Introduction
Patients with haematological diseases occasionally exhibit liver dysfunction during treatment. This liver dysfunction can have various causes such as therapy-related drugs and hepatitis B and C infections, although the cause is unclear in some cases. It was recently reported that some patients initially diagnosed with drug-induced liver dysfunction actually had hepatitis E1. Several cases of transfusion-transmitted hepatitis E infections have also been reported1,2. In Japan, screening for hepatitis E does not appear to be performed at the initial examination of patients with acute hepatitis. This might be because hepatitis E is believed to be orally transmitted and to occur mainly in developing countries and rarely in developed countries. However, hepatitis E is a zoonotic infectious disease. Cases of regional endemic hepatitis E virus (HEV) infection have been increasing in Europe, the United States, and Japan1.
Although HEV usually causes self-limited acute hepatitis, it sometimes progresses to a chronic infection. Most cases of chronic infection occur in patients undergoing solid organ or haematopoietic stem cell transplantation, in those receiving anti-cancer or immunosuppressant drugs, and in patients with human immunodeficiency virus infection, in whom the condition may progress to liver cirrhosis3. HEV RNA persisted for a long period during treatment in a patient with T-cell lymphoma4. Reactivation of HEV hepatitis was reported after an allogeneic haematopoietic stem cell transplant in a patient with Philadelphia chromosome-positive acute lymphoblastic leukaemia5. On the other hand, a low risk of HEV reactivation after haematopoietic stem cell transplantation was also reported6. More studies on the risk of HEV reactivation are, therefore, required.
Here, we report the case of a patient with a myelodysplastic syndrome (MDS) who developed acute hepatitis due to transfusion-transmitted HEV infection. We also review the literature on the topic.
Case report
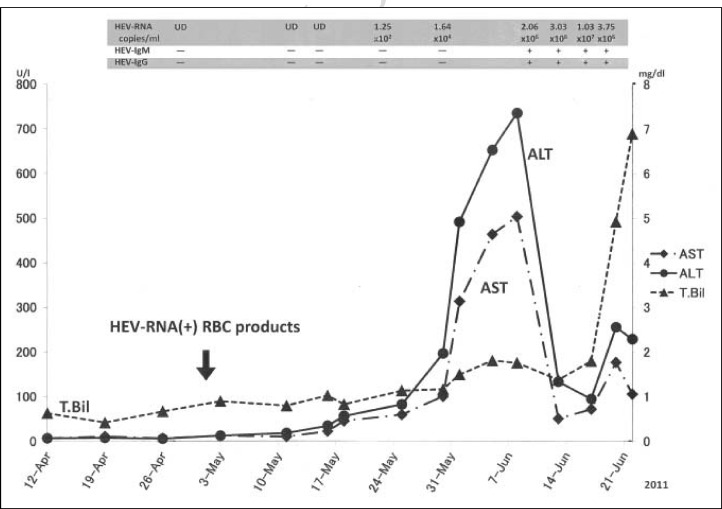
The patient was a 70-year old Japanese man who attended our hospital for Parkinson’s disease in June 2001. In July 2001, he was referred to the Haematological Department because of thrombocytopenia. Haematological examinations revealed that he had pancytopenia with a white blood cell count of 2.9×109/L, haemoglobin level of 9.0 g/dL, and a platelet count of 36×109/L. Bone marrow findings showed 8.8% myeloblasts and trilineage dysplastic features. Chromosome abnormalities with [46,XY, −10, +marker] were detected in 15 of 22 mitotic bone marrow cells. He was, therefore, diagnosed with MDS. According to the French-American-British criteria, he was classified as having refractory anaemia with excess of blasts (RAEB)-1 and was given a score of intermediate-2 according to the International Prognostic Scoring System at that time. Because he was suffering from Parkinson’s disease, he received combination therapy with oral vitamin K2 (menatetrenone, 45 mg/day) and vitamin D3 (alfacalcidol, 1 μg/day)7 instead of chemotherapy. This treatment resulted in no progression to leukemic transformation over the next 10 years. However, the pancytopenia gradually worsened, and protein anabolic steroids (metenolone, 20 mg/day) were added to the treatment in 2009. Over the next 12 months, he received repeated red cell and platelet transfusions because of anaemia and haemorrhagic symptoms. Bacterial infections often occurred during medical home care, and his Parkinson’s disease worsened. On April 28th, 2011, the patient was admitted to hospital with a lung abscess and aspiration pneumonia. He had a gastrointestinal bleed after admission to hospital and the volume of blood transfusions consequently increased. Although hepatic function was within the normal range on admission, serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) began to increase from May 18th, peaking at 504 and 736 IU/L, respectively, on June 8th. Although these levels decreased transiently, they increased again from June 20th together with a rise in total bilirubin level. On June 22nd, the patient died of exacerbation of the lung abscess (Figure 1).
Figure 1.
Clinical course of the patient. (UD: undetectable)
After the patient had died, the stocked plasma split from one of the donors of red blood cell (RBC) products given to our patient was screened for viruses before utilisation in plasma-fractionated products. The results revealed HEV RNA in the stocked plasma. We, therefore, performed complete examinations of the stocked donated blood and identified the HEV RNA-positive donor. The RBC product derived from this donor had been transfused into our patient on May 2nd. Serological examinations using the stored sera from this donor revealed an ALT level of 26 IU/L; the sera were negative for immunoglobulin (Ig)G anti-HEV and IgM anti-HEV assayed by enzyme immunoassay (IgG/IgM anti-HEV, Institute of Immunology, Tokyo, Japan) as well as hepatitis B virus (HBV) DNA and hepatitis C virus (HCV) RNA. The HEV RNA copy number quantitatively assayed with a TaqMan reverse transcription polymerase chain reaction (QIAGEN) was 1.2×103. These findings suggest that the blood had been donated during the “window period” of HEV infection in this donor.
We retrospectively investigated HEV RNA in our patient’s stocked sera. As shown in Figure 1, HEV RNA began to rise on May 23rd, peaked on June 16th, and started to decrease on June 18th. The patient’s serum from June 10th was found to be positive for IgG anti-HEV and IgM anti-HEV, whereas the sera stocked before May 30th were all negative for IgG anti-HEV and IgM anti-HEV. In addition, the results of viral examinations conducted when the patient presented with liver dysfunction were negative for HBV DNA, HCV RNA, cytomegalovirus and Epstein–Barr virus. These results strongly suggest that the patient had acquired transfusion-transmitted hepatitis E.
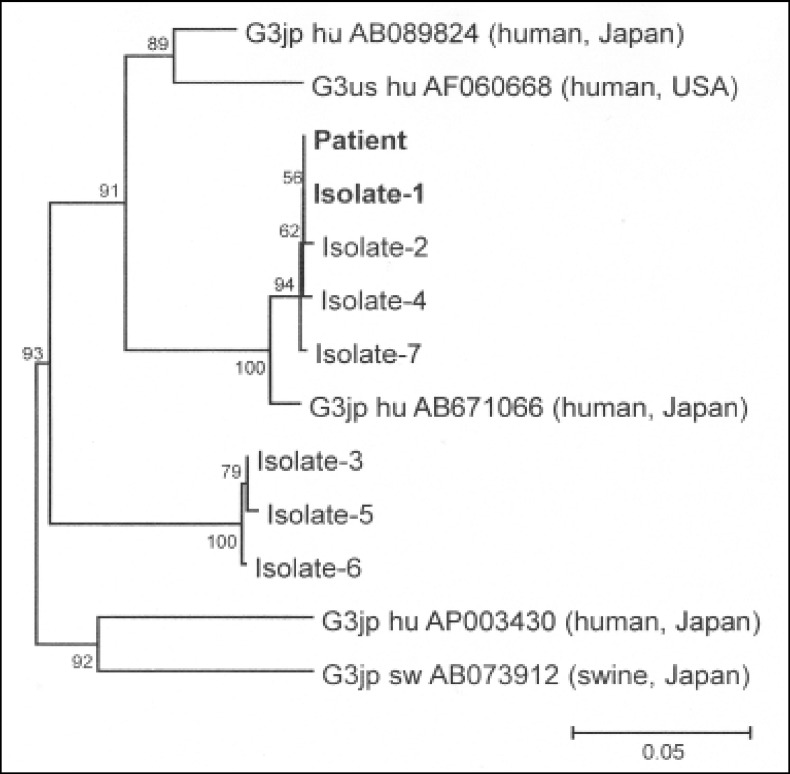
We then compared the sequence of the viral RNA detected from the stocked sera of the donor and patient using reverse transcription-polymerase chain reaction followed by direct sequencing. We also compared the sequence of the amplification products of open reading frame 1 (ORF1) (326 bp, nt 123–448) and ORF2 (412 bp, nt 5,987–6,398) of HEV between the patient’s and donor’s samples. Many different HEV sequences were determined among the sequences from the donor’s sample. For precise analysis, the donor’s HEV was cloned and sequenced. A total of 28 polymerase chain reaction products were obtained and seven HEV strains were isolated from the donor’s stocked sera, all of which were genotype 3. The phylogenetic tree according to the ORF2 sequence is shown in Figure 2. Interestingly, the tree suggests that the donor carried two distinct phylogenies of HEV. Of these, one strain (isolate-1, in Figure 2) showed 100% sequence homology with the strain isolated from the patient’s stocked serum with respect to both ORF1 and ORF2.
Figure 2.
Phylogenic analysis of ORF2 region Phylogenetic tree of HEV constructed by neighbor-joining method. A 100% homology is observed between the patient and isolate-1. Isolate-1 to −7: HEV clones from the blood donor.
Discussion
HEV is an RNA virus comprising approximately 7.2 kb and has four different genotypes1. HEV genotypes vary regionally. The epidemic form is related to genotypes 1 and 2, which cause severe acute disease and are spread via drinking water in developing countries. The regional endemic or autochthonous form is related to genotypes 3 and 4, which are zoonotic and cause mild asymptomatic disease that is spread via food in developed countries2. Cases of transfusion-transmitted HEV infection were recently reported in the United Kingdom, France, and Japan8–10. Among these, five patients including the present one had haematological diseases4,8,11,12. These cases comprised only male patients and the causative blood transfusion products were RBC and platelet products. The HEV genotypes were type 3 in four cases and type 4 in one case. In developed countries, most cases are reported to be of genotype 3, like the present case2. However, these cases are very likely to be regional endemic diseases rather than infections imported from developing countries.
Recent studies demonstrate that some cases of HEV infection were initially misdiagnosed as drug-induced liver dysfunction. A British study13 found acute HEV infection in 13% (6/47 cases) of cases initially diagnosed as drug-induced liver dysfunction. Similarly, an American study reported HEV infection in 3% (9/318 cases) of cases initially diagnosed as drug-induced liver injury14. HEV RNA analysis was performed in four of these nine cases and revealed that all of the cases were caused by HEV genotype 314. These data suggest that HEV infection should be included in the differential diagnosis of patients with liver dysfunction.
The prevalence rates of HEV vary among countries and even among regions within a country15–19. In Japan, out of 12,600 samples, 431(3.4%) were positive for IgG anti-HEV. The results of that study showed that the prevalence of IgG anti-HEV was significantly higher in eastern Japan (5.6%) than in western Japan (1.8%) (P<0.001), indicating marked regional variation19. Indeed, the infected donor involved in this case report was from Tokyo, which is in eastern Japan. Furthermore, the Hokkaido area is reported to have higher incidence of IgG anti-HEV than other eastern areas including Tokyo. Besides the high prevalence of HEV RNA18, the incidence of HEV transmission is also high with a predominance of genotype 4, which is the viral genotype that causes severe symptoms11,20. Thus, HEV RNA screening of donated blood was experimentally initiated in Hokkaido in 2005.
Patients who have received many blood transfusions are reported to have a significantly higher incidence of markers of HEV infection (i.e., IgG/IgM anti-HEV and HEV RNA) than those who have received fewer blood transfusions21. Since HEV screening is not performed to prevent haematologically transmitted infections in developed countries, the frequency of transfusion-transmitted HEV infection might be underestimated. The present and previously reported cases indicate that any blood product, including RBC products4,8, p latelet products11,12 and fresh-frozen plasma10 can transmit HEV. However, the viral load required to induce transfusion-transmitted hepatitis E in recipients is unclear. Further investigation is required to clarify this point. Therefore, HEV screening for blood transfusion donors should be considered in areas in which the seroprevalence of HEV is high. However, the present case revealed that HEV can be transmitted via blood products from donors during the “window period”. Moreover in two previous cases11,12, blood products that transmitted HEV were positive for HEV RNA but negative for anti-HEV antibody. Thus, HEV RNA should be investigated at the onset of liver dysfunction in patients receiving frequent blood transfusions. Furthermore, HEV RNA screening among blood donors might be effective for the prevention of transfusion-transmitted HEV.
Acknowledgements
We thank Dr. Keisuke Miyazawa (Department of Biochemistry, Tokyo Medical University) for his critical reading of our manuscript. We also thank Ms. Ayako Hirota for her assistance with preparing the manuscript.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet. 2012;379:2477–88. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237–44. doi: 10.1056/NEJMra1204512. [DOI] [PubMed] [Google Scholar]
- 3.Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–9. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 4.Tamura A, Shimizu YK, Tanaka T, et al. Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol Res. 2007;37:113–20. doi: 10.1111/j.1872-034X.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 5.le Coutre P, Meisel H, Hofmann J, et al. Reactivation of hepatitis E infection in a patient with acute lymphoblastic leukaemia after allogeneic stem cell transplantation. Gut. 2009;58:699–702. doi: 10.1136/gut.2008.165571. [DOI] [PubMed] [Google Scholar]
- 6.Abravanel F, Mansuy JM, Huynh A, et al. Low risk of hepatitis E virus reactivation after haematopoietic stem cell transplantation. J Clin Virol. 2012;54:152–5. doi: 10.1016/j.jcv.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama N, Miyazawa K, Kanda Y, et al. Multicenter phase II trial of vitamin K2 monotherapy and vitamin K2 plus 1α-hydroxyvitamin D3 combination therapy for low-risk myelodysplastic syndromes. Leuk Res. 2010;34:1151–7. doi: 10.1016/j.leukres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Boxall E, Herborn A, Kochethu G, et al. Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus Med. 2006;16:79–83. doi: 10.1111/j.1365-3148.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 9.Colson P, Coze C, Gallian P, et al. Transfusion-associated hepatitis E, France. Emerg Infect Dis. 2007;13:648–9. doi: 10.3201/eid1304.061387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubayashi K, Nagaoka Y, Sakata H, et al. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion. 2004;44:934–40. doi: 10.1111/j.1537-2995.2004.03300.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsubayashi K, Kang JH, Sakata H, et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368–75. doi: 10.1111/j.1537-2995.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 12.Haïm-Boukobza S, Ferey MP, Vétillard AL, et al. Transfusion-transmitted hepatitis E in a misleading context of autoimmunity and drug-induced toxicity. J Hepatol. 2012;57:1374–8. doi: 10.1016/j.jhep.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Dalton HR, Fellows HJ, Stableforth W, et al. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:1429–35. doi: 10.1111/j.1365-2036.2007.03504.x. [DOI] [PubMed] [Google Scholar]
- 14.Davern TJ, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665–72. doi: 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajpai M, Gupta E. Transfusion-transmitted hepatitis E: is screening warranted? Indian J Med Microbiol. 2011;29:353–8. doi: 10.4103/0255-0857.90158. [DOI] [PubMed] [Google Scholar]
- 16.Mansuy JM, Bendall R, Legrand-Abravanel F, et al. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309–12. doi: 10.3201/eid1712.110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scotto G, Giammario A, Centra M, et al. Seroprevalence of hepatitis E virus among blood donors in a distinct of southern Italy. Blood Transfus. 2012;10:565–6. doi: 10.2450/2012.0154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakata H, Matsubayashi K, Takeda H, et al. A nationwide survey for hepatitis E virus prevalence in Japanese blood donors with elevated alanine aminotransferase. Transfusion. 2008;48:2568–76. doi: 10.1111/j.1537-2995.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- 19.Takeda H, Matsubayashi K, Sakata H, et al. A nationwide survey for prevalence of hepatitis E virus antibody in qualified blood donors in Japan. Vox Sang. 2010;99:307–13. doi: 10.1111/j.1423-0410.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- 20.Mizuo H, Yzaki Y, Sugawara K, et al. Possible risk facors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol. 2005;76:341–9. doi: 10.1002/jmv.20364. [DOI] [PubMed] [Google Scholar]
- 21.Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol. 2004;19:778–84. doi: 10.1111/j.1440-1746.2004.03437.x. [DOI] [PubMed] [Google Scholar]