Abstract
Based on in vitro synergy studies, the addition of nafcillin to daptomycin was used to treat refractory methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. Daptomycin is a de facto cationic antimicrobial peptide in vivo, with antistaphylococcal mechanisms reminiscent of innate host defense peptides (HDPs). In this study, the effects of nafcillin on HDP activity against MRSA were examined in vitro and in vivo. Exposures to β-lactam antimicrobials in general, and nafcillin in particular, significantly increased killing of S. aureus by selected HDPs from, keratinocytes, neutrophils and platelets. This finding correlated with enhanced killing of MRSA by whole blood, neutrophils and keratinocytes after growth in nafcillin. Finally, nafcillin pretreatment ex vivo reduced MRSA virulence in a murine subcutaneous infection model. Despite the lack of direct activity against MRSA, these studies show potent, consistent, and generalized nafcillin-mediated ‘sensitization’ to increased killing of MRSA by various components of the innate host response. The use of nafcillin as adjunctive therapy in MRSA bacteremia merits further study and should be considered in cases refractory to standard therapy.
Keywords: MRSA, innate immunity, beta-lactams, nafcillin, host defense peptides
Introduction
The treatment of bacterial infections has become increasingly challenging due to continued emergence of antibiotic resistance. Emblematic of this problem are strains of methicillin-resistant Staphylococcus aureus (MRSA) that have reached epidemic proportions in many countries [1–3]. In the United States, S. aureus is the most common cause of hospital- and community-associated bacterial infections of the bloodstream, skin and soft tissue and other sites, with MRSA strains comprising a large majority in many locales [4, 5]. Clinical outcomes of patients with invasive MRSA infections are significantly worse than those with methicillin-susceptible isolates, including longer hospitalizations and higher mortality (20–30%) despite modern therapeutic interventions [6, 7]. Both hospital- and community-associated MRSA may exhibit broad resistance to multiple classes of antibiotics [3, 5], and a recent upward “creep” in vancomycin MICs among MRSA isolates is associated with even higher treatment failure rates [8, 9].
The ability of MRSA to produce severe invasive disease, frequently in otherwise healthy individuals, reflects multiple virulence mechanisms for host tissue invasion and immune evasion [10–12]. Normal innate antistaphylococcal immunity depends significantly upon cationic host defense peptides (HDPs) such as cathelicidins and defensins, produced by epithelial cells (e.g. skin keratinocytes) and phagocytes (e.g. neutrophils), to provide a critical first line of defense against invasive infection [13, 14]. S. aureus partially counteracts HDP killing through mechanisms such as modification of cell envelope charge and hydrophobicity, expression of drug efflux pumps, HDP-binding proteins or proteases, or modulation of cell membrane order (fluidity-rigidity) [15–18]. Notably, recent surveys have found that MRSA strains are significantly more resistant to human cathelicidin LL-37 compared to methicillin-sensitive strains [19, 20] perhaps contributing to their enhanced survival in vivo during skin or bloodstream infections.
Optimal combination antibiotic therapy for MRSA bacteremia has not been established [21]. We recently documented a salutary clinical experience in seven patients with refractory MRSA bacteremia, using a combination of daptomycin plus the anti-staphylococcal β-lactams, nafcillin or oxacillin [22]. In that report, we demonstrated a net reduction in the in vitro bacterial surface charge induced by diverse anti-staphylococcal β-lactams, with an associated enhancement of daptomycin binding to the staphylococcal envelope [22]. Although the native daptomycin molecule is an anionic lipopeptide, it complexes in vivo with divalent calcium (Ca2+) to become positively charged [23], and target bacterial membranes in a fashion similar to cationic HDPs [24, 25]. Indeed, in recent analyses of MRSA strains that develop resistance to daptomycin during the course of therapy, concomitant resistance to cationic HDPs has been noted [26, 27]. We hypothesized that anti-staphylococcal β-lactams might also sensitize MRSA to enhanced clearance by innate HDPs and the host cells that deploy them.
In the current study, we explore the capacity of the commonly prescribed β-lactam agent nafcillin to sensitize a cadre of MRSA strains to killing by cathelicidin and other prototypical HDPs. The relevance of these outcomes is extended to ex vivo analyses of whole blood, neutrophil and keratinocyte killing, and the effect of nafcillin exposure on MRSA survival is examined in a murine challenge model. For this foremost of contemporary bacterial pathogens, the combined results highlight potential utilities of a safe existing antibiotic otherwise dismissed by standard antimicrobial testing.
Methods
Bacterial strains
Daptomycin-susceptible USA100 MRSA D592 and its isogenic daptomycin-nonsusceptible, vancomycin-intermediate (VISA) strain, D712, from the Case Study and described previously [22]. Additional well-characterized MRSA strains studied were: ATCC33591, TCH1516 (USA300), UAMS 1182 (USA300), Sanger 252 (USA200), VRSA-MI and VRSA-PA (exhibiting high-level vanA-mediated vancomycin resistance), and VISA-NJ HIP5836, obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA), http://www.narsa.net/control/member/repositories
Antimicrobial peptide killing assays
Cationic host defense peptides differing in anatomic and host source, molecular mass, net charge at pH 7.5, and proposed mechanism(s) of action were studied. Human cathelicidin LL-37 (net charge +6 at pH 7.5) and the murine cathelidicin, mCRAMP (net charge +6 at pH 7.5) were purchased from AnaSpec, Inc. (Fremont, CA); human neutrophil α-defensin-1, hNP-1 (net charge +3 at pH 7.5) was purchased from Peptide International (Louisville, KY); and RP-1 (a synthetic 18 amino-acid congener modeled after the α-helical microbicidal domain of the platelet factor IV family of peptides; net charge +8 at pH 7.5) was synthesized and authenticated as previously detailed [28]. The concentrations of HDPs utilized in the formal killing assays were based on extensive pilot studies, and represented levels that did not rapidly kill the starting inoculum in the absence of antibiotic pre-sensitization.
Bacteria were grown to stationary phase (14–16 hr) at 37°C with shaking in 5mL of antibiotic-free LB broth or LB containing varying sub-inhibitory concentrations of the antibiotic-of-interest. Beta-lactams were purchased from Sigma-Aldrich (St. Louis, MO), while anti-MRSA antibiotics vancomycin, linezolid, and daptomycin were purchased from the University of California, San Diego hospital pharmacy. For antibiotics with in vitro activity against MRSA and used clinically to treat MRSA infections, a concentration of 0.25 × MIC was used. Bacteria were pelleted by centrifugation for 10 min, washed in 5 mL sterile phosphate-buffered saline (PBS), and resuspended to an OD600nm of 0.5, approximating 108 cfu/mL. Bacteria were then serially diluted to ~104 cfu/mL using RPMI + 5% LB media and deployed in specific antimicrobial peptide killing assays at a final density of 103 cfu/mL in 100 uL in RPMI + 5% LB. Details of these HDP killing assays have been previously published [22]. At time 0 and after selected exposure time-points, 10 uL samples were plated on tryptic soy agar (TSA) plates and colonies enumerated after 24 h. HDP exposure times were optimized, based on a number of pilot studies as either 60 min (LL-37; mCRAMP) or 90 min (RP-1; hNP-1). The mean percentage survival (± SD) was quantified and expressed in relation to cfus at time 0.
Cathelicidin LL-37 binding studies
MRSA Sanger252 was grown to OD600nm 0.6–0.8 in phenol-free RPMI media with and without nafcillin 5 or 10 mg/L to which was then added rhodamine-labeled LL-37 (Phoenix Pharmaceuticals, Burlingame, CA) at 1 µM (5 mg/L). After 30 minutes at 37°C with shaking 200 rpm, cells were washed 3 times with phenol-free RPMI. The final wash contained 1 mg/L DAPI (4',6-diamidino-2-phenylindole) nucleic acid stain and the cells were visualized microscopically [22].
Whole blood killing assays
Blood from healthy donors was collected using 50µg/mL lepirudin (Refludan, Schering, Kenilworth, NJ) as anti-coagulant to preserve complement activity [29]. MRSA strains 592, 712 and Sanger 252 were grown to log phase in either 20 µg/mL nafcillin or media alone, and 104 cfu were incubated in a total volume of 400 µL blood, rotating at 37°C. After 60 min., 25 µL was removed and blood was lysed in water. Remaining bacteria were enumerated on Todd-Hewitt agar (THA) plates and survival was calculated as the percentage of the initial inoculum. Experiments were performed using blood from at least three individual donors. These studies were approved by the University of California San Diego Human Research Protections Program.
Neutrophil killing assays
Neutrophils were freshly isolated from the blood of healthy donors using PolyMorphPrep-kit (Fresenius Kabi), and erythrocytes lysed with sterile H2O as previously described [30]. Organisms prepared identically to those used in the whole blood killing assays above were grown to log phase in 20 µg/ml (D592 and D712) or 2 µg/ml (Sanger 252) nafcillin, 0.5 µg/ml vancomycin, 0.1 µg/ml ceftaroline or media alone (untreated) and incubated at a multiplicity of infection (MOI; MRSA:neutrophil ratio) = 1, with 5 × 105 neutrophils in RPMI containing 2% (vol/vol) of 70°C heat-inactivated FBS [31]. After 90 min, incubation at 37°C + 5% CO2, cells were lysed with 0.025% Triton X-100, and the total number of remaining bacteria were enumerated on Todd-Hewitt agar (THA) plates. Survival was calculated as the mean percentage (± SD) of the initial inoculum. Where indicated, in parallel studies, neutrophils were treated with 10 µg/mL diphenyleneiodonium chloride (DPI, Sigma) vs. vehicle control (DMSO) for 1 h prior (in order to block the respiratory burst), or 5 µg/mL cytochalasin D (cyt D, Sigma) vs vehicle control (DMSO) for 10 min prior to the addition of bacteria (in order to block phagocytosis); survival was calculated as the mean percentage (± SD) of the bacterial growth control, grown under the same conditions without neutrophils. Experiments were performed using blood from at least three individual donors. These studies were approved by the University of California San Diego Human Research Protections Program.
Neutrophil phagocytosis
In order to assess the impacts of nafcillin pretreatment on neutrophil phagocytic capacity, neutrophils prepared as described above were resuspended to 106 cells/mL in Opti-MEM culture medium (Invitrogen, Grand Island, NY) containing 1 mg/mL pHrodo S. aureus BioParticles Conjugates (Invitrogen, Grand Island, NY) prepared according the manufacturer instructions. Samples were prepared with 0, 5, or 20 µg/ml nafcillin. Each sample was aliquoted 100 µL in triplicate to 96 well plates (105 cells/well), plates were centrifuged 350 × g for 5 min, and incubated at 37°C with 5% CO2. Fluorescence was measured every 15–30 minutes at 544 nm excitation/590nm emission.
H2O2 sensitivity assays
To quantify the impact of β-lactam pre-exposures on subsequent resistance to oxidative stress, MRSA Sanger 252 was grown overnight to stationary phase (12–16 hr) in LB broth with or without sublethal nafcillin. Cells were pelleted, washed in PBS, and resuspended in PBS containing 1.5% H2O2. After 90 minutes, cells were diluted and plated on THA plates, incubated overnight, and viable cfus enumerated to determine mean percent of surviving bacteria (± SD). A minimum of 3 independent assays was carried out.
HaCaT Keratinocyte killing assay
We used HaCaT keratinocyte cells to examine the effects of nafcillin on the cathelicidin-mediated anti-staphylococcal activity of keratinocytes. HaCaT keratinocyte cells passaged in the absence of antibiotics were seeded 2.5 × 105 onto 24-well tissue culture plates. After 24 hr, cells were washed with 1× PBS and 450 µL RPMI + 2% 70°C heat-inactivated FBS was added to each well. MRSA Sanger 252 was grown to log phase (OD 600nm 0.5) in LB broth, resuspended in PBS to OD 600nm 0.4, and diluted in PBS such that 50 µL added to each well (this represented 2.5x × 105 cfu [MOI = 1.0]; or 2.5 × 104 cfu [MOI = 0.1]). Bacteria were centrifuged onto cells at 350× g for 10 min and incubated at 37°C in humidified 5% CO2 incubator for 2 hr. At the end of the incubation, 50µl 0.25% Triton X-100 was added to each well, cells were removed with a cell scraper, resuspended in the media, diluted 1:10–10,000, and 25 µL plated onto THA plates for bacterial enumeration. Experiments were performed twice in triplicate, and results expressed as the ratio of bacterial cfu in HaCaT/bacterial cfu in cell-free control.
Animal model of MRSA cutaneous infection
We used a murine subcutaneous infection model in order to translate our in vitro and ex vivo studies above to in vivo relevance. For ex vivo antimicrobial exposure studies in these investigations, MRSA Sanger 252 was grown overnight to stationary phase in 40 mL of antibiotic-free LB or LB containing sublethal nafcillin (5 µg/ml) or vancomycin (0.25 µg/ml). Bacteria were then washed in 40 mL PBS, and resuspended in 2 mL of PBS + 2 mL of Cytodex beads (1 µg/ml), yielding approximately 1010 cfu/ml. Bacterial inocula were confirmed for bacteria grown in the presence or absence of antibiotic, with less than 2-fold variability in colony counts and identical OD600nm. We point out that we have repeatedly observed slightly lower CFU/OD600nm ratios for nafcillin-grown MRSA due to increases in cellular aggregation by microscopy. Then, 0.1 mL of the bacterial suspension was injected subcutaneously (sc) into 8 week old (~25 g) female CD1 mice, and developing lesion sizes were manually measured for 3 days and expressed as mm2. For in vivo antimicrobial exposure studies, MRSA Sanger 252 grown in antibiotic-free LB broth was prepared and injected sc as above into female CD1 mice that received either sterile PBS or nafcillin 2.5 mg (100 mg/kg) sc, dosed 8, 4, and 0.5 hr before bacterial injection, 4 hr after the bacterial injection, and every 12 hr thereafter for 3 days. Lesion sizes were recorded on days 2–4 after bacterial injection, animals were sacrificed and their lesions harvested and quantitatively cultured for enumeration of surviving cfu (± SD) at day 4. All animal studies were performed under protocols that were reviewed and approved by the UCSD Institutional Animal Use and Care Committee. All animal work in this investigation was performed in accordance with national and local guidelines that are in place to maximize humane animal treatment.
Statistics
Data were analyzed using GraphPad Prism 5.03 (GraphPad Software). Analyses were performed using either the nonparametric Kruskall-Wallis, one-way ANOVA with Tukey’s post-test multiple comparison modification, or the unpaired Student’s t test where appropriate. Correlation analysis was performed using Spearman’s rho where indicated. P values < 0.05 was considered statistically significant.
Case Study
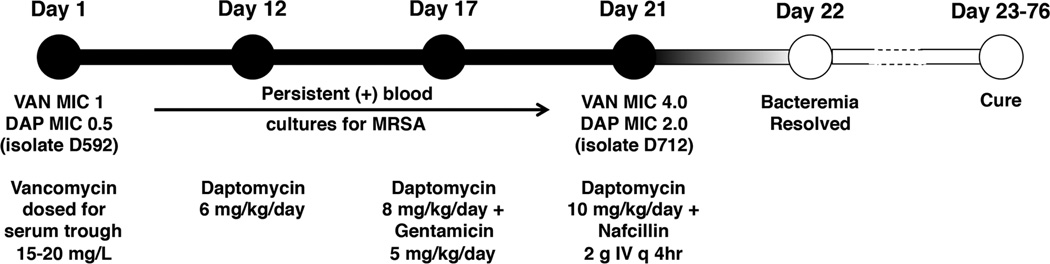
A 55 year-old male was admitted to a hospital in New York with fever, cough and dysuria. He had a prior history of poorly controlled diabetes mellitus, emphysema, tracheomalacia, hypothyroidism, idiopathic thrombocytopenia on >15 years of steroids, severe osteoporosis with concomitant multiple pathogenic fractures, and left foot osteomyelitis requiring below knee amputation one year prior. His clinical course was characterized by prolonged and persistent MRSA bacteremia (Fig. 1), though beginning on admission he underwent an exhaustive diagnostic workup that was unrevealing for any possible endovascular or surgical focus. After 21 d of persistent positive MRSA blood cultures, he was placed on the combination of high dose daptomycin and nafcillin resulting in resolution of bacteremia within 48 h. Although increased daptomycin MIC at day 21 was determined during retrospective analysis of the MRSA isolate, the combination therapy was continued since the patient had a sustained clinical and microbiological response. Other antibiotic choices were limited by thrombocytopenia, resistance to rifampin early in the course of bacteremia, and nephrotoxicity associated with gentamicin. On day 28 of his hospitalization, he was radiologically diagnosed with sacral osteomyelitis. Nafcillin was transiently changed to piperacilllin/tazobactam to treat nosocomial Pseudomonas aeruginosa pneumonia, and prior to discharge patient was also placed on trimethoprim/sulfamethoxazole for pneumocystis (PCP) prophylaxis. No adverse events associated with antibiotics were noted for duration of combination therapy. Of note, expert opinion suggests that high-dose daptomycin (10 mg/kg/day) is recommended for vancomycin-refractory MRSA bacteremia [21], and underdosing early in the course may have been a contributing factor to the protracted bacteremia.
Fig. 1.
Timeline of case study of refractory MRSA bacteremia. 55-year old male with multiple pre-existing medical conditions on steroid therapy who suffered prolonged and persistent MRSA/VISA bacteremia for 21 days failing vancomycin, daptomycin, and daptomycin + aminoglycoside therapy, but resolving upon addition of the β-lactam nafcillin to daptomycin. Isolates D592 (daptomycin-susceptible) and D712 (daptomycin-nonsusceptible) were subjected to in vitro analysis.
Results
Nafcillin and other β-lactam antibiotics sensitize MRSA to killing by LL-37 and other mammalian HDPs
To explore potential host factors in the dramatic clinical response of the Case Study, we tested how exposure to nafcillin affected the activity of human cathelicidin LL-37 against the patient’s original (strain D592, daptomycin- and vancomycin-susceptible) and the day 21 (strain D712, daptomycin-nonsusceptible and vancomycin-intermediate-resistant) MRSA isolates. The concentration of LL-37 can be severely increased at sites of inflammation, and levels up to 25 µg/ml have been reported in bronchoalveolar lavage fluid from infants with pulmonary infections [32] and cystic fibrosis patients [33] and much higher levels (over 1 mg/ml) has been reported from inflammatory skin lesions (e.g. psoriasis) [34]. A variety of different heat-killed Gram-positive and Gram-negative bacterial species induced production of 1 to 8 g/ml of LL-37 from freshly isolated human neutrophils in upon 24 h in vitro exposure [35].
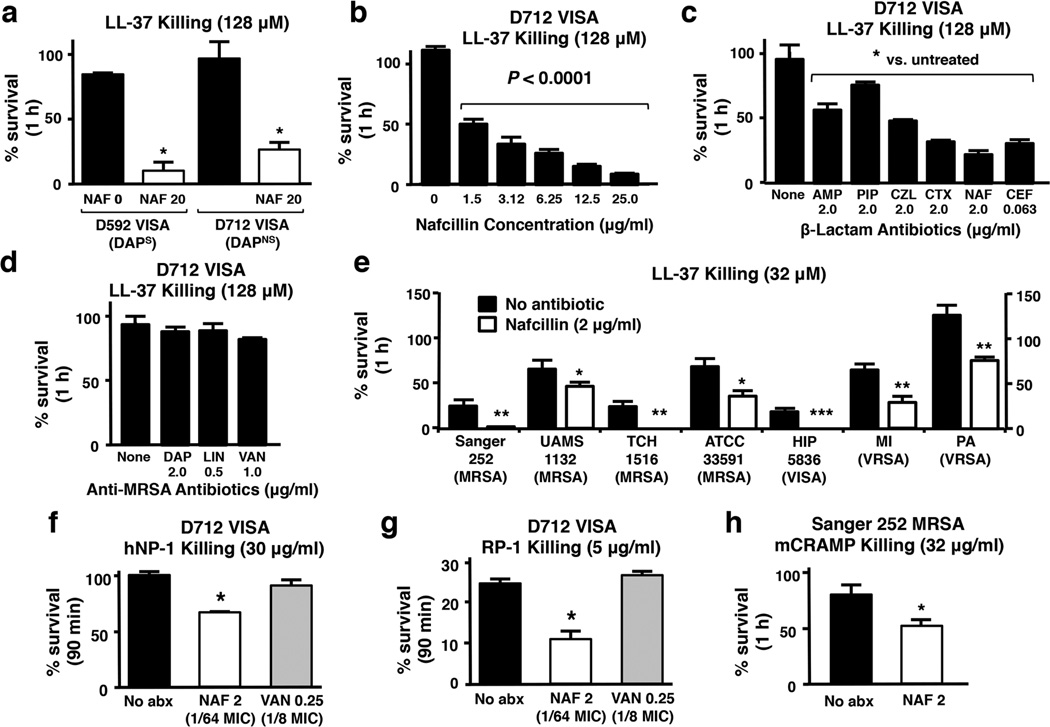
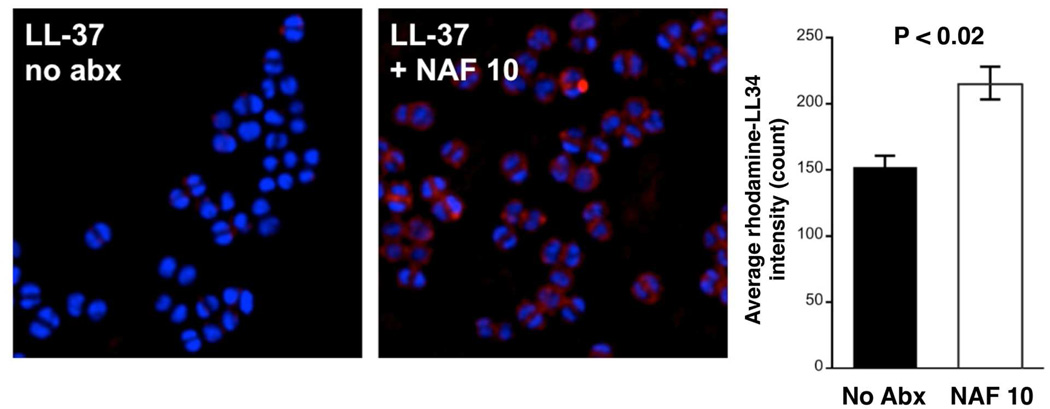
While the direct MIC for nafcillin against each isolate by standard testing was > 128 µg/ml, exposure to 20 µg/ml of nafcillin sensitized both MRSA strains to LL-37 killing (Fig. 2a). In growth curves monitored by spectrophotometry (OD600nm), no appreciable effects on growth were noted for D712 at nafcillin < 20 mg/L. At nafcillin concentrations 20 mg/L-128 mg/L, there were dose-dependent reductions in growth rate appreciated and incremental reductions in final cell density at 24 h. Sensitization to LL-37 killing by nafcillin was dose-dependent, and more pronounced with increasing antibiotic concentration (Fig. 2b); however, significant synergistic effects were noted even at extremely low nafcillin concentrations (i.e. at 0.01 × MIC = 1.5 µg/ml). This effect extended to a variety of other β-lactam antibiotics including: ampicillin (AMP), piperacillin (PIP), cefazolin (CZL), ceftriaxone (CTX) and ceftaroline (CEF). Growth of the VISA D712 strain in these antibiotics (2 µg/ml, except for the anti-MRSA cephalosporin CEF used at 0.25 × MIC = 0.063 µg/ml) led to a significant increase in killing by LL-37, with the outcomes for CEF and CTX being the most pronounced (Fig. 2c). In contrast, growth at proportionately higher (0.25–0.50 × MIC) of three prototypical non-β-lactam, anti-MRSA agents, each with a distinct mechanism of action, produced no significant sensitizing effect to LL-37 killing for MRSA D712: vancomycin (a cell wall-active glycopeptide); daptomycin (a cell membrane-targeting lipopeptide) and linezolid (a ribosomal-targeting oxazolidinone) (Fig. 2d). To verify that the effect of nafcillin was not MRSA strain-dependent, we repeated the above HDP killing assays using a panel of well-characterized MRSA strains, including both hospital-and community-associated isolates grown in LB media, with or without sub-MIC nafcillin (2 µg/mL = 0.016 × MIC). Although the baseline susceptibility to LL-37 killing varied from strain-to-strain, prior growth in sublethal nafcillin markedly and uniformly increased LL-37 killing in all strains (Fig. 2e). To show that the principle of sensitization extended to other cationic HDPs, growth in sublethal nafcillin, but not in vancomycin, sensitized strain D712 to killing by the α-defensin, hNP-1 (Fig. 2f) and the synthetic cationic platelet HDP congener, RP-1 (Fig. 2g). It should be noted that even in the absence of nafcillin pre-sensitization, strain D712 was very susceptible to killing by RP-1 (~75% reduction from baseline). This potency was further enhanced by nafcillin (Fig. 2g). By comparison, LL-37 efficacy against this strain was minimal. Finally, as a prelude to murine testing, we found that sublethal nafcillin pre-exposures sensitized MRSA strain Sanger 252 [36] to killing by cathelicidin mCRAMP (Fig. 2h), an essential component of host defense against invasive bacterial skin infection in mice [37]. Lastly, rhodamine-labeled human cathelicidin LL-37 exhibited significantly greater binding to the surface of MRSA Sanger 252 grown in 10 µg/mL of nafcillin compared to the same strain grown in antibiotic-free media (Fig. 3).
Fig. 2.
Nafcillin and additional β-lactam antibiotics sensitize MRSA to killing by LL-37 and other mammalian HDPs Sequential MRSA/VISA blood stream isolates D592 (daptomycin-susceptible) and D712 (daptomycin-nonsusceptible) in LL-37 killing assays (128 µM, 1h) (a) when grown to stationary phase in LB broth containing no antibiotic or nafcillin 20 µg/ml or (b) when grown to stationary phase in LB broth containing varying concentrations of nafcillin, analyzed statistically by Spearman’s rho. (c) Growth of MRSA/VISA D712 in media containing various β-lactam antibiotics at stated concentrations with consequent effects on LL-37 killing (128 µM, 1h); ampicillin (AMP), piperacillin (PIP), cefazolin (CZL), ceftriaxone (CTX), nafcillin (NAF), CEF (ceftaroline). (d) Growth of MRSA/VISA D712 in media containing anti-MRSA antibiotics at 0.25 × MIC with consequent effects on LL-37 killing (128 µM, 1h); daptomycin (DAP), linezolid (LIN), vancomycin (VAN). Results in A–D are expressed as mean % survival ± SEM of 3 independent experiments. (e) Various MRSA strains grown to stationary phase in LB broth containing no antibiotic or nafcillin (NAF) 2 µg/ml and tested for survival after 1 h in LL-37 (32 µM); results expressed as mean % survival ± SEM of 4 independent experiments. Percent survival at 90 min of MRSA D712 upon exposure to (f) α-defensin human neutrophil peptide-1 (hNP-1, 30 µg/ml) or (g) synthetic platelet-related HDP RP-1 (5 µg/ml) after growth to stationary phase in no antibiotic, nafcillin (NAF) 2 µg/ml (0.016 × MIC), or vancomycin 0.25 µg/ml (0.125 × MIC). (H) Percent survival of MRSA Sanger 252 in murine cathelidin mCRAMP (32 µM, 1 h) after growth in antibiotic-free LB broth vs. LB + nafcillin (NAF) 2 µg/ml. Results of F–H are expressed as mean % survival ± SEM of 2 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. Statistical analysis by Mann-Whitney U test.
Fig. 3.
Nafcillin increases surface binding of human cathelicidin LL-37. MRSA Sanger 252 was grown to late log phase in phenol-free RPMI with nafcillin 10 µg/mL (left) or without antibiotic (right) and subjected to rhodamine-labeled human cathelicidin LL-37 (red stain) for 30 min. After 3 washes, the cells were counterstained with DAPI nucleic acid stain (blue stain) and visualized microscopically. Significantly more LL-37 was noted on the surface of nafcillin grown MRSA.
Nafcillin sensitizes MRSA to killing by human whole blood, neutrophils and keratinocytes
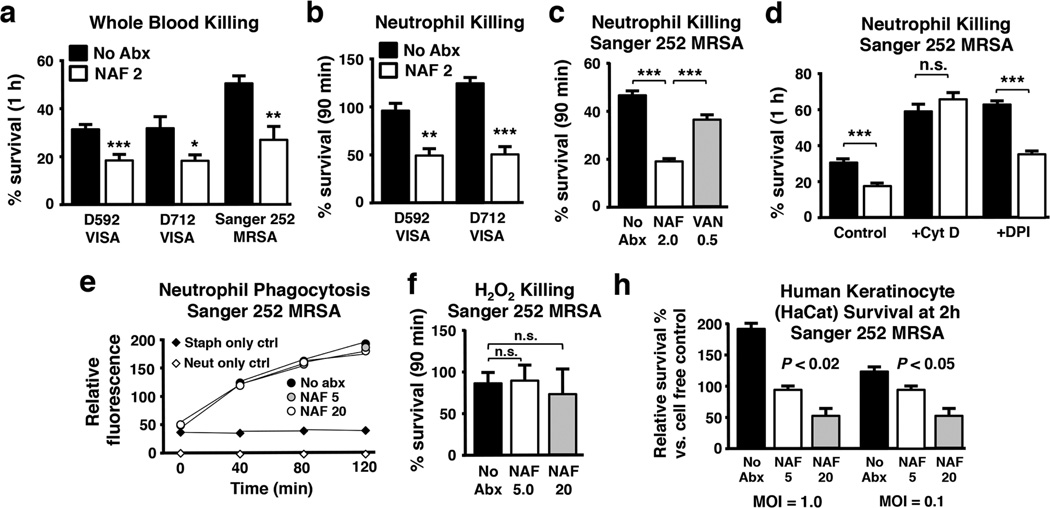
To further analyze the unanticipated effect of adjunctive nafcillin therapy to eradicate persistent MRSA bacteremia [22], we undertook specific ex vivo studies. Neutrophils are the most abundant leukocyte in blood circulation, and harbor abundant stores of cationic HDPs, LL-37 and hNP-1, within the specific and azurophilic granules, respectively [38, 39]. Blood platelets are also a rich source of small cationic HDPs, especially those released from α-granules upon thrombin activation [40, 41]. We found that MRSA prior growth in sublethal nafcillin (~0.016 × MIC) enhanced sensitivity of the strain pair, D592/D712, as well as MRSA Sanger 252 to whole blood killing (Fig. 4a) and killing by human neutrophils (Fig. 4b, c). In contrast, enhancement of neutrophil killing was modest in the presence of MRSA strain Sanger 252 preexposed to sublethal vancomycin (0.5 × MIC) (Fig. 4c). Increased neutrophil killing following sublethal nafcillin likely involved an intracellular mechanism, since blockage of phagocytosis using cytochalasin D eliminated the observed nafcillin-sensitizing effect (Fig. 4d). However, nafcillin itself did not significantly alter the rate of phagocytic uptake of fluorescently-labelled MRSA (Fig. 4e).
Fig. 4.
Nafcillin sensitizes MRSA to killing by human whole blood, neutrophils and keratinocytes. Clinical isolates of MRSA grown in 20 µg/mL nafcillin are more susceptible to killing by (a) human whole blood (60 min) or (b) isolated neutrophils (90 min). (c) MRSA Sanger 252 grown in 2 µg/mL nafcillin is more sensitive to neutrophil killing than bacteria grown in 0.5 µg/mL vancomycin. (d) Sensitivity to neutrophil killing is mediated by an intracellular mechanism, as the bacteria survive equally well in the absence of phagocytosis (cytochalasin D treatment), but is independent of oxidative burst (DPI treatment). Nafcillin exposure does not affect neutrophil phagocytosis of MRSA (e) nor sensitivity of MRSA to hydrogen peroxide killing (f). For each panel, data are shown as the mean ± SEM of 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparison post-test (C, E) or unpaired t test (A, B, D). (g) Nafcillin sensitized MRSA to killing by human keratinocyte cell line HaCaT, analyzed statistically by Spearman’s rho.
Cathelicidin LL-37 exerts its anti-staphylococcal activity within the phagolysosome [42], and the effect of nafcillin sensitization of MRSA to killing by LL-37 and other neutrophil HDPs (e.g. hNP-1) likely plays a major role in neutropihls intracellular killing. Notably, nafcillin sensitization to neutrophil killing was preserved even when oxidative burst funtion was blocked pharmacologically by diphenyleneiodonium (DPI) (Fig. 4d); furthermore, growth in sublethal nafcillin did not increase the susceptibility of MRSA to hydrogen peroxide killing (Fig. 4f). Keratinocytes also produce abundant cathelicidin HDP in response to injury or bacterial infection, contributing in a critical fashion to the cutaneous innate immune barrier [14, 43]. Prior growth in sublethal nafcillin increased the susceptibility of MRSA to killing by human keratinocyte cell line HaCaT (Fig. 4g).
Nafcillin modifies MRSA pathogenesis in a murine subcutaneous infection model
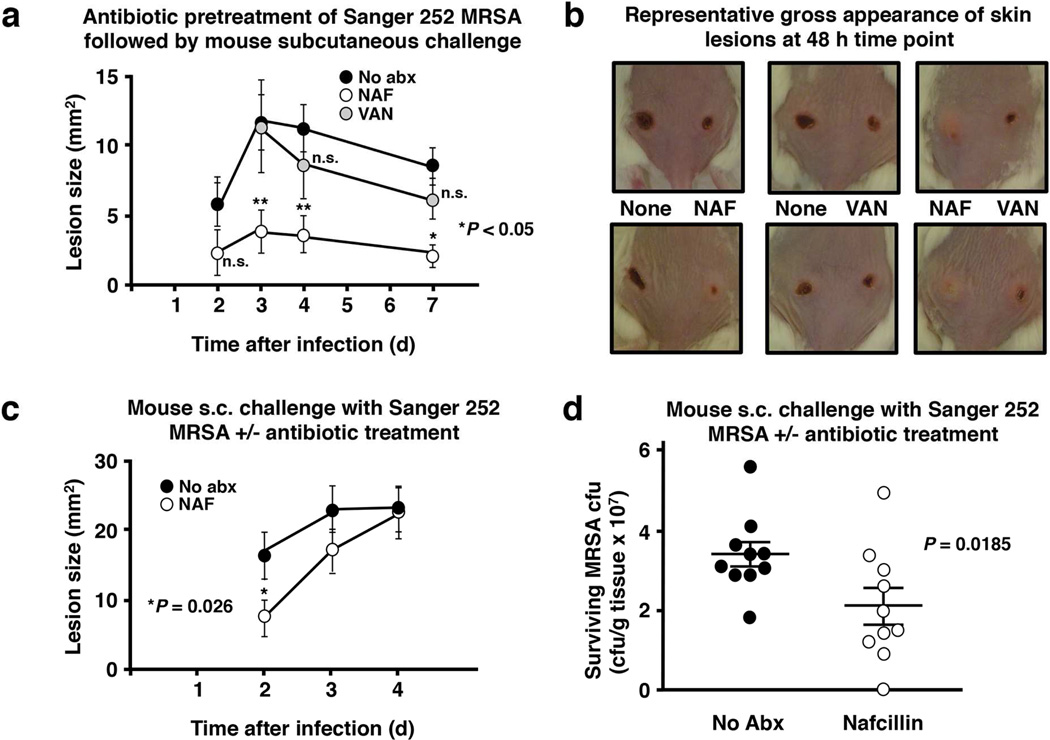
We next determined whether nafcillin could sensitize MRSA for host defense-mediated clearances in vivo, paralleling the observed effects in vitro (Fig. 2 and 3) and ex vivo (Fig. 4). In a first experiment, female CD-1 mice were infected subcutaneously on their bilateral flanks with MRSA Sanger 252 that had either been grown to stationary phase in antibiotic-free LB or LB containing either sublethal nafcillin (5 µg/mL) or sublethal vancomycin (0.25 µg/mL). Mean size (mm2) of the resulting necrotic skin lesions at days 2–4 were significantly smaller in the nafcillin-pre-exposed MRSA challenge group as compared to the vancomycin-pre-exposed or no antibiotic control challenge groups (Fig. 5a, b). In a second set of experiments, nafcillin or PBS controls were administered to groups of mice that were challenged with MRSA 252 not pre-exposed to nafcillin, but in whom nafcillin was administered systemically before and after induction of infection. Lesions were significantly smaller in the nafcillin-treated groups vs controls at day 2 of treatment (Fig. 5c). However, perhaps as a result of the increased dosing interval of nafcillin from every 4 h to every 12 h after the bacterial inoculum was administered, this difference was not durable over the following two days of observation. A modest, but statistically significant reduction in lesional MRSA burden (cfu/g tissue) was also observed in the nafcillin treatment group upon lesion harvest at day 4 (Fig. 5d).
Fig. 5.
Nafcillin exposure modifies MRSA pathogenesis in a murine subcutaneous infection model. (a) Growth of MRSA Sanger 252 to stationary phase in LB broth containing no antibiotic (control), nafcillin 5 µg/ml (NAF), or vancomycin 0.25 µg/ml (VAN) and subsequent subcutaneous injection (1 × 109 CFU) into the shaved flanks of female CD-1 mice, showing significantly smaller lesions produced by NAF-treated MRSA at 2 d (P = 0.006), 3 days (P = 0.03), 4 days (P = .004), and 7 days (P= 0.01); representative lesions at 48 hours shown in (b). (c) Lesions sizes of female CD-1 mice treated with nafcillin (see methods for dosing regimen) or PBS control and infected with MRSA; (d) CFU/g of MRSA recovered from skin lesions harvested from animals at 96 h time point. Statistical analysis by Mann Whitney U.
Discussion
For the past several decades, clinical medicine has observed the emergence of multidrug-resistant pathogens of increasing concern [44–46]. Alarm over antimicrobial resistance among Gram-negative bacteria centers on the paucity of novel compounds with activity against increasingly common Acinetobacter baumanii and carbapenem-resistant Enterobacteriaciae. For Gram-positive bacteria in general, and for MRSA in particular, the concern is less centered on the lack of novel antibiotics, but rather on the millions of infections caused in both healthcare and community settings, with increasing adverse consequences individually to patients and to the healthcare system overall. Despite the fact that the U.S. Food and Drug Administration has approved five new antibiotics for treatment of MRSA infection in the last 12 years, data consistently show increased mortality of MRSA bacteremia compared to MSSA bacteremia [6, 7]. Invasive MRSA infection was recently estimated to account for more deaths per year in the U.S. than HIV/AIDS [47]. In infections caused by MRSA, clinicians have had no choice but to resort to “second or third tier” antimicrobials, in particular vancomycin, bemoaning extensive data and common appreciation of the superiority of β-lactam antibiotics over glycopeptides and other classes as anti-staphylococcal agents for MSSA [48, 49].
In the current study, we have shown that nafcillin, at concentrations far below MIC, sensitize MRSA to killing by human cathelicidin LL-37 and other cationic HDPs, in association with increased LL-37 binding to the MRSA cell envelope. Nafcillin-mediated sensitization of MRSA was subsequently observed in killing assays where enhanced HDP killing is anticipated, such as whole blood, neutrophils, and keratinocytes. This effect of sensitization to LL-37-mediated killing, while induced at various degrees by all other β-lactams tested, was not induced by sub-MIC concentrations of other commonly prescribed non-β-lactam, anti-MRSA antibiotics, in particular vancomycin, consistent with prior observations [19]. Along these lines, we recently reported that addition of the β-lactam ampicillin to ongoing daptomycin therapy led to rapid clearance of a protracted bacteremia caused by ampicillin- and vancomycin-resistant Enterococcus faecium (VRE) and potentiated killing of the isolate by LL-37 and other cationic HDPs [50]. Thus it appears that β-lactams, unlike glycopeptides, may exert their in vivo anti-staphylococcal (and anti-enterococcal) activity in two ways: in vivo: (a) directly against susceptible organisms, and (b) indirectly by boosting the pharmacodynamic effects of endogenous antimicrobial HDPs against both non-susceptible and susceptible organisms. This may at least partially explain the clinical superiority of β-lactams over vancomycin against MSSA, and underscores the importance of prioritizing β-lactam therapy over glycopeptide therapy in serious MSSA infections [49].
While detailed mechanism of this “sensitizing” effect is still unknown, it has been hypothesized that β-lactams may prompt the release of lipoteichoic acid (LTA) from the cell envelope, which, could either increase cell wall autolysin activity or reduce substrate availability for dlt-mediated LTA D-alanylation. A lack of D-alanylated LTA may in turn enhance relative net negative envelope charge and thus increase susceptibility to killing by cationic daptomycin and HDPs [51]. Critical events defining β-lactam and HDP interaction may also lie in the divisome complex. Daptomycin insertion into the Gram-positive membrane results in patches of membrane defects triggering recruitment of cell division proteins such as divIVA to these sites [52]. Pharmacological inhibition of the ClpXP protease that regulates the cell division protein FtsZ increases S. aureus susceptibility to daptomycin and LL-37 [53], while inhibition of FtsZ directly can restore methicillin susceptibility to MRSA [54].
These current data, along with our previously published case series [22], suggest significant potential therapeutic benefit of using β-lactams as adjunctive MRSA therapy in combination with daptomycin or other possibly anti-MRSA antibiotics. In addition, these data suggest that the choice of surgical antibiotic prophylaxis in patients who are colonized with MRSA deserves further scrutiny. While clinicians have frequently used vancomycin prophylaxis in this setting, our present data, particularly in the animal model, support the notion that β-lactams may more effective in preventing the establishment of an MRSA infection than in vitro susceptibility metrics might suggest [55].
While there have been extensive reporting of drug-drug interactions for synergies and antagonisms between traditional antibiotics, few such studies have explored such interactions between conventional with endogenous antibiotics (i.e., HDPs). An early report by Yeaman et al. [56] suggested that platelet microbicidal proteins could synergize with and prolong the post-antibiotic effect of cell wall-acting antibiotics against S. aureus. Conversely, Kristian et al demonstrated that various bacteriostatic antibiotics (e.g. erythromycin, tetracycline, etc.) acted antagonistically to impair killing of Gram-positive and –negative bacteria by LL-37 and other HDPs, as well as by serum and whole blood [57].
As our previous case series outlined, the addition of an anti-staphylococcal β-lactam to daptomycin therapy in refractory MRSA bacteremia resulted in rapid bacteremia clearance [22]. We have continued to employ this combination successfully using cefazolin and ceftaroline in combination with daptomycin for refractory MRSA and MSSA bacteremias as well as refractory methicillin-resistant Staphylococcus epidermidis bacteremia in cases of infected left ventricular assist devices that cannot be removed (unpublished data). We originally attributed the benefit of this regimen to direct synergy between β-lactams and daptomycin against MRSA, a phenomenon recently confirmed by others and shown to prevent selection of daptomycin-resistant derivatives [58]. Moreover, recent clinical data suggests that for patients with underlying renal insufficiency and endovascular S. aureus infections, the addition of β-lactam to daptomycin may improve outcome compared to daptomycin monotherapy [59]. The current study provides an additional explanation for the clinical efficacy findings, i.e., the potential for boosting HDP-mediated and/or neutrophil-mediated killing of MRSA by nafcillin. Our findings indicate that β-lactam antibiotics deemed ineffective by conventional susceptibility testing could in fact have significant pharmacodynamic interactions with endogenous HDPs that translate to clinical utility.
One potential caution to this approach is that in vitro subinhibitory nafcillin can increase expression of S. aureus exotoxins, including toxic shock syndrome toxin-1, Panton-Valentine leukocidin, and α-hemolysin [60]. However, it is encouraging that concomitant β-lactams did not negatively influence clinical outcome in cases of MRSA bacteremia derived from soft tissue infection [59]. Larger scale prospective controlled clinical studies are needed to formally evaluate the clinical benefit of adjunctive β-lactam therapy and the optimal combination therapies for MRSA bacteremia.
Key Messages.
Nafcillin has been used as adjunctive therapy to clear persistent MRSA bacteremia
Nafcillin enhances killing of MRSA by a cadre of innate host defense peptides
Nafcillin increases binding of human cathelicidin LL-37 to the MRSA membrane
Nafcillin enhances killing of MRSA by polymorphonuclear leukocytes
Nafcillin reduces virulence of MRSA an a mouse subcutaenous infection model
Acknowledgements
We thank Anna Salvioni for assistance with peroxide assays. This research was supported by National Institutes of Health grants HD071600 (GS), AI057153 (VN), AI052453 (VN), R01GM073898 (JP), AI39108 (ASB), AI39001 (MRY), and AI48031 (MRY). CYO was supported through the UCSD/SDSU IRACDA Postdoctoral Fellowship Program (GM06852). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest G.S. has received speaking honoraria from Cubist, Forest, and Pfizer Pharmaceuticals, consulting fees from Cubist and Forest Pharmaceuticals, and research grant support from Forest Pharmaceuticals. A.S.B. has current research grants from Cubist Pharmaceuticals and Trius Therapeutics, and has received speaking honoraria from Cubist Pharmaceuticals. M.R.Y. is a founder of NovaDigm Therapeutics, Inc., and has participated in research programs supported in part by grants from Cubist Pharmaceuticals; V.N. was on the Scientific Advisory Board of Trius Therapeutics.
References
- 1.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 2.Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) Curr Opin Microbiol. 2012;15:588–595. doi: 10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Como-Sabetti K, Harriman KH, Buck JM, Glennen A, Boxrud DJ, Lynfield R. Community-associated methicillin-resistant Staphylococcus aureus: trends in case and isolate characteristics from six years of prospective surveillance. Public Health Rep. 2009;124:427–435. doi: 10.1177/003335490912400312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Cont Hosp Epidemiol. 2005;26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 7.Reed SD, Friedman JY, Engemann JJ, Griffiths RI, Anstrom KJ, Kaye KS, Stryjewski ME, Szczech LA, Reller LB, Corey GR, et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Cont Hosp Epidemiol. 2005;26:175–183. doi: 10.1086/502523. [DOI] [PubMed] [Google Scholar]
- 8.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 10.Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Ann Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 11.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins RR, David MZ, Salata RA. Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. J Med Microbiol. 2012;61:1179–1193. doi: 10.1099/jmm.0.043513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cederlund A, Gudmundsson GH, Agerberth B. Antimicrobial peptides important in innate immunity. FEBS J. 2011;278:3942–3951. doi: 10.1111/j.1742-4658.2011.08302.x. [DOI] [PubMed] [Google Scholar]
- 14.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peschel A, Collins LV. Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin. Peptides. 2001;22:1651–1659. doi: 10.1016/s0196-9781(01)00500-9. [DOI] [PubMed] [Google Scholar]
- 16.Kraus D, Peschel A. Staphylococcus aureus evasion of innate antimicrobial defense. Future Microbiol. 2008;3:437–451. doi: 10.2217/17460913.3.4.437. [DOI] [PubMed] [Google Scholar]
- 17.Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, Mond JJ, Tarkowski A, Foster SJ. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1:199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8:11–26. [PubMed] [Google Scholar]
- 19.Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, Yamazaki K, Sayama K, Taubman MA, Kurihara H, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–3739. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouhara K, Komatsuzawa H, Kawai T, Nishi H, Fujiwara T, Fujiue Y, Kuwabara M, Sayama K, Hashimoto K, Sugai M. Increased resistance to cationic antimicrobial peptide LL-37 in methicillin-resistant strains of Staphylococcus aureus. J Antimicrob Chemother. 2008;61:1266–1269. doi: 10.1093/jac/dkn106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 22.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis. 2011;53:158–163. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayer AS, Schneider T, Sahl HG. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann NY Acad Sci. 2013;1277:139–158. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilhena C, Bettencourt A. Daptomycin: a review of properties, clinical use, drug delivery and resistance. Mini-Rev Med Chem. 2012;12:202–209. doi: 10.2174/1389557511209030202. [DOI] [PubMed] [Google Scholar]
- 25.Tedesco KL, Rybak MJ. Daptomycin. Pharmacotherapy. 2004;24:41–57. doi: 10.1592/phco.24.1.41.34802. [DOI] [PubMed] [Google Scholar]
- 26.Mishra NN, Bayer AS, Moise PA, Yeaman MR, Sakoulas G. Reduced susceptibility to host-defense cationic peptides and daptomycin coemerge in methicillin-resistant Staphylococcus aureus from daptomycin-naive bacteremic patients. J Infect Dis. 2012;206:1160–1167. doi: 10.1093/infdis/jis482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra NN, McKinnell J, Yeaman MR, Rubio A, Nast CC, Chen L, Kreiswirth BN, Bayer AS. In vitro cross-resistance to daptomycin and host defense cationic antimicrobial peptides in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:4012–4018. doi: 10.1128/AAC.00223-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong YQ, Bayer AS, Elazegui L, Yeaman MR. A synthetic congener modeled on a microbicidal domain of thrombin- induced platelet microbicidal protein 1 recapitulates staphylocidal mechanisms of the native molecule. Antimicrob Agents Chemother. 2006;50:3786–3792. doi: 10.1128/AAC.00038-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 30.Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. D-alanylation of teichoic acids promotes group A Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol. 2005;187:6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Kockritz-Blickwede M, Chow OA, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–5246. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002;165:992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- 33.Chen CI, Schaller-Bals S, Paul KP, Wahn U, Bals R. Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros. 2004;3:45–50. doi: 10.1016/j.jcf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 35.Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB, Karimbux N, Napimoga MH, Seki M, Ouhara K, Sugai M, Taubman MA, et al. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006;146:218–225. doi: 10.1111/j.1365-2249.2006.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci USA. 2004;101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 39.Faurschou M, Sorensen OE, Johnsen AH, Askaa J, Borregaard N. Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochim Biophysica Acta. 2002;1591:29–35. doi: 10.1016/s0167-4889(02)00243-4. [DOI] [PubMed] [Google Scholar]
- 40.Yeaman MR. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–968. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 41.Yeaman MR, Bayer AS. Antimicrobial peptides versus invasive infections. Curr Top Microbiol Immunol. 2006;306:111–152. doi: 10.1007/3-540-29916-5_5. [DOI] [PubMed] [Google Scholar]
- 42.Jann NJ, Schmaler M, Kristian SA, Radek KA, Gallo RL, Nizet V, Peschel A, Landmann R. Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J Leuk Biol. 2009;86:1159–1169. doi: 10.1189/jlb.0209053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 45.Spellberg B. Antibiotic resistance and antibiotic development. Lancet Infect Dis. 2008;8:211–212. doi: 10.1016/S1473-3099(08)70048-3. [DOI] [PubMed] [Google Scholar]
- 46.Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(Suppl 5):S397–S428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 48.Chang FY, Peacock JE, Jr, Musher DM, Triplett P, MacDonald BB, Mylotte JM, O'Donnell A, Wagener MM, Yu VL. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine. 2003;82:333–339. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 49.Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, Thom KA, Cosgrove SE, Sakoulas G, Perencevich EN. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279. doi: 10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 2012;56:838–844. doi: 10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.al-Obeid S, Gutmann L, Williamson R. Correlation of penicillin-induced lysis of Enterococcus faecium with saturation of essential penicillin-binding proteins and release of lipoteichoic acid. Antimicrob Agents Chemother. 1990;34:1901–1907. doi: 10.1128/aac.34.10.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pogliano J, Pogliano N, Silverman JA. Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J Bacteriol. 2012;194:4494–4504. doi: 10.1128/JB.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGillivray SM, Tran DN, Ramadoss NS, Alumasa JN, Okumura CY, Sakoulas G, Vaughn MM, Zhang DX, Keiler KC, Nizet V. Pharmacological inhibition of the ClpXP protease increases bacterial susceptibility to host cathelicidin antimicrobial peptides and cell envelope-active antibiotics. Antimicrob Agents Chemother. 2012;56:1854–1861. doi: 10.1128/AAC.05131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan CM, Therien AG, Lu J, Lee SH, Caron A, Gill CJ, Lebeau-Jacob C, Benton-Perdomo L, Monteiro JM, Pereira PM, et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to beta-lactam antibiotics. Science Trans Med. 2012;4:126ra135. doi: 10.1126/scitranslmed.3003592. [DOI] [PubMed] [Google Scholar]
- 55.rawford T, Rodvold KA, Solomkin JS. Vancomycin for surgical prophylaxis? Clin Infect Dis. 2012;54:1474–1479. doi: 10.1093/cid/cis027. [DOI] [PubMed] [Google Scholar]
- 56.Yeaman MR, Norman DC, Bayer AS. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kristian SA, Timmer AM, Liu GY, Lauth X, Sal-Man N, Rosenfeld Y, Shai Y, Gallo RL, Nizet V. Impairment of innate immune killing mechanisms by bacteriostatic antibiotics. FASEB J. 2007;21:1107–1116. doi: 10.1096/fj.06-6802com. [DOI] [PubMed] [Google Scholar]
- 58.Mehta S, Singh C, Plata KB, Chanda PK, Paul A, Riosa S, Rosato RR, Rosato AE. Beta-lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother. 2012;56:6192–6200. doi: 10.1128/AAC.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moise PA, Amodio-Groton M, Rashid M, Lamp KC, Hoffman-Roberts HL, Sakoulas G, Yoon MJ, Schweitzer S, Rastogi A. Multicenter evaluation of the clinical outcomes of daptomycin with and without concomitant beta-lactams in patients with Staphylococcus aureus bacteremia and mild to moderate renal impairment. Antimicrob Agents Chemother. 2013;57:1192–1200. doi: 10.1128/AAC.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis. 2007;195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]