciably different after undergoing treatment and freeze-drying (Fig. 1A). After over-night incubation in PBS, both CON and EDC scaffolds were saturated in the solution. The elastic modulus of the scaffolds was significantly increased from ~0.6kP (CON) to ~ 2.5kP (EDC) after EDC-treatment (Fig. 1B). This difference in mechanical strength was also visually demonstrated by placing scaffolds on the edge rigid structure. The EDC-treated scaffolds were able to resist deformation due to gravitational force, while control scaffolds were flaccid and demonstrated a loss of structural integrity (Fig. 1C). Therefore, EDC-treatment significantly improved the stiffness of gelatin 3D scaffolds without changing their internal macro-structure.
Fig. 1. Scaffold mechanical integrity is significantly increased by EDC mediated crosslinking.
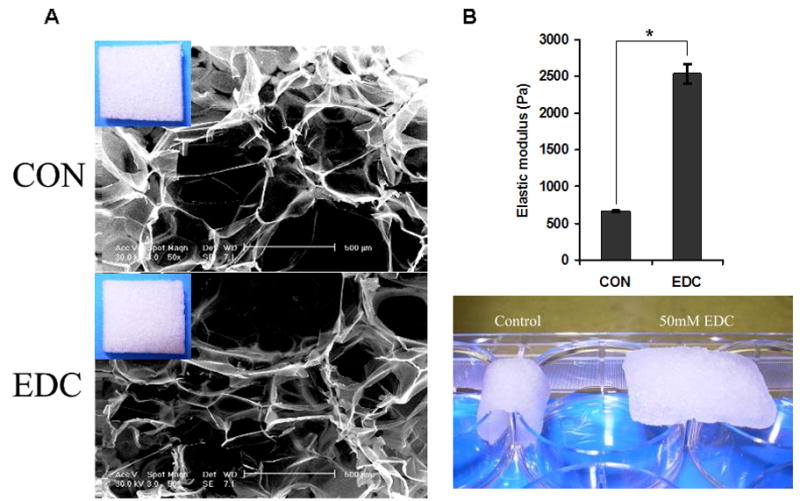
(A) The scaffold microstructures as viewed by SEM were not appreciably affected by EDC-treatment. Similar macrostructures were observed in the EDC-treated (EDC) and non-treated control (CON) scaffolds after they were freeze-dried (inserts at up-left corner). (B) The elastic modulus of the scaffolds was significantly increased by EDC-treatment. The scaffolds were saturated in PBS and quantified by a rheometer (n=4). This significant difference in mechanical strength was demonstrated by placing the scaffolds on the edges of a six-well plate. EDC-treated scaffolds were able to resist deformation due to gravitational force, while control scaffolds were not. Data are expressed as means ± SD. *p<0.001.
3.2. Cell - scaffold interactions in vitro
Control and EDC-treated scaffolds maintained a similar morphology during sterilizing and washing prior to cell seeding. The structure of EDC-treated scaffolds was preserved, while control scaffolds significantly contracted after cell seeding (data not shown). Histologic analysis immediately after cell seeding demonstrated that the cells were approximately evenly distributed throughout the entire scaffold, although a slightly higher number of cells were observed on the periphery compared to the central portion of the scaffolds (Fig. 2A). It was noted that the total volume and pore size of the control scaffolds decreased during the cell culture period, while no change was observed in EDC-treated scaffolds. Accordingly, local cell density in CON scaffolds was relatively higher than in EDC scaffolds due to the change in size of the scaffolds (Fig. 2A). An equivalent number of cells (~1×106) were seeded in CON and EDC scaffolds and this was confirmed by quantifying the total DNA content 4 hours after cell seeding (Fig. 2B). Likewise, the rhBMP2 levels in the supernatant produced by the transduced cells were not significantly different (p>0.05) in CON and EDC scaffolds (Fig. 2C).
Fig. 2. Cell seeding and growth on scaffolds in vitro.
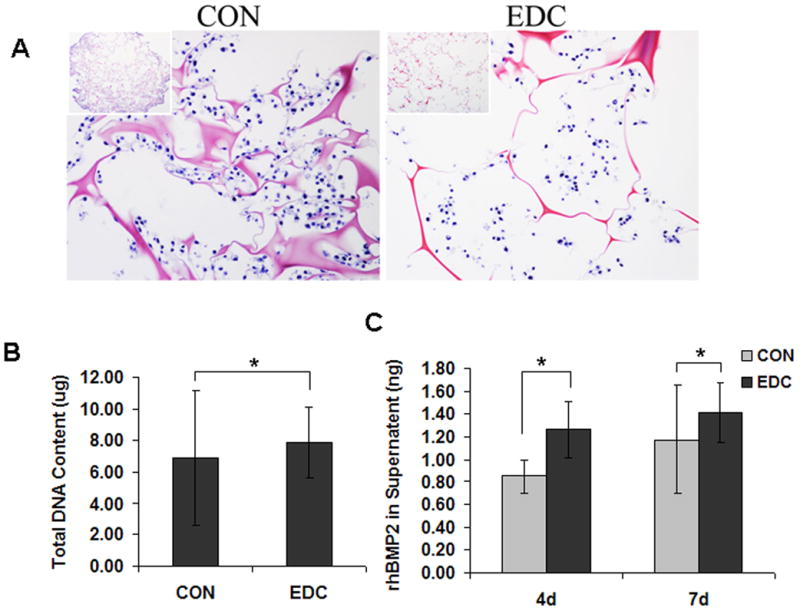
(A) Histological observation revealed that EDC-treatment significantly changed the scaffold deformation due to cell seeding and growth. (B) The same number of cells (1 × 106) were seeded in CON and EDC scaffolds and was confirmed by total DNA content 4 hours after cell seeding (n=4). (C) The rhBMP2 levels in the supernatant produced by the transduced cells were not significantly different in CON and EDC scaffolds (n=4). Data are expressed as means ± SD. *p>0.05.
3.3. Cell differentiation on scaffolds in vitro
Osteoblastic differentiation of MC3T3-E1 cells was determined, in part, by osteoblast-related gene expression on the 4th and 7th day of culture on CON and EDC-treated scaffolds. RUNX2 and osteocalcin (OCN) expression levels were significantly higher on EDC scaffolds compared to CON scaffolds on both day 4 and 7. In contrast, the adipogenic-related gene, PPARγ, did not significantly change (Fig. 3A). Consistent with the increased osteoblastic gene expression, the total calcium content was also significantly elevated in the EDC-treated cell-scaffold construct compared the CON group, suggesting more rapid mineralization (Fig. 3B). Differentiation of the mouse mesenchymal stem cell line, C3H10T1/2 was investigated in addition to pre-osteoblastic MC3T3-E1 cells. For C3H10T1/2 cells, the osteogenic marker gene, OCN was significantly lower on EDC scaffolds on 4th day after cell seeding compared to on the CON scaffolds. In contrast, the chondrogenic gene collagen type 2 (COL2A1) was significantly increased by EDC scaffolds while slightly elevated Sox9 gene expression was observed. However, chondrogenic genes, including Sox9, COL2A1 and Aggrecan, and osteogenic genes, including Runx2 and OCN, were significantly increased when cultured on EDC-treated scaffolds on day 7. PPARγ was also more strongly expressed by cells on EDC-treated scaffolds on day 7, although expression of FABP4 was not altered (Fig. 3C). Surprisingly, protein levels of Sox9 were dramatically enhanced when cells were cultured with EDC-treated scaffolds. Sox9 protein was detected as early as 4h after cell seeding on the scaffolds and was maintained for at least 7 days (Fig. 3D). In contrast to the significantly enhanced Sox9 protein levels, no obvious difference was observed in Runx2 protein levels. Consistent with the gene expression data, protein levels of OCN in the EDC group were significantly higher than in the CON group at early time points, including 4h, 15h, 1d and 2d. After 4 days in culture, significant differences were not noted by Western blot analysis (Fig. 3D). The gene expression and protein data consistently indicated that EDC-treated scaffolds significantly improved chondrogenic differentiation at earlier time points and both chondrogenic and osteogenic differentiation at later time points.
Fig. 3. Osteogenic and chondrogenic differentiation on scaffolds in vitro.
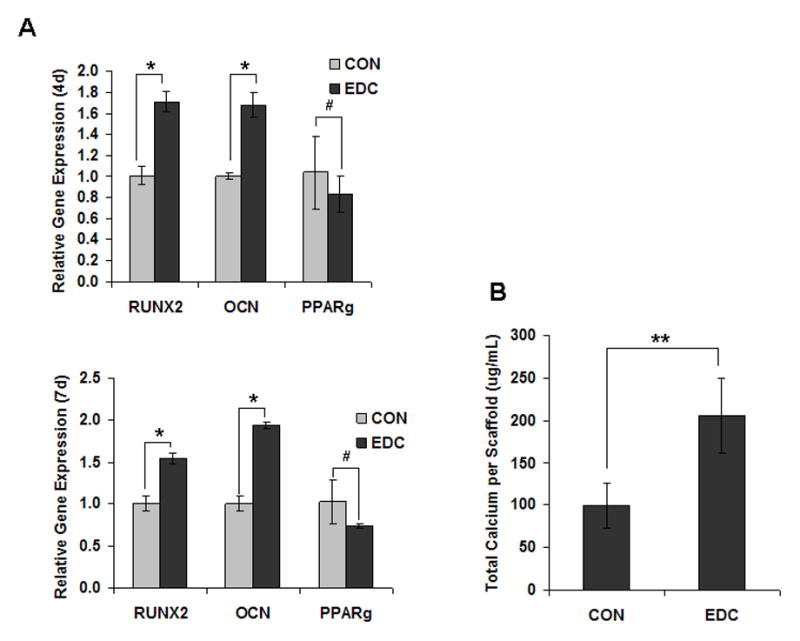
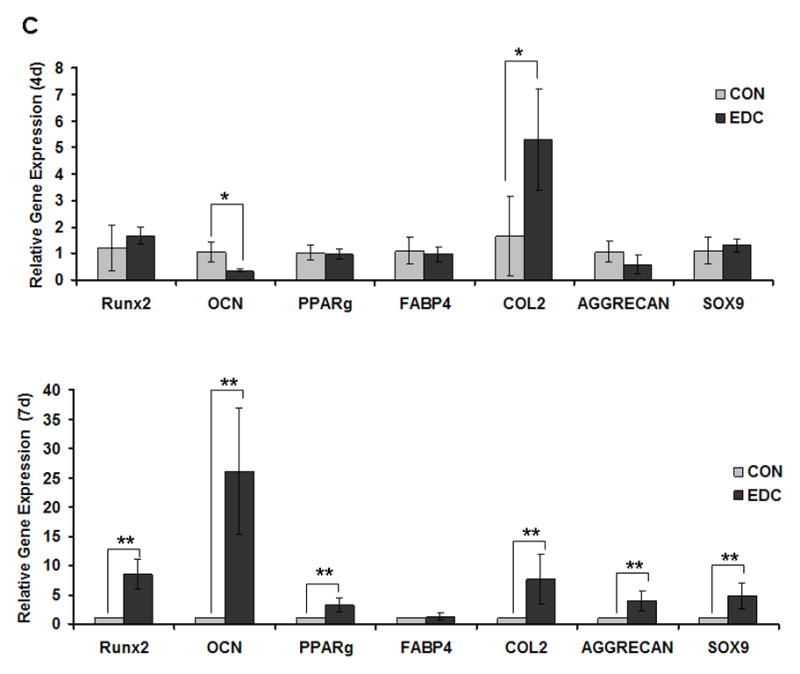
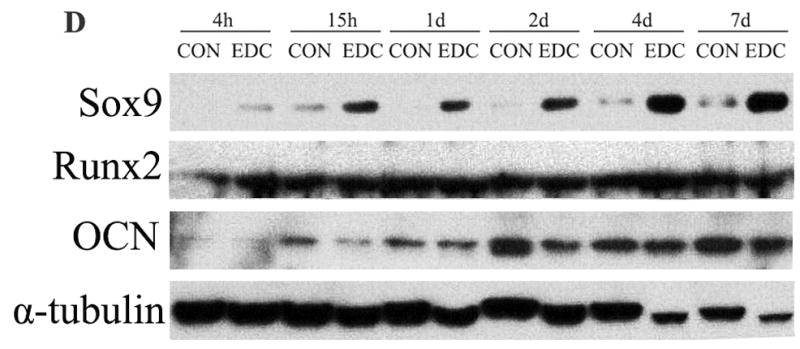
(A) EDC-treatment significantly increased osteoblast differentiation from cells seeded in vitro. Osteoblastic differentiation of MC3T3-E1 cells was determined by osteoblast-related gene expression on the 4th and 7th day after culture on CON and EDC scaffolds (n=3). *p<0.001; #p>0.05. (B) Total calcium was increased by EDC-treatment in osteoblasts (n=3). **p<0.01. (C) Differentiation of stem cells (C3H10T1/2) cells was determined by gene expression on the 4th and 7th day after culture on CON and EDC scaffolds (n=5). Data are expressed as means ± SD. *p<0.05; **p<0.005. (D) Chondrogenic and osteogenic protein levels on different scaffolds were detected by Western blot analysis. The experiments were repeated at least 2 times, and the representative data are shown.
3.4. Chondrogenesis on scaffolds in vivo
Implanted cell-scaffold constructs were harvested at 4, 7, 10 and 14d after surgery. Significantly higher total protein from the EDC-treated group was found compared to the CON group at almost all time points (Fig. 4A). Two weeks after implantation, immature bone structure developed in both EDC and CON scaffolds. Consistent with the protein measurement data, a greater number of cells and developing tissue were observed inside the EDC scaffold compared to the CON group. Interestingly, most of the newly formed bone surrounded the scaffold (the dash line indicates the boarder of the scaffold) in both CON and EDC scaffolds. However, bone formation in either scaffold was not substantial at 2 weeks (Fig. 4B). In contrast, a significantly greater number of hypertrophic-like chondrocytes were observed inside the scaffold in the EDC group compared to the CON group. Chondrocytes were confirmed by collagen type 2 (Col2) staining (Fig. 4B). The contribution of EDC-treated scaffolds in supporting chondrogenesis was further investigated using in vitro passaged chondrocytes. Four weeks after implantation, the implanted chondrocytes were negative for COL2 staining in CON scaffolds. In contrast, the cells on EDC scaffolds stained strongly for COL2 and many cells exhibited the typical hypertrophic chondrocyte morphology (Fig. 4C). As a positive control, passaged chondrocytes were strongly positive for COL2 when Sox-9 was over-expressed by transduction with Ad-Sox9. It was noted that more hypertrophic cells were observed in the EDC group compared to the Ad-Sox-9 group. Therefore, EDC-treated gelatin scaffolds likely provided a more favorable microenvironment for chondrogenesis compared to the control scaffolds in vivo.
Fig. 4. EDC-Treatment increased chondrogenesis in vivo.
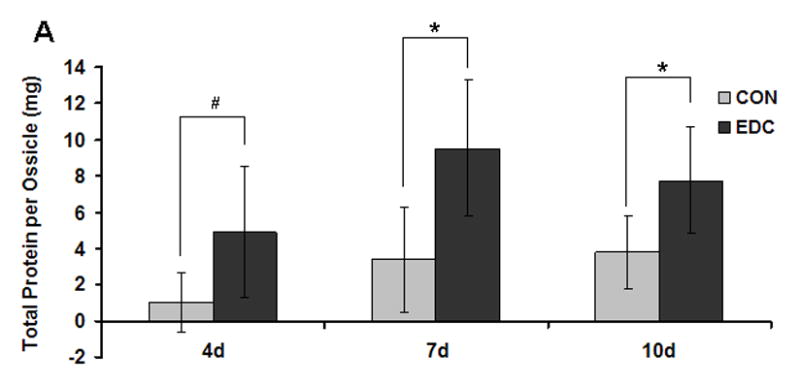
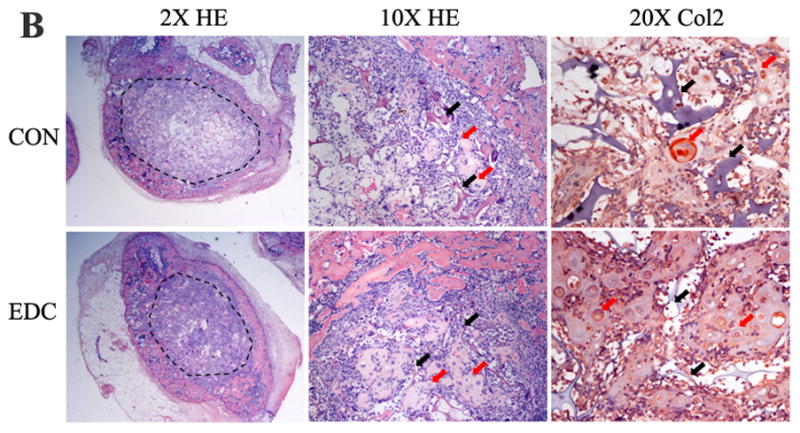

(A) Significantly more new tissue grew in EDC-treated scaffolds. Total protein within the ossicles demonstrated that the EDC group had higher protein content compared to the CON group on day 4, 7, 10 and 14 (n=5). *p<0.001; #p>0.05. (B) EDC-treatment promoted chondrogenic differentiation in vivo. The dash line indicates the scaffold border. The black and red arrows indicate scaffold and chondrocytes, respectively. The chondrocytes were confirmed by collagen type 2 (Col2) staining (red) (n=5). (C) EDC-treatment promoted chondrocyte differentiation in vivo. Bovine chondrocytes were seeded on CON and EDC scaffolds and implanted for four weeks. As a positive control, chondrocytes were strongly positive for COL2 staining when Sox-9 was over-expressed by Ad-Sox9 transduction (n=3).
3.5. Bone formation on scaffolds in vivo
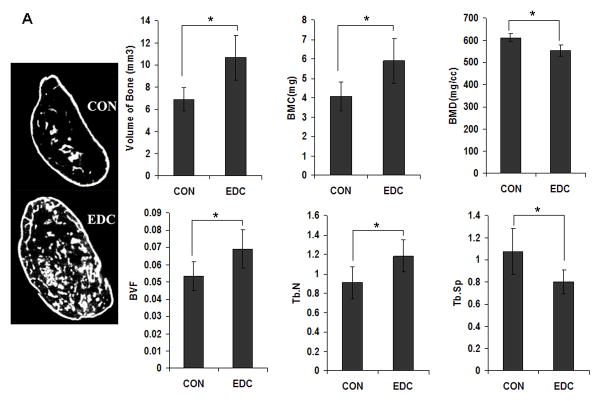
To study the role of scaffold stiffness in bone formation in vivo, an equivalent number of primary mouse BMSCs transduced with Ad-BMP2 were seeded on EDC or CON scaffolds and were subcutaneously implanted into mice. After 6 weeks, ossicles developed in both EDC and CON scaffolds and were then retrieved and quantitatively analyzed by μCT and histology. As the μCT data demonstrated, both bone volume and bone mineral content (BMC) were significantly higher in the EDC group compared to the CON group (Fig. 5A). Other indicators of bone quality also improved in EDC-treated scaffolds. The EDC group had an elevated bone volume fraction (BV/TV) and greater trabecular bone numbers (Tb.N.), while trabecular spacing (Tb.S.) was significantly less than controls (Fig. 5A). Although most of the tested parameters indicated trabecular bone formation was improved in the EDC scaffolds, bone mineral density (BMD) was slightly lower in EDC group compared to the CON group and trabecular thickness (Tb.Th) and bone surface density (BS/TV) were not significantly different (P>0.05, data not shown). Representative images derived from reconstructed μCT data also demonstrated the enhanced bone development in the EDC scaffolds (Fig. 5A). Consistent with the μCT data, histologic data also indicated that EDC scaffolds significantly enhanced the levels of new bone formation in vivo. Moreover, the adipocyte number in EDC group was significantly lower than in CON groups (Fig. 5B). It was also noted that the gelatin scaffold was nearly completely degraded in the CON group after 4 weeks in vivo, while a greater amount of residual scaffold was observed in EDC scaffolds, suggesting that EDC treatment increased the mechanical strength and stability of the collagen scaffolds.
Fig. 5. EDC-treated scaffolds significantly increased trabecular bone formation in vivo.
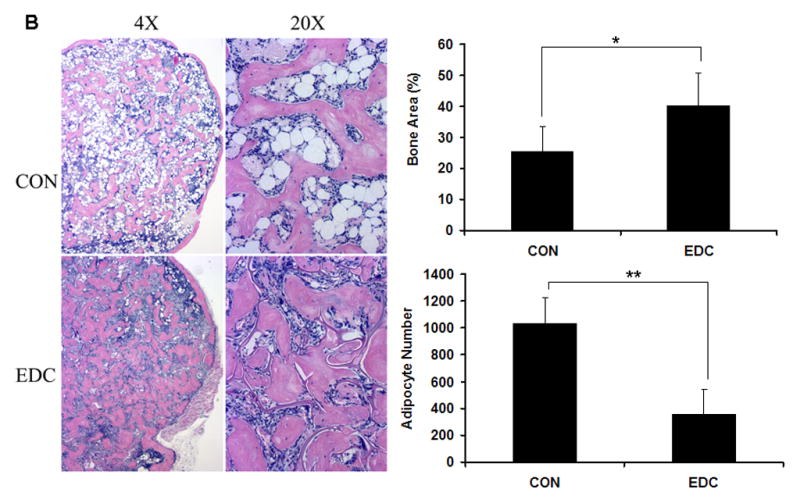
(A) μCT analysis of trabecular bone. Representative images reconstructed from μCT data (n=7 or 9). Data are expressed as means ± SD. *p<0. 01. BMC, bone mineral content; BMD, bone mineral density; BVF, bone volume fraction; Tb.N., trabecular number; Tb.Sp., trabecular spacing. (B) Histologic analysis of new bone formation. EDC scaffold significantly enhanced bone volume fraction (BVF). Moreover, the adipocyte number in EDC group was significantly lower than in CON group (n=7 or 9). Data are expressed as means ± SD. *p<0.01; **p<0.001.
4. Discussion
To address the challenge of studying the contribution of scaffold mechanical strength in stem cell-mediated bone formation in vivo, we used the ossicle model in our study. Compared to in vitro studies, the in vivo ossicle model is more relevant to the natural bone repair process. Dexamethasone (DEX) is widely used as an in vitro supplement to induce the osteogenic differentiation of BMSCs, while it inhibits bone formation and induces bone resorption in vivo [45, 46]. Therefore, we instead chose BMP2 to direct stem cell differentiation in our study because BMPs are powerful morphogens that induce robust bone development and regeneration in vivo. It has been reported that BMP2 can induce C3H10T1/2 cells to differentiate to osteoblasts, adipocytes and chondrocytes in vitro [47–49]. The cell fate was determined by the cell culture conditions and cell-cell interactions. In the present study, the contribution of scaffold mechanical strength in stem cell differentiation was easy distinguished when using the same cells (C3H10T1/2 cells) driven by the single factor (BMP-2), rather than a complex cocktail of growth factors. It is also noted that most bone repair in vivo is through an endochondral ossification process, while transplanted BMSCs form bone without first passing through an intermediate chondrogenic phase. Consistent with previous findings [47, 50], our data suggest that BMP-2 induced C3H10T1/2 cells to undergo a sequential pattern of chondrogenesis, followed by osteogenic differentiation. Therefore, the BMP2-transduced cell system is an appropriate model to study the endochondral ossification in vivo, and its equivalent in vitro, because it is relevant to the natural bone repair process.
The mechanical properties of the matrix have been shown to play an essential role in osteogenic and chondrogenic differentiation of stem cells in vitro. Most of the available data supports the notion that a softer matrix is better for chondrogenic differentiation, while stiffer matrices better support osteogenic differentiation [2, 51–54]. Our data suggested a different phenomenon; that is, improved mechanical strength supported chondrogenic differentiation. There are a number of possible reasons for this apparent discrepancy. First, we used BMP2 to induce stem cells towards chondrogenic and osteogenic differentiation, while other groups have used osteogenic and chondrogenic supplements in the culture medium. We raised the hypothesis that a BMP2-induced stem cell model represented a more natural correlate to tissue regeneration compared to using synthetic compounds including DEX and other medium supplements. Second, although EDC-treatment significantly increased the mechanical strength of the gelatin scaffolds, the elastic moduli of the treated scaffolds (~2.5 kP) were much lower than the mechanical strength of biomaterials used for osteogenic differentiation in many other studies (>100 kP) [2]. Third, it was noted that previous studies used either 2D substrates [2, 51, 52], or hydrogels [54, 55], which are different than the 3D porous gelatin scaffolds used in this study. From the histologic data, we found that the control scaffolds were more like hydrogels. These scaffolds were very soft and were unable to maintain their pore structure and shape after cell seeding. In contrast, the EDC-treated scaffolds were elastic and able to maintain their shape and micro-structure after cell seeding and in vitro culture. One of the limitations of using hydrogels for cartilage repair is that it is difficult to generate anatomically shaped grafts due the poor mechanical properties of most hydrogels [56]. Therefore, an improved load bearing, clinically relevant sized 3D porous scaffold is desirable for tissue engineering applications.
Both our in vitro and in vivo data demonstrated that EDC-treatment was able to significantly increase chondrogenic differentiation, which suggested EDC-treated scaffolds represent a more pro-chondrogenic microenvironment compared to control scaffolds. It has been reported that primary chondrocytes quickly lose their differentiated phenotype in 2D culture [57]. However, the dedifferentiation process may be reversible because dedifferentiated chondrocytes can recover their phenotype when they are subjected to a favorable 3D environment [58]. In our study, the passaged chondrocytes on EDC-treated scaffolds displayed positive cartilage markers after 4-weeks in vivo, while the same cells on control scaffolds did not express these makers. These in vivo data suggest that EDC-treatment contributes to generate a favorable microenvironment for chondrogenesis. To achieve chondrogenic differentiation, stem cells are required to be cultured at high density in 3D environments that favors cell condensation and significant cell–cell interactions analogous to that which occurs during embryonic development [56]. This may explain why most studies on chondrogenic differentiation of stem cells have been performed when chondrocytes are restricted in micromass or pellet cultures and encapsulated in a hydrogel [59]. EDC-treatment significantly increased the mechanical strength of collagen scaffolds. In control groups, the cells were entrapped within the contracted scaffold. Due to the decreased or collapsed pore structure, the cell-matrix interactions were accordingly increased which has been reported to reduce chondrogenic differentiation [60]. In contrast, cells in EDC-treated groups aggregated into larger clusters that may reduce cell-matrix interactions and favor the cell-cell interactions that support chondrogenesis. In addition to the cell-cell interactions, EDC-treated scaffolds provided larger spaces for cells and thus may provide for better nutrient transport compared to non-treated scaffolds. Therefore, the scaffold structural differences due to different mechanical strength were one of the possible reasons that led to the distinct cell behavior on the scaffolds.
Based on the in vitro and in vivo data, we reasoned that the EDC-treatment increased trabecular bone formation in vivo by promoting chondrogenesis, a prerequisite for endochondral bone formation. Although stem cell-based bone tissue engineering holds great promise for large bone defect repair, the clinical application of this alternative strategy is impeded by significant challenges [61]. For example, the low viability of grafted cells, poor vascularization and limited integration with host bone [62–64] have been difficult to overcome. To address these challenges, a new strategy has recently been developed. In these studies, stem cells were directed to hypertrophic chondrocytes rather than osteoblasts in vitro prior to in vivo transplantation. The chondrogenic-primed stem cells not only showed a higher viability than osteoblasts, but also induced the typical endochondral bone formation, a sequential physiological processes, including angiogenesis, osteoclastogenesis, hematopoiesis and bone remodeling [30, 33, 65]. All of these physiological processes are known to be essential for generating functional bone in vivo. Compared to the chondrognic priming model, our stem cell/BMP2 system relied on the direct transplantation of stem cells with Ad-BMP2 without the in vitro chondrognic differentiation that may take up to several weeks. Although both the control and EDC-treated scaffolds supported BMP2-stem cell-mediated endochondral bone formation and hematopoiesis after implantation, EDC-treatment significantly promoted early chondrognesis and increased bone formation. The nature of the bone marrow was also affected by EDC-treatment of the scaffolds. Following an increase in bone formation, the marrow was less cellular and contained significantly fewer adipocytes. Moreover, EDC-treatment increased the stability of the scaffold. The control scaffolds were completely degraded by six weeks. In contrast, small amounts of residual EDC-treated scaffold remained at 6 weeks. This more slowly resorbing scaffold may have contributed to increased bone formation by providing longer and more stable support for differentiating osteoblasts in vivo.
5. Conclusions
In summary, both in vitro and in vivo model systems support the conclusion that the higher mechanical strength of the EDC-treated 3D scaffolds supported cell-mediated bone regeneration by promoting an endochondral ossification process. Our research presents a paradigm for modifying stem cell behavior for bone regeneration in vivo by tailoring the mechanical properties of 3D biomaterial scaffolds.
Acknowledgments
This study was supported by NIH grants R01 DE018890 and R01 DK082481 (PHK). This study was also supported by National Natural Science Foundation of China (31301207, Natural Science Foundation of Jiangsu Province (BK20130080) and Nanjing Medical Science and technique Development Foundation. The authors also thank Dr. Xiaohua Liu, Zhanpeng Zhang, Michael J Solomon and Leo Pavlovsky for their outstanding technical assistance.
Footnotes
Disclosure of potential conflicts of interest
The authors state no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Even-Ram S, Artym V, Yamada KM. matrix control of stem cell fate. Cell. 2006;126:645–7. doi: 10.1016/j.cell.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Villa-Diaz LG, Lam RHW, Chen W, Krebsbach PH, Fu J. Mechanics regulates fate decisions of human embryonic stem cells. PLoS One. 2012;7:e37178. doi: 10.1371/journal.pone.0037178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suh JKF, Matthew HWT. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–98. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 9.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 10.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–22. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;74:1487–510. [Google Scholar]
- 13.Burg KJL, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–59. doi: 10.1016/s0142-9612(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Wu CT, Dai KR, Chang J, Tang TT. Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics. Biomaterials. 2006;27:5651–7. doi: 10.1016/j.biomaterials.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Jin X, Zhang X, Sun H, Tu J, Tang T, et al. In vitro and in vivo evaluation of akermanite bioceramics for bone regeneration. Biomaterials. 2009;30:5041–8. doi: 10.1016/j.biomaterials.2009.05.077. [DOI] [PubMed] [Google Scholar]
- 16.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 17:424–32. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Benders KEM, Weeren PRv, Badylak SF, Saris DBF, Dhert WJA, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–76. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol-Cell Physiol. 2008;295:C1037–C44. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 19.Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cells Mater. 2009;18:1–14. doi: 10.22203/ecm.v018a01. [DOI] [PubMed] [Google Scholar]
- 20.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28:1123–U168. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 21.Zemel A, Rehfeldt F, Brown AEX, Discher DE, Safran SA. Optimal matrix rigidity for stress-fibre polarization in stem cells. Nat Phys. 2010;6:468–73. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du J, Chen X, Liang X, Zhang G, Xu J, He L, et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci U S A. 2011;108:9466–71. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trappmann B, Gautrot JE, Connelly JT, Strange DGT, Li Y, Oyen ML, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–9. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 24.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–8. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrie RJ, Yamada KM. At the leading edge of three-dimensional cell migration. J Cell Sci. 2012;125:5917–26. doi: 10.1242/jcs.093732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao F, Yin YJ, Lu WW, Leong JC, Zhang WJ, Zhang JY, et al. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials. 2002;23:3227–34. doi: 10.1016/s0142-9612(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 27.Kim SS, Park MS, Jeon O, Choi CY, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28:3338–48. doi: 10.1016/j.biomaterials.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–69. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 30.Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010;107:7251–6. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun H, Jung Y, Shiozawa Y, Taichman RS, Krebsbach PH. Erythropoietin Modulates the Structure of Bone Morphogenetic Protein 2-Engineered Cranial Bone. Tissue Eng Part A. 2012;18:2095–105. doi: 10.1089/ten.tea.2011.0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrell E, Both SK, Odoerfer KI, Koevoet W, Kops N, O’Brien FJ, et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang WX, Yang F, Wang YN, Both SK, Jansen JA. In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta Biomater. 2013;9:4505–12. doi: 10.1016/j.actbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Lengner CJ, Hassan MQ, Serra RW, Lepper C, van Wijnen AJ, Stein JL, et al. Nkx3. 2-mediated repression of Runx2 promotes chondrogenic differentiation. J Biol Chem. 2005;280:15872–9. doi: 10.1074/jbc.M411144200. [DOI] [PubMed] [Google Scholar]
- 35.Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, et al. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci U S A. 2006;103:19004–9. doi: 10.1073/pnas.0605170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RVB, Rowe DW, Robey PG. Bone formation in vivo: Comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–69. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 37.Song J, Kiel MJ, Wang Z, Wang J, Taichman RS, Morrison SJ, et al. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood. 2010;115:2592–600. doi: 10.1182/blood-2009-01-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieper JS, Hafmans T, Veerkamp JH, van Kuppevelt TH. Development of tailor-made collagen-glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials. 2000;21:581–93. doi: 10.1016/s0142-9612(99)00222-7. [DOI] [PubMed] [Google Scholar]
- 39.Park SN, Park JC, Kim HO, Song MJ, Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide cross-linking. Biomaterials. 2002;23:1205–12. doi: 10.1016/s0142-9612(01)00235-6. [DOI] [PubMed] [Google Scholar]
- 40.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–34. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Smith LA, Hu J, Ma PX. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30:2252–8. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi JS, Harley BAC. The combined influence of substrate elasticity and ligand density on the viability and biophysical properties of hematopoietic stem and progenitor cells. Biomaterials. 2012;33:4460–8. doi: 10.1016/j.biomaterials.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31:1133–9. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–8. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- 45.Hahn TJ, Halstead LR, Teitelbaum SL, Hahn BH. Altered mineral metabolism in glucocorticoid-induced osteopenia – effect of 25-hydroxyvitamin-D administration. J Clin Invest. 1979;64:655–65. doi: 10.1172/JCI109506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 47.Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem. 2003;90:1112–27. doi: 10.1002/jcb.10734. [DOI] [PubMed] [Google Scholar]
- 48.Date T, Doiguchi Y, Nobuta M, Shindo H. Bone morphogenetic protein-2 induces differentiation of multipotent C3H10T1/2 cells into osteoblasts, chondrocytes, and adipocytes in vivo and in vitro. J Orthop Sci. 2004;9:503–8. doi: 10.1007/s00776-004-0815-2. [DOI] [PubMed] [Google Scholar]
- 49.Huang HY, Song TJ, Li X, Hu LL, He Q, Liu M, et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106:12670–5. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seghatoleslami MR, Tuan RS. Cell density dependent regulation of AP-1 activity is important for chondrogenic differentiation of C3H10T1/2 mesenchymal cells. J Cell Biochem. 2002;84:237–48. doi: 10.1002/jcb.10019. [DOI] [PubMed] [Google Scholar]
- 51.Schuh E, Kramer J, Rohwedel J, Notbohm H, Muller R, Gutsmann T, et al. Effect of Matrix Elasticity on the Maintenance of the Chondrogenic Phenotype. Tissue Eng Part A. 2010;16:1281–90. doi: 10.1089/ten.TEA.2009.0614. [DOI] [PubMed] [Google Scholar]
- 52.Park JS, Chu JS, Tsou AD, Diop R, Tang ZY, Wang AJ, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32:3921–30. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy CM, Matsiko A, Haugh MG, Gleeson JP, O’Brien FJ. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen–glycosaminoglycan scaffolds. J Mech Behav Biomed Mater. 2012;11:53–62. doi: 10.1016/j.jmbbm.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Liu SQ, Tian Q, Hedrick JL, Po Hui JH, Rachel Ee PL, Yang YY. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials. 2010;31:7298–307. doi: 10.1016/j.biomaterials.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Kwon HJ. Chondrogenesis on sulfonate-coated hydrogels is regulated by their mechanical properties. J Mech Behav Biomed Mater. 2013;17:337–46. doi: 10.1016/j.jmbbm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Wang YZ, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082–94. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 57.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425–32. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Malda J, Van Blitterswijk CA, Grojec M, Martens DE, Tramper J, Riesle J. Expansion of bovine chondrocytes on microcarriers enhances redifferentiation. Tissue Eng. 2003;9:939–48. doi: 10.1089/107632703322495583. [DOI] [PubMed] [Google Scholar]
- 59.Jukes JM, Moroni L, Van Blitterswijk CA, De Boer J. Critical steps toward a tissue-engineered cartilage implant using embryonic stem cells. Tissue Eng Part A. 2008;14:135–47. doi: 10.1089/ten.a.2006.0397. [DOI] [PubMed] [Google Scholar]
- 60.Connelly JT, Garcia AJ, Levenston ME. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071–83. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4:e9. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akiyama M, Nakamura M. Bone regeneration and neovascularization processes in a pellet culture system for periosteal cells. Cell Transplant. 2009;18:443–52. doi: 10.3727/096368909788809820. [DOI] [PubMed] [Google Scholar]
- 63.Verseijden F, Posthumus-van Sluijs SJ, Farrell E, van Neck JW, Hovius SER, Hofer SOP, et al. Prevascular structures promote vascularization in engineered human adipose iissue constructs upon implantation. Cell Transplant. 2010;19:1007–20. doi: 10.3727/096368910X492571. [DOI] [PubMed] [Google Scholar]
- 64.Bueno EM, Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nat Rev Rheumatol. 2009;5:685–97. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- 65.Farrell E, van der Jagt OP, Koevoet W, Kops N, van Manen CJ, Hellingman CA, et al. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods. 2009;15:285–95. doi: 10.1089/ten.tec.2008.0297. [DOI] [PubMed] [Google Scholar]