Abstract
Background
Requirements for allogeneic red cell transfusion after total lower limb arthroplasty are still high (20–50%), and post-operative intravenous iron has been shown to reduce transfusion requirements for this surgery. We performed a cost analysis to ascertain whether this alternative is also likely to be cost-effective.
Materials and methods
Data from 182 matched-pairs of total lower limb arthroplasty patients, managed with a restrictive transfusion protocol and without (control group) or with post-operative intravenous iron (iron group), were retrospectively reviewed. Acquisition and administration costs of iron (iron sucrose or ferric carboxymaltose) and allogeneic red cell concentrates, haemoglobin measurements, and prolonged stay in hospital were used for blood management cost analysis.
Results
Patients in the iron group received 600 mg intravenous iron, without clinically relevant incidents, and had a lower allogeneic transfusion rate (11.5% vs 26.4% for the iron and control groups, respectively; p=0.001). The reduction in transfusion rate was more pronounced in anaemic patients (17% vs 40%; p=0.015) than in non-anaemic ones (9.6% vs 21.2%; p=0.011). There were no differences with respect to post-operative infection rate. Patients receiving allogeneic transfusion stayed in hospital longer (+1.9 days [95% CI: 1.2–2.6]). As intravenous iron reduces the allogeneic transfusion rate, both iron formulations were cost-neutral in the different cost scenarios (−25.5 to 62.1 €/patient for iron sucrose, and −51.1 to 64.4 €/patient for ferric carboxymaltose).
Discussion
In patients presenting with or without pre-operative anaemia, post-operative intravenous iron after total lower limb arthroplasty seems to be safe and is associated with reduced transfusion rates, without incremental costs. For anaemic patients, its efficacy could be increased by associating some other blood-saving method.
Keywords: allogeneic red cell transfusion, post-operative intravenous iron, length of hospital stay, cost-effectiveness, lower limb arthroplasty
Introduction
Unilateral lower limb arthroplasty (total knee arthroplasty [TKA] and total hip arthroplasty [THA]) can result in a substantial blood loss (20–40% of the circulating blood volume). Allogeneic red blood cell transfusion (ARCT) is frequently used for treating acute intra- and post-operative anaemia, and 20–50% of these patients receive at least one unit of red blood cells1–4. However, as highlighted by several studies, there is a large inter-centre variability in the percentage of patients who receive ARCT when undergoing a particular orthopaedic surgical procedure. In order to reduce variability in transfusion practice, scientific societies have developed evidence-based guidelines and recommendations on the indications for ARCT5–10. Although the use of patient-based restrictive transfusion criteria seems to be safe and should be the cornerstone of any blood conservation programme for orthopaedic surgery, it is not the only strategy to reduce both the frequency and volume of ARCT and, consequently, ARCT-related risks11.
In this regard, a recent consensus statement suggested peri-operative administration of intravenous (IV) iron in patients undergoing major orthopaedic surgery who are expected to develop severe post-operative anaemia (GRADE recommendation 2B)12. In fact, when used together with a restrictive transfusion protocol, very short term peri-operative treatment with IV iron, with or without recombinant human erythropoietin, was associated with improvement of peri-operative anaemia lower transfusion requirements, and faster recovery from post-operative anaemia in patients undergoing lower limb orthopaedic surgery13–19. However, no formal cost analysis of this therapeutic option has been performed to date.
The purpose of this study was to examine blood management costs in a cohort of patients undergoing TKA or THA for whom a transfusion protocol was defined and post-operative IV iron (PIVI) administration used, and to compare them with those in a matched cohort of patients managed without PIVI (i.e. ARCT when appropriate). In addition, we evaluated in which patients this blood-sparing method is more likely to produce cost savings, according to the IV iron compound used, their pre-operative haemoglobin (Hb) concentration, and the different ARCT rates in patients managed with each treatment option.
Materials and methods
Patients and surgery
Data from primary TKA or THA patients, who underwent surgery between January 2004 and December 2011, were retrospectively reviewed. There was no need of ethical committee approval in this only retrospective observational study without any modification of treatment and using only non-identifiable, disaggregated data, maintaining confidentiality.
All patients underwent surgery using standardised anaesthetic and surgical protocols, antibiotic and antithrombotic prophylaxis, transfusion protocols, and post-operative analgesia. All TKA were performed using a pneumatic tourniquet, which was deflated after wound closure. No patient was operated on using minimally invasive techniques. Closed suction drains, which were removed on the second post-operative day, were placed in all operations.
Patients with any contraindication to receiving IV iron (history of anaphylaxis, iron overload, active infection, etc) were excluded. Patients presenting with a pre-operative Hb <10 g/dL were considered at very high risk of requiring ARCT and were also excluded. No patient was in an autologous blood donation programme, received salvaged blood, anti-fibrinolytic agents or recombinant human erythropoietin, or underwent acute normovolaemic haemodilution.
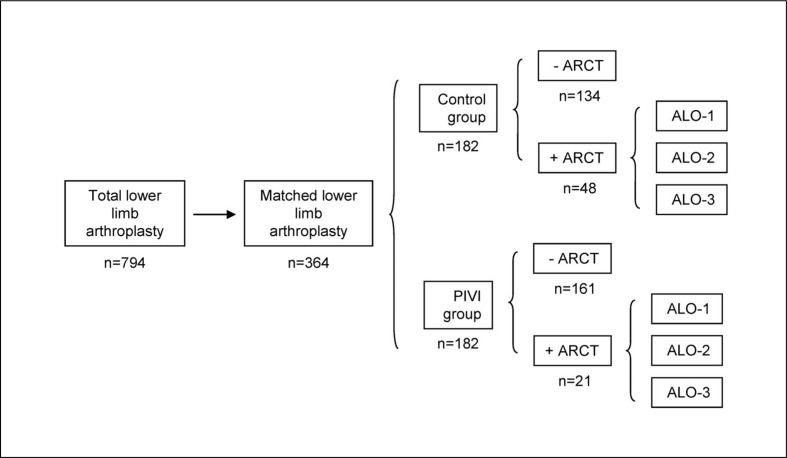
Included patients (n=794) were classified into two groups: the PIVI group if they received PIVI (n=257) and the control group if they did not receive PIVI (n=537). Each PIVI patient was matched by investigators with a control patient, based on the fulfilment of all of the following criteria: having the same age (± 2 years), gender, type of arthroplasty, surgery time (±10 min), and pre-operative Hb level (±0.5 g/dL). One hundred and eighty-two matched pairs were found (Figure 1).
Figure 1.
Distribution of patients according to group (control or post-operative IV iron administration [PIVI]), and need for allogeneic red cell transfusion (ARCT).
For patients receiving ARCT, three possible cost scenarios were considered (ALO-1, ALO-2, ALO-3) (see Methods section for further details). N=number of patients in each subgroup.
Intravenous iron supplementation
The IV iron formulations used were iron sucrose (IS, Venofer, Vifor France, Neully-sur-Seine, France), administered at doses of 200 mg in 100–200 mL saline over 30–60 min on 3 consecutive post-operative days, or ferric carboxymaltose (FCM, Ferinject, Vifor France, Neully-sur-Seine, France), administered as 600 mg in 100–200 mL saline over 15–30 minutes on the first morning after surgery.
Allogeneic blood transfusion protocol
Although elderly patients may tolerate anaemia poorly, they were not intended to receive ARCT if their Hb level was >8 g/dL, unless they developed signs and/or symptoms of acute anaemia (hypotension, tachycardia, tachypnoea, dizziness, fatigue, etc)5–10. This transfusion protocol was uniformly applied by anaesthesiologists and surgeons to all patients in the operating theatre, the post-operative care unit, and the ward for the entire duration of hospitalisation. Only leucodepleted units of blood were given.
Clinical data
Demographic and clinical data were collected for all patients. The information collected included age, gender, type of arthroplasty, surgery time, patients receiving ARCT (ARCT rate, %), number of allogeneic red cell units, both total and units per patient (ARCT index), peri-operative and pre-transfusion Hb levels, post-operative infection rate and type (urinary tract infection, respiratory tract infection, surgical wound infection, or other infection), and length of hospital stay.
Economic data
For the purpose of this study, we considered fixed and variable costs related to patients’ blood management. All costs were expressed in Euros (€), updated to 2012 according to changes in the consumer price index in Spain, and included allogeneic red cell acquisition costs, transfusion service costs, haemoglobin assessment costs, PIVI acquisition and administration costs and hospitalisation costs.
Allogeneic red cell acquisition costs
These costs were obtained from the METIS study, which used a time-driven activity-based costing (TDABC) methodology to develop the cost model because of its ability to capture a wide spectrum of indirect costs and the cost for unused capacity as well, as detailed elsewhere20,21. These costs included the facilities, material, equipment and personnel costs incurred at the Regional Transfusion Centre for blood collecting on mobile units, blood collecting on site, blood processing and leucodepletion, serological and nucleic acid testing, immunohaematology testing, storage, distribution, and societal cost for donors (Table I).
Table I.
Direct supply, operating and hospitalisation costs associated with leucodepleted allogeneic red cell concentrate (ARC) and post-operative intravenous (IV) iron therapy.
| Supply costs | ||
|---|---|---|
| ARC acquisition cost (per unit) | € | 155 |
| Iron sucrose (per 100 mg)* | € | 8.5 |
| Ferric carboxymaltose (per 100 mg) | € | 20 |
| Iron isomaltoside 1,000 (per 100 mg) | € | 20 |
| Low molecular weight iron dextran (per 100 mg) | € | 10.3 |
| Saline and giving set (per infusion) | € | 2 |
|
| ||
| Operating costs | ||
|
| ||
| ARC transfusion cost (per unit) | € | 52 |
| IV iron infusion (per infusion)** | € | 16 |
| Haemoglobin assessment (per measurement) | € | 36 |
|
| ||
| Hospitalisation cost | ||
|
| ||
| Hospitalisation in the orthopaedic ward (per day) | € | 320 |
Mean cost of three available products;
Except for low molecular weight iron dextran which is estimated at €48.
Transfusion service costs
These included the facilities, material, equipment and personnel costs incurred at the hospital blood bank for selecting the red cell unit, performing cross-matching, and releasing the unit, and in the hospital orthopaedic ward for bed-side checking of the patient’s blood group, transfusion-giving set, and transfusing the unit to the patient. These costs were also obtained using the TDABC methodology. At our institution, all patients scheduled for TKA have a type and screening, irrespectively of whether they are going to be managed with a blood conservation strategy or not. The cost for type and screening was not included in the cost analysis21 (Table I).
Haemoglobin assessment costs
All patients have their Hb level measured within 24–48 hours after surgery to evaluate the need for ARCT. The costs for these determinations were not, therefore, included in the model. We considered only those Hb measurements requested for monitoring the effect of ARCT. These costs included the facilities, material, equipment and personnel costs for blood drawn, Hb measurement and data interpretation21 (Table I).
Postoperative IV iron administration costs
For the present cost analysis we considered the acquisition costs of the two preparations used (IS and FCM), and those of two other compounds available in Spain, iron isomaltoside 1000 (MNF; Monofer, Pharmacosmos, Holbaek, Denmmark) and low molecular weight iron dextran (LMWID, Cosmofer, Pharmacosmos, Holbaek, Denmmark). The costs of administration of IV iron were also estimated. These included the facilities, material, equipment and personnel costs incurred in the hospital orthopaedic ward for preparing and infusing the IV iron solution to the patient (Table I).
Hospitalisation costs
The cost of 1 day of hospitalisation in the orthopaedic ward was obtained from Servicio Andaluz de Salud (Spain)21 (Table I).
Blood management cost scenarios
In the basic cost scenario (ALO-1), blood management costs were calculated taking into account the costs of acquisition and transfusion of allogeneic red cells, the costs of acquisition and infusion of IV iron, and the cost of extra analytical measurements (Hb assessments). We considered another two possible cost scenarios by adding the cost of 1 (ALO-2) or 2 extra days (ALO-3) of hospitalisation in patients receiving ARCT. Furthermore, blood management costs per patient in the three scenarios were also analysed after stratifying patients according to their pre-operative Hb (two Hb strata: Hb <13 g/dL and Hb ≥13 g/dL). Finally, a sensitivity analysis was performed for the three scenarios by varying the percentage of patients receiving ARCT in the control group (15%, 20%, 25%, 30%, and 35%) and in the PIVI group (10%), and assuming a mean transfusion index of two red blood cell units per patient. All the cost analyses in the different scenarios were performed separately for the four IV iron compounds.
Statistics
Data were expressed as incidence (n) and percentage (%), as the mean ± standard deviation (SD), or as mean and 95% confidence interval (CI). Pearson’s chi-square test or Fisher’s exact test was used for comparison of qualitative variables. Parametric analysis of variance (ANOVA) or the non-parametric Kruskall-Wallis test was used for comparison of quantitative variables, after consideration of distributional characteristics. The effect size, as measured by Cohen’s d, is provided where appropriate to avoid the recognition of small and irrelevant differences22. All statistics were performed with IBM SPSS Statistics 19 (Licensed to the University of Málaga, Spain) and a p value <0.05 was considered statistically significant.
Results
Efficacy of post-operative intravenous iron administration
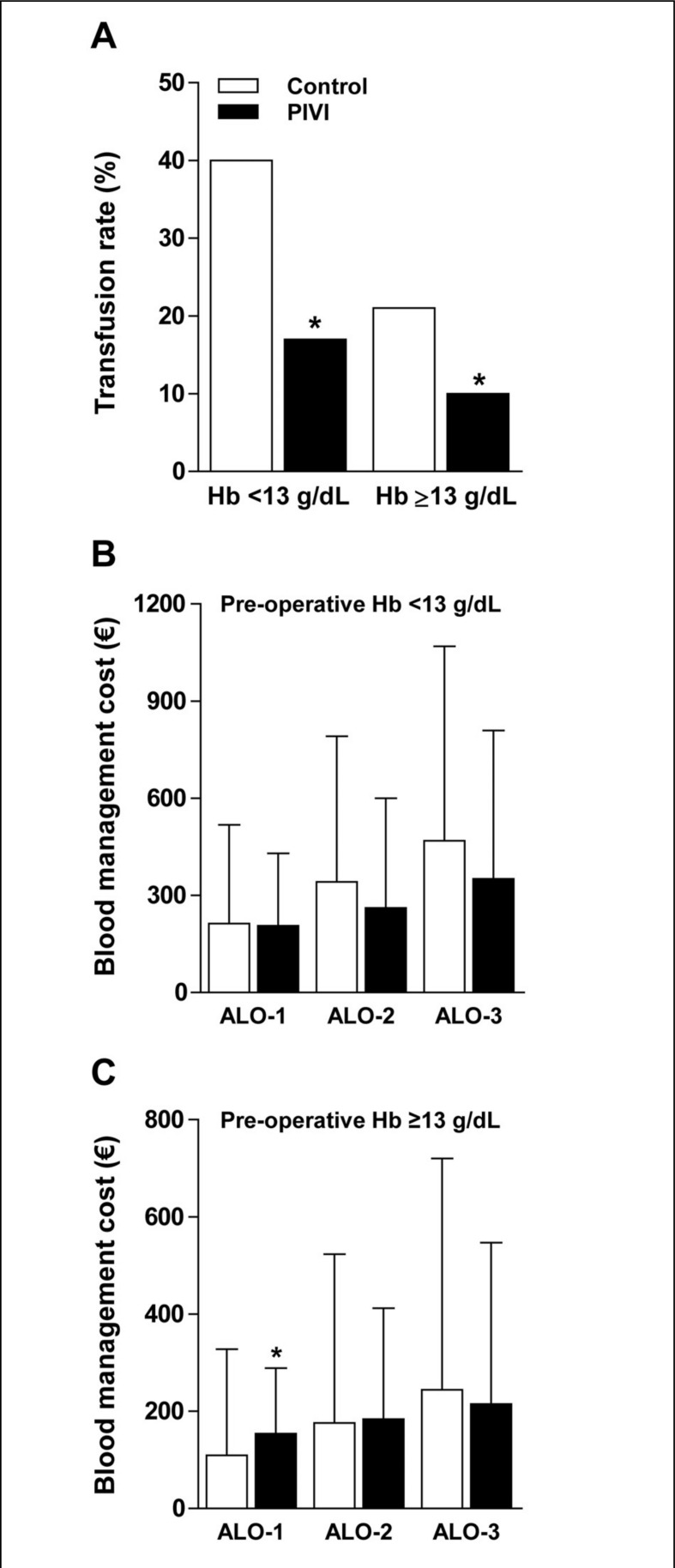
There were no differences in patients’ characteristics between groups, except for a slightly higher pre-operative Hb level in the PIVI group, although the effect size was small (Cohen’s d=0.167) (Table II). At least one ARCT was needed in 21 patients from the PIVI group and 48 patients from the control group (Figure 1). Most of these transfusions were given within 48 hours after surgery. The percentage of patients receiving ARCT was lower in the PIVI group than in the control group (11.5% vs 26.4%, respectively; p=0.001) as was the number of transfused allogeneic red cell units (Table II). There were no differences in transfusion rates between patients receiving IS or FCM (12.7% vs 10%; p=0.599). Despite the lower ARCT rate, Hb levels on post-operative days 3 and 7 were higher in patients from the PIVI group than in those from the control group (Table II). In addition, after stratifying patients according to their pre-operative Hb concentration, the differences in ARCT rate between groups remained statistically significant for both Hb <13 g/dL and Hb ≥13 g/dL (Figure 2A).
Table II.
Demographic and clinical data of two series of patients undergoing surgery for total hip or knee arthroplasty, managed without (control group) or with (PIVI group) post-operative administration of intravenous iron (iron sucrose, 3×200 mg; ferric carboxymaltose, 1×600 mg; see Methods section for details).
| Control group | PIVI group | p | |
|---|---|---|---|
| Patients (n) | 182 | 182 | ---- |
| Gender (M/F) | 75/107 | 75/107 | 1.000 |
| Age (years) | 68±10 | 68±9 | 0.988 |
| Operation (THA/TKA) | 95/87 | 95/87 | 1.000 |
| Preoperative Hb (g/dL) | 13.6±1.2 | 13.8±1.2 | 0.048 |
| 72h postoperative Hb (g/dL) | 9.2±1.5 | 10.4±1.6 | 0.001 |
| 7d postoperative Hb (g/dL) | 9.2±2.2 | 10.6±1.3 | 0.001 |
| ARCT rate, n (%) | 48 (26.4) | 21 (11.5) | 0.001 |
| ARCT units, n (%) | 0.001 | ||
| 0 | 134 (73.6) | 161 (88.5) | |
| 1 | 4 (2.2) | 3 (1.6) | |
| 2 | 34 (18.7) | 16 (8.8) | |
| 3 | 5 (2.7) | 1 (0.5) | |
| 4 | 9 (4.9) | 1 (0.5) | |
| Pre-ARCT Hb (g/dL) | 7.9±0.6 | 7.9±0.8 | 0.682 |
| Surgical time (min) | 94±30 | 96±30 | 0.661 |
| Post-operative infection n (%) | 6 (3.3) | 3 (1.6) | 0.502 |
| Surgical wound | 3 | 1 | |
| Pneumonia | 2 | 2 | |
| Urinary tract | 1 | 0 | |
| Length of hospital stay (days) | 8.4±3.1 | 7.9±2.1 | 0.072 |
Legend ARCT: allogeneic red cell transfusion; F: female; Hb: haemoglobin; M: male; Pre-ARCT Hb: haemoglobin level prior to ARCT; THA: total hip arthroplasty; TKA: total knee arthroplasty. Data are expressed as mean ± SD, or incidence and %.
Figure 2.
Allogeneic red cell transfusion (ARCT) rate (%) and blood management costs in two series of patients undergoing lower limb arthroplasty, managed with (PIVI group) or without (control group) post-operative intravenous iron administration, according to pre-operative haemoglobin level (preOP Hb) (A) and three possible cost scenarios (ALO-1, ALO-2, ALO) (B and C) (see Methods section for further details).
*p<0.05, PIVI group vs control group.
There were no statistically significant differences in post-operative infection rates between patients in the control group and PIVI group, but there was a trend to a shorter duration of hospital stay in the latter (Table II). Among the whole series, the post-operative infection rate was higher among transfused patients than among non-transfused patients (7.2% vs 1.4%, respectively; p=0.014) and trans fused patients spent longer in hospital than did non-transfused ones (+1.9 days [95% CI: 1.2–2.6]; p=0.001).
Cost analysis of post-operative intravenous iron administration
As stated in the Methods section, blood management costs were calculated taking into account the costs of acquisition and transfusion of allogeneic blood units, the costs of acquisition and infusion of IV iron compounds, and the cost of extra Hb measurements (ALO-1), plus the costs of prolonging the hospital admission by 1 (ALO-2) or 2 days (ALO-3) in patients receiving ARCT (Table III). In the control group, mean blood management costs per patient were € 137.5, € 221.9, and € 306.3, for ALO-1, ALO-2, and ALO-3, respectively (Table III). The corresponding figures for patients managed with PIVI were € 163.0, € 203.6, and € 244.2 for IS, and € 188.6, € 215.3, and € 241.9 for FCM, respectively, thus causing no significant incremental costs with respect to the control group in any of the three cost scenarios tested (Table III).
Table III.
Estimation of blood management costs and cost savings in patients undergoing total hip or knee arthroplasty, managed with 600 mg intravenous iron sucrose (IS) or ferric carboxymaltose (FCM), compared to those from the control group.
| N. | Cost scenarios | |||
|---|---|---|---|---|
|
|
||||
| ALO-1 | ALO-2 | ALO-3 | ||
| Blood management costs | ||||
| Control | ||||
| Without ARCT (€/patient) | 134 | 0 | 0 | 0 |
| With ARCT(€/patient) | 48 | 521 | 841 | 1,161 |
| Mean cost (€/patient) | 182 | 137.5 | 221.9 | 306.3 |
| Range | 0–972 | 0–1,292 | 0–1,612 | |
|
| ||||
| IS (600 mg) | ||||
| Without ARCT (€/patient) | 117 | 105 | 105 | 105 |
| With ARCT (€/patient) | 17 | 562 | 882 | 1,202 |
| Mean cost (€/patient) | 134 | 163.0 | 203.6 | 244.2 |
| Range | 105–834 | 105–1,154 | 105–1,474 | |
|
| ||||
| FCM (600 mg) | ||||
| Without ARCT (€/patient) | 44 | 138 | 138 | 138 |
| With ARCT (€/patient) | 4 | 745 | 1,065 | 1,385 |
| Mean cost (€/patient) | 48 | 188.6 | 215.3 | 241.9 |
| Range | 138–1,110 | 138–1,430 | 138–1,750 | |
|
| ||||
| Cost differences | ||||
| IS vs control | ||||
| Mean (€/patient) | −25.5 | +18.3 | +62.1 | |
| 95% CI | (−73.8–22.8) | (−57.4–93.9) | (−41.7–165.9) | |
| p | 0.299 | 0.635 | 0.240 | |
|
| ||||
| FCM vs control | ||||
| Mean (€/patient) | −51.1 | +6.6 | +64.4 | |
| 95% CI | (−126.8–24.6) | (−109.4–122.6) | (−92.9–221.7) | |
| p | 0.185 | 0.910 | 0.421 | |
|
| ||||
| FCM vs IS | ||||
| Mean (€/patient) | −25.6 | −11.7 | +2.2 | |
| 95% CI | (−80.1–28.9) | (−99.2–75.9) | (−118.9–123.4) | |
| p | 0.356 | 0.793 | 0.971 | |
Data calculations take into account both allogeneic blood and intravenous iron management costs (ALO-1), plus a hospital stay prolonged by 1 day (ALO-2) or 2 days (ALO-3) in patients receiving allogeneic red cell transfusion (ARCT). Cost differences: control costs – IV iron costs, expressed as mean and 95% confidence interval (CI); (+) cost savings, (−) incremental cost.
This analysis was repeated after stratification of patients according to their pre-operative Hb level. Again, the use of PIVI was cost neutral for patients presenting with a pre-operative Hb <13 g/dL or ≥13 g/dL in all three cost scenarios tested, except for ALO-1 in patients with a pre-operative Hb ≥13 g/dL (Figure 2B and 2C).
The sensitivity analysis offered different results depending on the cost scenario (ALO-1, ALO-2, or ALO-3), the ARCT rate in control group (15% – 35%), the ARCT rate in the PIVI group (10%), and the IV iron compound (IS, LMWID, FCM, or MNF). Overall, cost savings increased from cost scenario ALO-1 to cost scenario ALO-3, especially for IS and LMWID (Table IV). Conversely, as the ARCT rate in the control group decreased, the mean blood management costs per patient in the PIVI group approached (reduced savings or cost-neutral) and even exceeded that of the control group (net incremental costs). For all ARCT rates and cost scenarios, savings were higher or incremental costs were lower for IS and LMWID than for FCM and MNF (Table IV).
Table IV.
Estimation of blood management cost savings (€+) or incremental costs (€−) per patient undergoing surgery for total hip or knee arthroplasty, managed with four different intravenous iron formulations (n=100), compared to those from the control group (n=100), at different allogeneic transfusion rates.
| Control | IS | LMWID | FCM | MNF |
|---|---|---|---|---|
|
| ||||
| 10% ALO-1 | 10% ALO-1 | 10% ALO-1 | 10% ALO-1 | |
|
|
||||
| 35% ALO-1 | € +28.9 | € +21.9 | € −4.1 | € −4.1 |
| 30% ALO-1 | € +2.8 | € −4.2 | € −30.2 | € −30.2 |
| 25% ALO-1 | € −23.2 | € −30.2 | € −56.2* | € −56.2* |
| 20% ALO-1 | € −49.3* | € −56.3* | € −82.3* | € −82.3* |
| 15% ALO-1 | € −75.4* | € −82.4* | € −108.4* | € −108.4* |
|
|
||||
| 10% ALO-2 | 10% ALO-2 | 10% ALO-2 | 10% ALO-2 | |
|
|
||||
| 35% ALO-2 | € +108.9* | € +101.9* | € +75.9 | € +75.9 |
| 30% ALO-2 | € +66.8 | € +59.8 | € +33.8 | € +33.8 |
| 25% ALO-2 | € +24.8 | € +17.8 | € −8.2 | € −8.2 |
| 20% ALO-2 | € −17.3 | € −24.3 | € −50.3 | € −50.3 |
| 15% ALO-2 | € −59.4 | € −66.4 | € −92.4* | € −92.4* |
|
|
||||
| 10% ALO-3 | 10% ALO-3 | 10% ALO-3 | 10% ALO-3 | |
|
|
||||
| 35% ALO-3 | € +188.9* | € +181.9* | € +155.9* | € +155.9* |
| 30% ALO-3 | € +130.8* | € +123.8* | € +97.8 | € +97.8 |
| 25% ALO-3 | € +72.8 | € +65.8 | € +39.8 | € +39.8 |
| 20% ALO-3 | € +14.7 | € +7.7 | € −18.3 | € −18.3 |
| 15% ALO-3 | € −43.4 | € −50.4 | € −76.4 | € −76.4 |
Legend IS: iron sucrose; LMWID: low molecular weight iron dextran; FCM: ferric carboxymaltose; MNF: iron isomaltoside 1000.
Data calculations take into account the mean cost per patient for both intravenous iron and allogeneic blood management (cost scenario ALO-1) and a hospital stay prolonged by 1 day (cost scenario ALO-2) or 2 days (cost scenario ALO-3) in patients receiving allogeneic red cell transfusion (see Table III).
p<0.05; bold font for cost saving, shadowed cell for incremental cost.
Discussion
In this matched-pair, cohort study, we found that patients treated with PIVI had a lower risk of receiving ARCT than patients in the control group (11.5% vs 26.4%, respectively; p<0.001) (Table II), an observation which is in agreement with those of previously published studies using short-term peri-operative IV iron and a similar transfusion protocol23. Available data do, therefore, seem to support a role for peri-operative IV iron in reducing the need for ARCT after lower limb arthroplasty. In addition, we did not observe clinically relevant adverse effects of PIVI, although these might have been under-reported, or an increase in postoperative infection rate (Table II). Again, these results are in agreement with those previously published, which did not report increased post-operative infection rates in patients receiving IV iron23.
Despite the observed efficacy of PIVI in reduce ARCT after TKA and THA, the question regarding which patients are more likely to benefit from this blood-saving strategy, without increasing total blood management costs, was still open. We, therefore, comparatively analysed blood management costs for the control and PIVI groups in three possible cost scenarios. For the basic cost scenario, ALO-1, the three main blood management cost drivers were the ARCT rate, the costs of acquisition and transfusion of allogeneic red cell units, and the costs of acquisition and use of IV iron compounds.
We used the TDABC methodology, instead of activity-based cost (ABC) methodology24, because the former is simpler since it only requires the estimation of two parameters: how much it costs per time unit to supply resources to the business’s activities (the total overhead expenditure of a department divided by the total number of minutes of employee time available) and how much time it takes to carry out one unit of each kind of activity (as estimated or observed by the manager). Thus, TDABC has the ability to capture a wide spectrum of indirect costs and as well as the cost for unused capacity20.
Using the TDABC method, the red cell acquisition cost was estimated to be € 155 per unit, which is similar to costs reported for the United Kingdom (€ 160), USA (€ 150 – € 190), Austria (€ 115), and Switzerland (€ 145)24,25. The costs incurred for transfusing one red cell unit, including those in the blood bank and orthopaedic ward and for Hb assessment, were estimated to be € 88. Thus overall costs for one ARCT was € 243, which are considerably less than those reported for Greece (€ 295 – € 414), Austria (€ 397), Switzerland (€ 440) or the USA (€ 523 – € 852)24,26. In contrast, in a cross-sectional survey of a randomized sample of hospital-based blood bank and transfusion service directors, it was found that the mean acquisition cost for one unit of red blood cells purchased from a supplier was € 158 and the mean charge to the patient was € 25827.
There is a variety of IV iron formulations available in Spain, with different acquisition costs. As it seems that they are all alike in terms of efficacy28, for this cost study we chose the two formulations used in our area: IS and FCM. The acquisition costs of FCM are uniform across Spain, whereas those of IS vary according to the manufacturer. Thus, the average ex-factory IS price was used (Table I). Finally, as neither Hb measurements nor compatibility tests are needed, the costs for one infusion of IV iron are mostly derived from nursing time costs and are, therefore, lower than those from transfusing an allogeneic red cell unit (€ 18 vs € 88, respectively). Again, though the acquisition costs of the different IV iron formulations are similar in several European countries, there are marked differences in administration costs. For in-patients, the total costs for the administration of 600 mg IV IS (Venofer) are € 117 in Spain (this study), and € 249 in Greece, whereas the corresponding figures for FCM are € 138 and € 144, and € 113 and € 203 for LMWID30. For out-patients, the total costs for the administration of 600 mg IV IS (Venofer) are € 263 in Spain, € 502 in Greece, and € 314 in UK, whereas the corresponding figures for FCM are €143, € 187 and € 208, and € 295, € 388 and € 265 for LMWID25,29,30. The different methodologies used for capturing costs are most probably behind the observed differences in costs of red cell transfusion and IV iron administration, thus indicating that estimated local costs have to be applied when reproducing this study at a particular hospital.
At the actual ARCT rates in the control and PIVI groups (Table II), the use of IS and FCM was cost neutral in cost scenario ALO-1 (Table III). However, as ARCT rates were influenced by pre-operative Hb level (Figure 2A), management costs for patients presenting with a pre-operative Hb <13 g/dL or Hb ≥13 g/dL were analysed separately. The use of PIVI was again cost neutral for patients with a pre-operative Hb <13 g/dL, but resulted in a significant incremental cost in patients with a Hb ≥13 g/dL (Figures 2B and 2C).
Allogeneic blood transfusion is not a risk-free therapy and may result in patients having a poorer clinical outcome. In our study, patients receiving ARCT had a higher rate of post-operative infections than those not given a transfusion (7.2% vs 1.4%, respectively; p=0.014) and spent longer in hospital (+1.9 days [95% CI: 1.2–2.6]; p=0.001), a finding that has been previously documented1,2,31. In contrast, we found a trend towards a shorter stay in hospital among patients receiving PIVI, which might reflect a faster post-operative recovery32,33. Therefore, as patients receiving ARCT may have a prolonged stay in hospital, we considered two other possible scenarios by adding the cost of 1 (ALO-2) or 2 extra days (ALO-3) of hospitalisation in transfused patients. Interestingly, in a previous study the costs for two-unit ARCT were € 721 and € 1041 for cost scenarios ALO-2 and ALO-3, respectively21, which approach those recently estimated from six studies in Western Europe (€ 878)34. Again, the use of PIVI was cost neutral for ALO-2 and ALO-3, regardless of the IV iron compound used (Table III) or the pre-operative Hb stratum (Figure 2B and 2C).
As expected, the sensitivity analysis provided different results depending on the cost scenario, the ARCT rate in the control and PIVI groups, and the IV iron preparation used. Overall, costs savings increased from cost scenario ALO-1 to cost scenario ALO-3 (Table IV). Conversely, as the ARCT rate in the control group decreased, the mean blood management cost per patient in the PIVI group approached (reduced savings or cost neutral) and then even exceeded that of the control group (net incremental costs). As their acquisition costs are considerably higher (Table I), savings were lower or incremental costs were higher for FCM and MNF when compared with IS and LMWID, for all ARCT rates and cost scenarios (Table IV).
Some limitations of the study are worth noting. First, this was a retrospective, matched cohort study, and as such it does not provide unbiased results. A cause and effect relationship between treatment with PIVI and the observed clinical benefits cannot be inferred. Thus, the trend towards a shorter time spent in hospital observed in the PIVI group, as well as the prolonged length of hospital stay in patients receiving ARCT, must be evaluated with caution, as without rigid criteria for discharge, it may be that standards changed slightly during the study period.
Second, although no severe adverse drug effects were witnessed, the number of patients included in this study is not big enough to draw definite conclusions regarding the safety of IV iron compounds in this clinical setting. However, according to an analysis of data from the Food and Drug Administration (FDA) (2001–2003; 30×106 doses), the incidence of life-threatening adverse drug effects (2.2 per million doses), including deaths (0.4 per million doses), associated with the use of four IV iron preparations (iron gluconate, iron sucrose, HMW iron dextran, and LMW iron dextran), is much lower than that associated with the use of allogeneic blood transfusion (10 and 4 per million units, respectively)35,36. However, no cost was estimated for possible complications associated with ARCT. On the other hand, no prospective safety trials of long-term IV iron have been adequately powered to examine rates of infection, cardiovascular events, and deaths among patients treated with these products. A long-term randomized IV iron safety study is more complicated than it appears. Only haemodialysis patients receive repeated iron, so the study should be in this population, and target Hb should be the same in both arms. However, an extended safety trial of IV iron vs no iron would become confounded by differences in doses of erythropoiesis-stimulating agents between the arms37. As our patients only received a short-course of post-operative IV iron, no long-term adverse events should be expected. Long-term safety studies on patients receiving ARCT are also scant. In a USA population-based, case-control study using 552,951 elderly cases identified from cancer registries and 100,000 frequency matched controls, cancer risk was elevated 0 to 12 months after blood transfusion and associated with multiple transfusions, but possibly due to reverse causation, that is, incipient cancers or cancer precursors causing anaemia38. The evaluation of long-term adverse effects of PIVI or ARCT and their economic impact in our study population is, therefore, challenging.
Third, as we performed a retrospective analysis, the required sample size was not determined beforehand. As a result, this study was not powered to detect statistically significant differences in the incidence of post-operative infections39, and no definitive conclusions regarding the role of PIVI administration on this outcome variable can be drawn. Fourth, patients’ readmission rates, which could have major financial repercussions, were not evaluated in this study. Fifth, no patient received recombinant human erythropoietin, while it has been shown that the addition of a single dose of recombinant human erythropoietin (40,000 IU) can boost the erythropoietic effect of IV iron, further reducing transfusion requirements40. More research is needed to ascertain whether this joint therapy could be more cost-effective than IV iron alone. Lastly, as approximately 200 mg iron are needed to increase a patient’s Hb concentration by 1 g/dL, the scheduled PIVI dose (600 mg) may not cover total iron requirements for restore pre-operative Hb levels, especially in patients with pre-operative iron deficiency. However, as iron status was not generally assessed in these patients, the scheduled iron dose seems to be conservative, thus minimising the risk of iron overload. Nevertheless, it has been reported that very short-term perioperative IV IS in TKR patients reduced ARCT rate and hastened the recovery from post-operative anaemia, without depleting iron stores41. The use of newer IV iron formulations (e.g. FCM or MNF), which allow the administration of single larger doses (up to 20 mg/kg body weight) will probably facilitate the implementation of a more accurate, patient-tailored iron replacement therapy. This may be especially important if a fast-track patient management programme is to be implemented42. In this regard, for TKR patients presenting with a pre-operative ferritin <100 ng/mL, preliminary results from a randomised controlled trial (EudraCT 2010-023038-22) showed significant improvements in Hb levels (11.6 g/dL vs 10.3 g/dL; p<0.001) and Barthel index (95 vs 92; p<0.03) on post-operative day 30 when FCM rather than oral iron was administered after surgery43. Complete data from this trial will be useful to ascertain in which patients PIVI is more likely to be cost-effective.
Acknowledgements
The Authors thank Mr. Emilio Alfonso from HICY Optimising Health Care, Bruge (Belgium) for his advice regarding the TDABC methodology for cost calculations.
Footnotes
The Authors declare no conflict of interest.
References
- 1.Bierbaum BE, Callaghan JJ, Galante JO, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81A:2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–69. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 3.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86-B:561–5. [PubMed] [Google Scholar]
- 4.Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47:1468–80. doi: 10.1111/j.1537-2995.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 5.British Committee for Standards in Haematology. Blood transfusion Task Force. Guidelines for the clinical use of red cell transfusion. Br J Haematol. 2001;113:24–32. [Google Scholar]
- 6.Sociedad Española de Transfusión Sanguínea y Terapia Celular. Guía sobre la transfusión de componentes sanguíneos y derivados plasmáticos. 4th Ed. Madrid: SETS; 2010. pp. 43–54. [Google Scholar]
- 7.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. II. The intra-operative period. Blood Transfus. 2011;9:189–217. doi: 10.2450/2011.0075-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology Working Party. Recommendations for the transfusion management of patients in the peri-operative period. III. The post-operative period. Blood Transfus. 2011;9:320–35. doi: 10.2450/2011.0076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz M, García-Erce JA, Villar I, Thomas D. Blood conservation strategies in major orthopaedic surgery: efficacy, safety and European regulations. Vox Sang. 2009;96:1–13. doi: 10.1111/j.1423-0410.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 12.Beris P, Muñoz M, García-Erce JA, et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 13.Cuenca J, García-Erce JA, Muñoz M, et al. Patients with pertrochanteric hip fracture may benefit from preoperative intravenous iron therapy: a pilot study. Transfusion. 2004;44:1447–52. doi: 10.1111/j.1537-2995.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- 14.Cuenca J, García-Erce JA, Martínez AA, et al. Role of parenteral iron in the management of anaemia in the elderly patient undergoing displaced subcapital hip fracture repair. Preliminary data. Arch Orthop Trauma Surg. 2005;125:342–7. doi: 10.1007/s00402-005-0809-3. [DOI] [PubMed] [Google Scholar]
- 15.García-Erce JA, Cuenca J, Muñoz M, et al. Perioperative stimulation of erythropoiesis with intravenous iron and erythropoietin reduces transfusion requirements in patients with hip fracture. A prospective observational study. Vox Sanguinis. 2005;88:235–43. doi: 10.1111/j.1423-0410.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 16.Cuenca J, García-Erce JA, Martínez F, et al. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol, reduce the need for allogeneic blood after knee replacement surgery. Transfusion. 2006;46:1112–9. doi: 10.1111/j.1537-2995.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz M, Naveira E, Seara J, et al. Role of parenteral iron in transfusion requirements after total hip replacement. A pilot study. Tranfus Med. 2006;16:137–42. doi: 10.1111/j.1365-3148.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz M, Naveira E, Seara J, Cordero J. Effects of postoperative intravenous iron on transfusion requirements after lower limb arthroplasty. Br J Anaesth. 2012;108:532–4. doi: 10.1093/bja/aes012. [DOI] [PubMed] [Google Scholar]
- 19.Serrano-Trenas JA, Font-Ugalde P, Muñoz-Cabello L, et al. Role of perioperative intravenous iron therapy in elderly hip fracture patients. A single center randomized controlled trial. Transfusion. 2011;51:97–104. doi: 10.1111/j.1537-2995.2010.02769.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82:131–8. [PubMed] [Google Scholar]
- 21.Muñoz M, Ariza D, Campos A, Martín-Montañez E, Pavía J. The cost of post-operative shed blood salvage after total knee arthroplasty: an analysis of 1,093 consecutive procedures. Blood Transfus. 2013;11:260–71. doi: 10.2450/2012.0139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. pp. 21–3. [Google Scholar]
- 23.Muñoz M, García-Erce JA, Cuenca J, et al. On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012;10:8–22. doi: 10.2450/2011.0061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 25.Bhandari S. Update of a comparative analysis of cost minimization following the introduction of newly available intravenous iron therapies in hospital practice. Ther Clin Risk Manag. 2011;7:501–9. doi: 10.2147/TCRM.S25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanavos P, Yfantopoulos J, Vandoros C, Politis C. The economics of blood: gift of life or a commodity? Int J Technol Assess Health Care. 2006;22:338–43. doi: 10.1017/s0266462306051233. [DOI] [PubMed] [Google Scholar]
- 27.Toner RW, Pizzi L, Leas B, et al. Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Policy. 2011;9:29–37. doi: 10.2165/11530740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107:477–8. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 29.Fragoulakis V, Kourlaba G, Goumenos D, et al. Economic evaluation of intravenous iron treatments in the management of anemia patients in Greece. Clinicoecon Outcomes Res. 2012;4:127–34. doi: 10.2147/CEOR.S30514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubio-Terrés C, Fernández-López A, Fernández-Montero A, et al. Análisis farmacoeconómico del tratamiento de la deficiencia de hierro con hierro carboximaltosa (Ferinject®) en España. Pharmacoeconomics (Spanish Research Articles) 2010;7:109–17. [Google Scholar]
- 31.Llewelyn CA, Taylor RS, Todd AA, et al. The effect of universal leukoreduction on postoperative infections and length of hospital stay in elective orthopedic and cardiac surgery. Transfusion. 2004;44:489–500. doi: 10.1111/j.1537-2995.2004.03325.x. [DOI] [PubMed] [Google Scholar]
- 32.Bellamy MC, Gednaey JA. Unrecognised iron deficiency in critical illness. Lancet. 1998;352:1903. doi: 10.1016/s0140-6736(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz M, García-Erce JA, Remacha ÁF. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol. 2011;64:287–96. doi: 10.1136/jcp.2010.086991. [DOI] [PubMed] [Google Scholar]
- 34.Abraham I, Sun D. The cost of blood transfusion in Western Europe as estimated from six studies. Transfusion. 2012;52:1983–8. doi: 10.1111/j.1537-2995.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- 35.Chertow GM, Mason PD, Vaaga-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–82. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 36.Stainsby D, Jones H, Asher D, et al. SHOT Steering Group. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–82. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Coyne DW. It’s time to compare anemia management strategies in hemodialysis. Clin J Am Soc Nephrol. 2010;5:740–2. doi: 10.2215/CJN.02490409. [DOI] [PubMed] [Google Scholar]
- 38.Riedl R, Engels EA, Warren JL, et al. Blood transfusions and the subsequent risk of cancers in the United States elderly. Transfusion. 2013 Jan 16; doi: 10.1111/trf.12071. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleiss JL. Determining sample sizes needed to detect a difference between two proportions. In: Fleiss JL, Lewin B, Chopaile M, editors. Statistical Methods for Rates and Proportions. 2nd ed. New York: Wiley; 1981. pp. 38–45. [Google Scholar]
- 40.García-Erce JA, Cuenca J, Haman-Alcober S, et al. Efficacy of preoperative recombinant human erythropoietin administration for reducing transfusion requirements in patients undergoing surgery for hip fracture repair. An observational cohort study. Vox Sang. 2009;97:260–7. doi: 10.1111/j.1423-0410.2009.01200.x. [DOI] [PubMed] [Google Scholar]
- 41.García-Erce JA, Cuenca J, Martínez F, et al. Perioperative intravenous iron preserves iron stores and may hasten the recovery from post-operative anaemia after knee replacement surgery. Transfus Med. 2006;16:335–41. doi: 10.1111/j.1365-3148.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 42.Kotzé A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–52. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 43.Bisbe E, Moltó L, Arroyo R, et al. The efficacy of intravenous iron for treating postoperative anemia and hastening functional recovery in patients undergoing total knee arthroplasty: preliminary results from a randomized controlled trial [Abstract] Transfus Altern Transfus Med. 2012;12:29–30. [Google Scholar]