Abstract
Background
In patients who have large bleeds, there is a tendency to transfuse more plasma and platelets than recommended in earlier guidelines, and accordingly many hospitals now provide “transfusion packages” with an intended red cell:platelet:plasma ratio of 1:1:1. The purpose of this study was to investigate in vitro functions of transfusion packs compared with fresh whole blood.
Material and methods
“Reconstituted whole blood” was prepared with the same ratio of red cells, platelets and plasma as used in local transfusion packages. The aggregation and thrombin-antithrombin complex formation responses to collagen stimulation of this reconstituted whole blood were compared with those of fresh whole blood. The storage time of red cells and platelets was varied in a systematic manner, giving nine different compositions of reconstituted whole blood that simulated transfusion packs.
Results
The responses varied significantly between whole blood and reconstituted whole blood -and between the reconstituted whole blood of different compositions. A significant decrease (p<0.005) in collagen-induced platelet count reduction was seen with increasing platelet and red blood cell age. Thrombin-antithrombin complex formation peaked in studies with platelets stored for 5 days. The red cells stored for the longest time induced the greatest thrombin-antithrombin complex formation. Fresh whole blood gives more consistent responses, and the aggregation response to collagen is stronger than in reconstituted whole blood.
Discussion
Our results indicate that in vitro responses of reconstituted whole blood vary substantially according to how long the red cells and platelets are stored for. As the responses obtained by testing whole blood are more consistent and usually stronger, the alternative use fresh whole blood in special conditions should not be excluded without further consideration.
Keywords: reconstituted whole blood, blood components, platelet function, aggregation, thrombin formation
Introduction
Except for special purposes, such as paediatric cardiac surgery and military operations, when blood components are not available, the use of whole blood (WB) has almost entirely been replaced by blood component therapy1. However, as many severely bleeding patients need red cells, platelets and plasma, civilian hospitals now often provide “transfusion packages” -containing these components in a 1:1:1 ratio. The aim of these packages is to mimic whole blood, and the term “reconstituted whole blood” is, therefore, used, although the transfusion packages may consist of bags in which all the components are mixed -or the components may be kept separately1.
As there are no universal criteria for the composition of transfusion packages, severely bleeding patients may be treated with red cell concentrates and platelet concentrates that have been stored for significantly different periods. Thus both the red cell storage lesion and the platelet storage lesion may cause different outcomes in patients.
The aim of this study was to elucidate the in vitro consequences of the differences in storage age of the cellular components -and to compare the responses of reconstituted WB to those of what could be regarded as “the golden standard” under these circumstances- fresh WB. Collagen was used to simulate in vivo stimulation and the responses tested were: platelet disappearance, which is an indicator of platelet function; thrombin-antithrombin (TAT) complex formation, which is an indicator of coagulation activation; and thrombelastography (TEG), which is often, not least in trauma patients, used as an indicator of overall haemostatic function.
Materials and methods
Study design
The platelet aggregation response and thrombin-antithrombin complex formation in fresh WB and reconstituted WB were compared. The reconstituted WB consisted of red cells and platelets that had been stored for different, defined periods with the addition of fresh-frozen plasma in all cases.
Volunteers, blood components, blood sampling, and assays
Fresh WB samples were obtained from ten healthy volunteers with their informed consent. The WB (450 mL) was drawn into bags containing 63 mL CPD-A (Fenwal Inc., Lake Zurich, IL, USA). Immediately after collection, a 10 mL aliquot was removed for analysis. The reconstituted WB was prepared from 6 mL aliquots from SAGM red cell concentrates (from a total of 90 healthy volunteers with their informed consent) stored for 0–4 days, 12–16 days, or 26–35 days, 3 mL aliquots from platelet concentrates made after pooling five buffy coats and stored for 1, 3, and 5 days and 6 mL aliquots of freshly thawed Octaplas (Octapharma, Lachen, Switzerland). Accordingly, by varying red cell age and platelet age in a matrix setup, nine variations of reconstituted WB were produced (Table I and Table II). Before sampling, each bag was thoroughly mixed. Aliquots of 1 mL were then stimulated with collagen (Chrono-Log Corporation, Havertown, PA, USA, 1 mg/mL) in final concentrations of 5 μg/mL, 10 μg/mL, and 20 μg/mL and gently mixed on a rotor (Labinco BV., Model L-26, Breda, The Netherlands) for 5 minutes. Stimulated fresh WB samples were mixed gently by hand inversion. Control samples were included for each donor.
Table I.
Overview of the composition of the groups containing reconstituted whole blood.
| Platelets | Red blood cells | Plasma | |
|---|---|---|---|
| Group A | 1 day | 0 – 4 days | Fresh |
| Group B | 1 day | 12 – 16 days | Fresh |
| Group C | 1 day | 26 – 35 days | Fresh |
| Group D | 3 days | 0 – 4 days | Fresh |
| Group E | 3 days | 12 – 16 days | Fresh |
| Group F | 3 days | 26 – 35 days | Fresh |
| Group G | 5 days | 0 – 4 days | Fresh |
| Group H | 5 days | 12 – 16 days | Fresh |
| Group I | 5 days | 26 – 35 days | Fresh |
Nine different groups of reconstituted whole blood with different composition of platelets, red blood cells, and plasma were compared during the study. Platelets stored for 1, 3, or 5 days were combined with red blood cells stored for 0–4 days, 12–16 days, or 26–35 days. Freshly thawed AB RhD positive plasma (Octaplast 200 mL, Octapharma, Lachen, Switzerland) was used in all groups.
Table II.
Characteristics of the units tested.
| Group | PLTi | SD | Hb, g/dL | SD | Hct, % | SD |
|---|---|---|---|---|---|---|
| A | 171.9 | 12.75 | 12.2 | 0.40 | 34.6 | 1.04 |
| B | 166.8 | 46.08 | 10.2 | 1.04 | 29.5 | 1.96 |
| C | 174.9 | 14.20 | 12.4 | 0.27 | 35.1 | 1.65 |
| D | 159.8 | 30.48 | 11.4 | 1.56 | 33.7 | 1.32 |
| E | 162.0 | 28.06 | 14.3 | 0.65 | 41.1 | 1.56 |
| F | 188.1 | 16.63 | 12.9 | 0.42 | 37.1 | 1.03 |
| G | 160.8 | 19.03 | 12.1 | 1.48 | 36.0 | 1.56 |
| H | 159.2 | 32.74 | 11.8 | 1.40 | 34.0 | 1.43 |
| I | 148.5 | 35.86 | 12.2 | 1.72 | 36.8 | 1.95 |
| WB | 137.5 | 31.94 | 13.0 | 1.95 | 38.7 | 1.91 |
All units tested were from blood donors at the Blood Bank at Haukeland University Hospital. Fresh WB samples were acquired directly from blood bags (Fenwal, Inc., Lake Zurich, IL, USA). The haematocrit values were measured as the volume percentage (%) of red blood cells in blood, whereas the haemoglobin values are given in g/dL. The values represent the average for the ten donors in each group, without collagen stimulation. An overview of the composition of the groups containing reconstituted whole blood is given in Table I. Abbreviations: PLTi: impedance platelet count; SD: standard deviation; Hb: haemoglobin; Hct: haematocrit.
The TEG analyses were performed on a TEG 5000, version 4.2 (Haemoscope Corporation, Niles, IL, USA). All analyses were performed with TEG disposable cups and pins as devised by the manufacturer. For each sample, 1 mL of blood was added to a kaolin vial and gently mixed by inversion. Then, 20 μL CaCl2 (B. Brown, Melsungen, Germany) and 340 μL of the kaolin-treated citrated blood in the vial were added to a neutral cup2.
Collagen-stimulated platelet count reduction
The collagen-induced platelet count reduction (CPCR) was calculated on the basis of pretesting platelet counts (n0=100% of platelets in sample) and platelet counts after collagen stimulation (n1). The CPCR percentage is defined as 100–(n1/n0)×100. Platelet concentrations were determined by the impedance method on a clinical blood cell counter (Cell-Dyn Sapphire, Abbott Diagnostic, IL, USA).
Thrombin-antithrombin complex formation
Plasma samples were made from both fresh WB and reconstituted WB. The samples were centrifuged for 10 minutes at 1,500 g on a Sigma 2–5 table centrifuge (DJB Labcare, Buckinghamshire, England) and stored at –20 °C until further use. Human TAT complex was quantified in the plasma in vitro using a commercially available TAT complex enzyme-linked immunosorbent assay kit (Cusabio Biotech Co., Ltd., Hubei Province, China), following the manufacturer’s guidelines. The optical density of each well was determined within 30 minutes, using a SpectraMax Plus384 Absorbance Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) set to 450 nm. In each assay, seven standard concentrations were run in duplicate. The relationship between concentration of standard and optical density was established by fitting the data to a four-parameter fit, as described by the manufacturer (Molecular Devices, Sunnyvale, CA, USA).
Statistics
All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). The Mann-Whitney test was used for statistical analyses, and p values <0.05 were considered statistically significant. Correlations between platelet ages were analysed via an intra-class correlation coefficient and Spearman’s correlation coefficient, and Bonferroni’s correction was applied to counteract the problem of multiple comparisons.
Results
Collagen-induced platelet count reduction
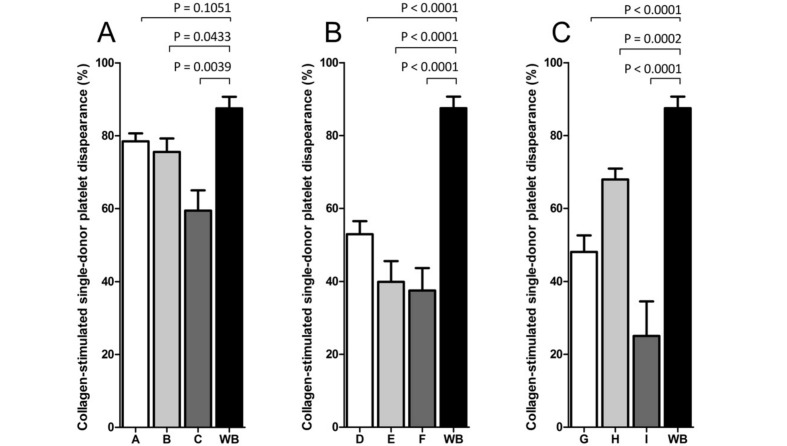
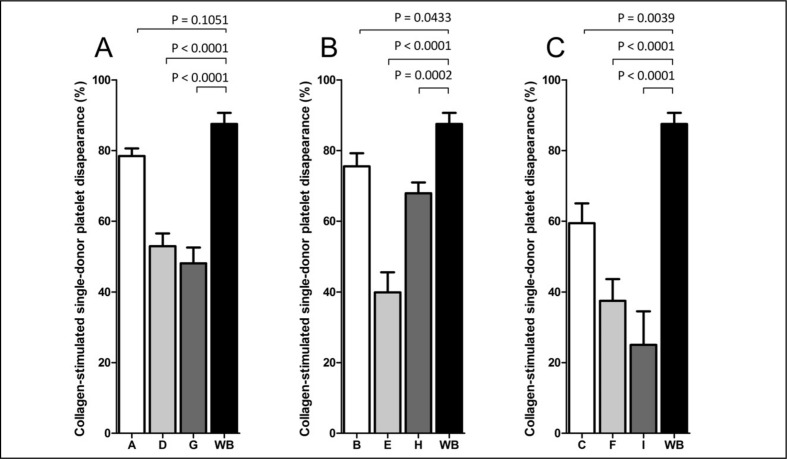
The CPCR was greater in fresh WB than in all of the nine formulations of reconstituted WB. In reconstituted WB, the CPCR decreased with increasing platelet storage age and increasing red cell age, as shown in Figure 1 and Figure 2. The exception from this trend was found only in group H, in which platelets had been stored for 5 days and the red cells had been stored in the medium range (12–16 days).
Figure 1.
The effect of collagen (10 μg/mL) (Chrono-log Corporation) stimulation on CPCR in reconstituted whole blood and fresh whole blood.
A matrix system (Table I) was set up to provide nine variations of reconstituted whole blood (varying storage age of red cells and platelets), whilst the fresh whole blood was stored for a maximum of 4 hours. The figure shows the results for platelets stored for 1 day (A), 3 days (B), and 5 days (C). The samples were stimulated for a minimum of 5 minutes prior to analysis. All groups show a decrease in CPCR after stimulation with collagen. A significant decrease in platelet response to agonist was observed in control samples compared with stimulated samples (p<0.05, Wilcoxon’s test for paired samples). The results are presented as CPCR of control samples and stimulated samples. A statistically significant decrease of CPCR was found between fresh whole blood and all groups of reconstituted whole blood (p<0.05, Wilcoxon’s test for paired samples). P-values are indicated above the graphs.
Figure 2.
The determination of CPCR in reconstituted whole blood and fresh whole blood after collagen (10 μg/mL) (Chrono-log Corporation) stimulation.
The figure shows the results for red blood cells stored for 0–4 days (A), 12–16 days (B), and 26–35 days (C). The samples were stimulated for a minimum of 5 minutes prior to analysis. All groups show a decrease in CPCR after stimulation with collagen. A significant decrease in platelet response to agonist was observed in control samples compared with stimulated samples (p<0.05, Wilcoxon’s test for paired samples). The results are presented as CPCR for control samples and stimulated samples. A statistically significant decrease of CPCR was found between whole blood and all groups of reconstituted whole blood (p<0.05, Wilcoxon’s test for paired samples). P-values are indicated above the graphs.
Thrombelastograhy
No significant abnormalities were seen in the TEG tracing of either fresh WB or reconstituted WB when compared to the standard values given by the manufacturer (data not shown).
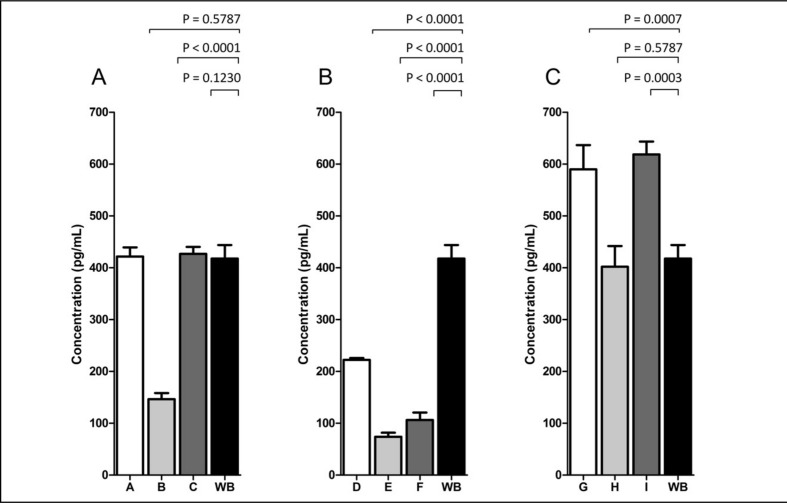
Thrombin-antithrombin complex formation
TAT complex formation was examined in all the groups, as well as in the fresh WB. Figure 3 shows that the highest concentrations of thrombin generation occurred in the samples of reconstituted WB with platelets stored for 5 days, whereas the lowest concentrations were found in the reconstituted WB with platelets stored for 3 days. The exception was group H, which contained old platelets (stored for 5 days) and red cells stored for a medium period of time. All the groups of reconstituted WB with platelets stored for 3 days (groups D, E, and F) showed concentrations of TAT-complex that were significantly lower than those in fresh WB.
Figure 3.
Determination of thrombin generation in reconstituted whole blood and fresh whole blood after collagen stimulation (10 μg/mL) (Chrono-log Corporation).
The figure shows the results for platelets stored for 1 day (A), 3 days (B), and 5 days (C). All results are presented as TAT complex levels (pg/mL). Significant differences were found between whole blood and groups B, D, E, F, G, and I (p<0.05). The Wilcoxon’s test for paired samples was used for statistical comparisons, and p-values are shown above the graphs.
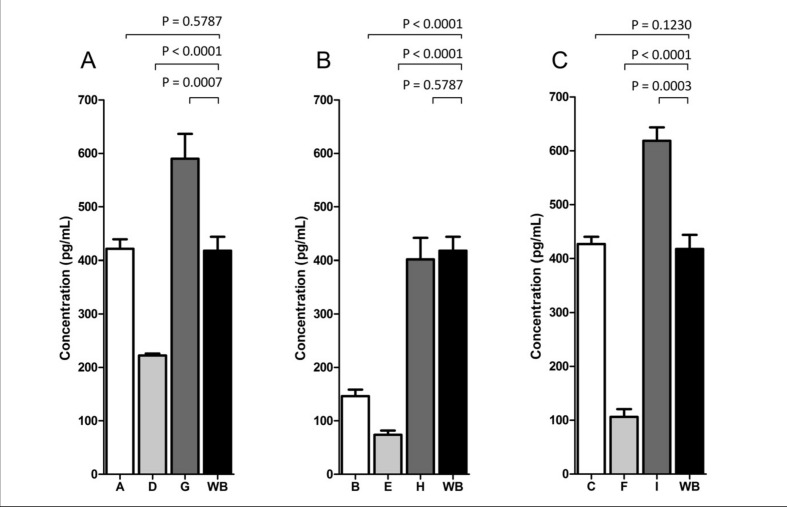
When analysing TAT-formation in relation to red cell age, the groups with the oldest erythrocytes had the highest concentration of thrombin generated (Figure 4). The concentrations of TAT-complex were significantly higher in groups G and I than in fresh WB (Figure 4).
Figure 4.
The effect of collagen (10 μg/mL) (Chrono-log Corporation) stimulation on thrombin generation in reconstituted whole blood and fresh whole blood.
The figure shows the results for red blood cells stored for 0–4 days (A), 12–16 days (B), and 26–35 days (C). All results are presented as TAT complex levels (pg/mL). Significant differences were found between whole blood and groups B, D, E, F, G, and I (p<0.05). Wilcoxon’s test for paired samples was used for statistical comparisons, and p-values are shown above the graphs.
A significant difference (p<0.005) in TAT-complex concentration between unstimulated samples and samples stimulated with collagen was seen in all groups (Figures 3 and 4). In all experiments, the response increased with increasing concentrations of collagen. All the statistical analyses were based on stimulation with 10 μg/mL of collagen.
Discussion
We have demonstrated that platelet aggregation response is greater in fresh WB than in reconstituted WB, even if the platelets and red cells involved are stored for only a few days. During prolonged storage of platelets, the aggregation responses diminish, which is in line with results from experiments involving platelet concentrates3,4. The reduction in aggregation responses during storage seems to be comparable to that found in a study in which refrigerated WB was used5.
Some studies from experience in battlefield traumatology, cardiac surgery and anecdotal case reports indicate that fresh WB has excellent haemostatic properties1–3.
Although in vitro data should not be extrapolated to in vivo conditions, the data from this study suggest that the fresh platelets in fresh WB are more reactive with collagen and may, therefore, be more able to adhere at the site of injury and participate in primary platelet plug formation. Similar results are reported as supernatants from stored red cells inhibit platelet aggregation6. This response is not part of the conventional tests of plasma coagulation or clot formation in TEG devices. Maximum clot strength in TEG devices is a function of fibrinogen concentration and the ability of the platelets to pull the cross-linked fibrin gel into a tight fibrin meshwork. Platelet counts at which this process becomes defective are of the order of 30×109/L, well below the counts observed in this study. It is not surprising that all of the TEG results were normal7.
TAT complex formation occurs at a very slow rate during blood storage even in anticoagulated plasma. The rate of TAT-complex formation is increased in the presence of blood cells whose aging membranes form negatively-charged phospholipid rafts which greatly accelerate early steps in plasma coagulation. However, the rate at which stored cellular products from different donors manifest these storage-related changes is highly variable in a donor-specific manner. Thus, while there was a general trend that TAT-complex concentrations were increased in the presence of platelets and red blood cells stored for longer periods, donor-specific effects heavily influenced the results. Similar results have been reported from studies involving platelet concentrates8, 9.
It is, however, interesting to note that TAT-complex formation was greater in experiments with red cells stored for 12–16 days. Our study does not explain this observation, but does point to the possibility that the storage age of red cells may have greater biological importance than we often consider, for example, as relates to pro-inflammatory effects6,10.
In our study, we did not find any significant differences between fresh WB and reconstituted WB evaluated by TEG, irrespective of cell storage ages. This is in line with results from another study using a different TEG method11. The results may, however, just reflect that TEG is a too insensitive a method for quality control of blood components. Clinically, TEG is used in the evaluation of patients with major haemorrhage2 or apparent platelet dysfunction12.
This study clearly indicates that patients receiving reconstituted WB -or transfusion packages- are treated with compositions of cellular components that cause, in vitro, significantly different responses with regards to platelet aggregation and TAT complex formation. These differences cannot be detected by standard TEG. Fresh WB yields more homogeneous responses, and the platelet aggregation response to collagen is consistently better than in reconstituted WB. These findings should not be interpreted as a support to the re-introduction of fresh WB, but they do indicate that the crucial question is still appropriate: is there a place for WB in some patients with massive bleeding?
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Young PP, Cotton BA, Goodnough LT. Massive transfusion protocols for patients with substantial hemorrhage. Transfus Med Rev. 2011;25:293–303. doi: 10.1016/j.tmrv.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reikvam H, Steien E, Hauge B, et al. Thrombelastography. Transfus Apher Sci. 2009;40:119–23. doi: 10.1016/j.transci.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Albanyan A-M, Murphy MF, Harrison P. Evaluation of the Impact-R for monitoring the platelet storage lesion. Platelets. 2009;20:1–6. doi: 10.1080/09537100802468653. [DOI] [PubMed] [Google Scholar]
- 4.Schlagenhauf A, Kozma N, Leschnik B, et al. Thrombin receptor levels in platelet concentrates during storage and their impact on platelet functionality. Transfusion. 2012;52:1253–9. doi: 10.1111/j.1537-2995.2011.03475.x. [DOI] [PubMed] [Google Scholar]
- 5.Jobes D, Wolfe Y, O’Neill D, et al. Toward a definition of “fresh” whole blood: an in vitro characterization of coagulation properties in refrigerated whole blood for transfusion. Transfusion. 2011;51:43–51. doi: 10.1111/j.1537-2995.2010.02772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matheu FA, McFaul SJ. Supernates from stored red blood cells inhibit platelet aggregation. Transfusion. 2010;50:1196–202. doi: 10.1111/j.1537-2995.2010.02582.x. [DOI] [PubMed] [Google Scholar]
- 7.Macafee B, Campbell JP, Ashpole K, et al. Reference ranges for thromboelastography (TEG®) and traditional coagulation tests in term parturients undergoing caesarean section under spinal anaesthesia. Anaesthesia. 2012;67:741–7. doi: 10.1111/j.1365-2044.2012.07101.x. [DOI] [PubMed] [Google Scholar]
- 8.Johansson PI, Svendsen MS, Salado J, et al. Investigation of the thrombin-generating capacity, evaluated by thrombogram, and clot formation evaluated by thrombelastography of platelets stored in the blood bank for up to 7 days. Vox Sang. 2008;94:113–8. doi: 10.1111/j.1423-0410.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- 9.Curvers J, van Pampus ECM, Feijge MAH, et al. Decreased responsiveness and development of activation markers of PLTs stored in plasma. Transfusion. 2004;44:49–58. doi: 10.1111/j.0041-1132.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 10.McFaul SJ, Corley JB, Mester CW, Nath J. Packed blood cells stored in AS-5 become proinflammatory during storage. Transfusion. 2009;49:1451–60. doi: 10.1111/j.1537-2995.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 11.Bartfeld G, Ellis M, Lubetzky A, et al. Storage of blood components does not decrease haemostatic potential: in vitro assessment of fresh versus stored blood components using thromboelastography. Transfus Med Hemoth. 2010;37:329–35. doi: 10.1159/000322256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apelseth TO, Hervig T, Bruserud O. Platelet transfusion in acute leukemia patients with severe chemotherapy-induced thrombocytopenia: the possible importance of hemoglobin levels and red blood cell transfusions for evaluation of clinical effects of transfusion. Transfusion. 2010;50:2505–6. [Google Scholar]