Abstract
Background
The treatment options in severe thrombocytopenia (platelet count ≤20×109/L) are limited. The aim of this study was to investigate ways of improving blood clotting and stability in reconstituted thrombocytopenia.
Materials and methods
Thrombocytopenia (platelets [16±4]×109/L) was created by differential centrifugation of normal blood followed by reconstitution of whole blood which was subjected to clotting in a rotation thromboelastometer by CaCl2 and tissue factor, and to fibrinolysis by tissue plasminogen activator (tPA). In separate experiments, blood was diluted by 40% with TRIS/saline solution. Blood was treated with fibrinogen (fib), factor XIII (FXIII), and thrombin-activatable fibrinolysis inhibitor (TAFI).
Results
The maximum clot firmness of thrombocytopenic blood was approximately 2-fold less than that of intact blood. Supplementation of blood with fib and FXIII improved clot formation. In the presence of tPA, among fib, FXIII and TAFI, only fib stimulated clot propagation whereas each of these agents increased clot strength. There was a synergistic effect when fib was added together with FXIII or TAFI. Fibrinolysis was inhibited by TAFI and to a greater extent by TAFI + FXIII. Fourty percent dilution of blood reduced clot strength and increased susceptibility to tPA. Clot strength was increased by the treatments in the following order: fib/FXIII/TAFI > fib/TAFI > fib > TAFI > FXIII. In the presence of tPA, TAFI and FXIII lysed the clots significantly more slowly. This effect was stronger when blood was treated with the combination of fib/FXIII/TAFI. Doubling the fib concentration, alone or together with other agents, did not improve clot strength or stability.
Discussion
Augmentation of clot formation and anti-fibrinolysis by combining fib, FXIII and TAFI may be beneficial for the treatment of patients with severe thrombocytopenia especially when complicated by haemodilution following introduction of fluids to compensate for massive blood loss.
Keywords: thrombocytopenia, thromboelastometry, fibrinogen, factor XIII, thrombin-activatable fibrinolysis inhibitor
Introduction
Thrombocytopenia is a common disorder mostly presenting as primary immune thrombocytopenia (ITP), which is characterised by a low platelet count due to accelerated platelet destruction and reduction of platelet production as a result of auto-antibodies against platelet surface antigens, as well as T-cell toxicity1,2. The consequence of elimination of platelets from the circulating blood is complex because platelets are important not only in building up the primary haemostatic plug but also in thrombin generation, clot formation and clot retraction3,4. Bleeding symptoms in severe thrombocytopenia are variable, ranging from mild to severe, and occasionally may even be life-threatening. The risk of bleeding becomes much higher when the thrombocytopenic patient undergoes severe trauma or surgery due to haemodilution caused by the introduction of fluids to counteract a reduction of blood pressure, followed by decreases of coagulation and anti-fibrinolytic factors. In addition, fibrinolytic agents are released from the endothelium5,6.
For many years, the treatments for ITP have targeted the immune and reticulo-endothelial systems and include corticosteroids, intravenous immunoglobulin, anti-RhD immunoglobulin, immunosuppressive drugs and splenectomy; thrombopoietic agents have also been used2,7. All these interventions are, however, more suitable for long-term therapy, while the possibilities for controlling acute bleeds are limited and mainly limited to platelet transfusion which was found to be useful in the emergency treatment of ITP8. However, some patients are refractory to platelet transfusions and there can be complications following these transfusions. Recombinant factor VIIa (rFVIIa) was demonstrated to stop bleeding in severe thrombocytopenia, promoting thrombin generation through an interaction with tissue factor and direct activation of factor X on the surface of activated platelets9,10. In conclusion of a systemic review of studies assessing the efficacy of rFVIIa in ITP patients, Salama et al. indicated that rFVIIa may help in the emergency treatment of such patients who do not respond to other therapies11. However, diminished response to rFVIIa was observed in patients with very low platelet counts12. Furthermore, there are some limitations to using rFVIIa in the treatment of thrombocytopenia including its high cost, depletion of coagulation factors, possible production of antibody to the factor and the risk of thrombosis. Thus, there is a need for additional effective agents to control bleeding in patients with severe thrombocytopenia. In this study we evaluated the haemostatic effect of fibrinogen, factor XIII (FXIII), and thrombin activatable fibrinolysis inhibitor (TAFI) in vitro using a model of severe thrombocytopenia based on reconstituted blood.
Among clotting factors fibrinogen has been found to be the most important limiting factor in massive haemorrhage. High fibrinogen concentrations increase clot stiffness through the production of greater fibers13. Fibrinogen concentrate has been widely used in experimental and clinical settings including thrombocytopenia14–17.
FXIIIa increases both fibrin polymer strength and resistance to proteolysis catalysing the formation of covalent bonds between glutamine and lysine residues in fibrin molecules18. There is evidence that FXIII incorporates α2-antiplasmin into fibrinogen and fibrin19. In vitro supplementation of blood with FXIII slightly increased clot strength and moderately decreased the susceptibility to tissue plasminogen activator (tPA)-induced fibrinolysis in Glanzmann’s thrombasthenia and a haemodilution model20,21. However, although FXIII is known to produce mechanical clot stability, it appears to be suboptimal in conferring full resistance to fibrinolysis22.
TAFI is a non-active zymogen which is converted to the active form (TAFIa) by thrombin and, even more so, by the thrombin/thrombomodulin complex2. TAFIa intensifies the antifibrinolytic system by decreasing plasminogen binding to fibrin and increasing plasmin inhibition by α2-antiplasmin23,24. A part of TAFI is synthesised in megakaryocytes, expressed by platelets and secreted on platelet activation25. This could be significant in areas of vascular damage, where activated platelets are known to accumulate26.
Over the last decade, thromboelastography emerged as a valuable tool for monitoring haemostasis in coagulopathy, blood transfusion and clotting factor replacement therapy27,28. A new approach involves evaluating clot susceptibility to fibrinolysis (ex vivo and in vitro) after the addition of tPA29,30. In this respect, thromboelastography has been used in different experimental models and clinical settings including thrombocytopenia to evaluate clot formation and lysis in whole blood.
The aims of this study were to: (i) characterise the coagulopathy in reconstituted blood with severe thrombocytopenia (platelet count [16±4]×109/L); (ii) evaluate the role of haemodilution, which is usually caused by the administration of massive amounts of fluid in order to support blood pressure in patients with severe bleeding6; and (iii) investigate the haemostatic responses of severely thrombocytopenic blood to spiking with fibrinogen, FXIII and TAFI separately and in combinations.
Material and methods
Preparation of native blood, reconstitution of thrombocytopenic blood and haemodilution
This study was approved by the Human Subjects Ethics Committee of the Sheba Medical Centre and all participants signed informed consent. Peripheral venous blood was drawn from 42 healthy volunteers without any history of coagulation or platelet disorders and who had not taken any medication known to affect haemostasis for at least 10 days prior to the blood sampling. Blood samples were considered suitable for the study when the haematocrit was within the range of 36–48% and the fibrinogen concentration in plasma was between 2.5 and 4.0 g/L. Blood was collected in polypropylene tubes using a 21-gauge butterfly needle and a Vacutainer system. The first tube was discarded to exclude coagulation activation during the vein puncture. The second portion was drawn into tubes containing EDTA for blood counts. The third and the forth portions were drawn into tubes containing sodium citrate solution (0.106 M) which was mixed with the blood in a ratio of 1:9. Prothrombin time, activated partial thromboplastin time, thrombin time, and fibrinogen clotting studies were performed using standard laboratory methods in plasma prepared by centrifugation of whole blood at 1,600 g for 15 minutes. Prothrombin, thrombin and fibrinogen for these assays were from Instrumental Laboratory (Milan, Italy).
To produce thrombocytopenia, native blood was centrifuged at 160 g for 12 minutes. Platelet-rich plasma was removed and subjected to high speed centrifugation (10,000 rpm for 3 minutes). The upper part of packed cells (10% of their volume) was discarded and the remaining packed cells mixed with plasma in the same ratio as it was after the first centrifugation of whole blood. The platelet count in the reconstituted whole blood was (16±4)×109/L. Fourty percent haemodilution was achieved by adding TRIS/saline solution. Products for spiking whole blood were fibrinogen essentially free of plasminogen (Sigma Aldrich), FXIII (Fibrogammin) and TAFI (CSL Behring GmbH, Marburg, Germany). tPA (Actilyse) was from Boehringer-Ingelheim (Paris, France). Fibrinogen was used at a concentration of 3 g/L, approximately doubling its normal concentration in blood. The chosen FXIII concentration (2.0 U/mL) was based on titration experiments and an observation of others showing that in vitro spiking of blood taken from intensive care patients with supraphysiological concentrations of FXIII increased clot strength31. tPA was used at a relatively high concentration (160 ng/mL) to reveal the possible anti-fibrinolytic potential of the haemostatic agents.
Rotation thromboelastometry
Before the assay, whole blood samples were rested for 40 minutes at room temperature. Rotation thromboelastometry (ROTEM) measurements were conducted by a ROTEM™ device (Pentapharm, Munich, Germany) using 300 μL of native or reconstituted whole blood placed into cups following the introduction of CaCl2 (20 mM) and a low concentration of tissue factor (EXTEM reagent diluted 1:1,700). ROTEM tests were performed according to the manufacturer’s instructions at 37 °C and ran for a minimum of 60 minutes. The following variables were used: alpha-angle (α-angle in degrees) reflecting clot propagation, maximum clot firmness (MCF), clot strength in mm, and lysis onset time (LOT), time in minutes from the beginning of clot formation to a fall in the clot amplitude by 15% from MCF. To ensure uniform methodology, all rotation thromboelastometry tests were performed by the same researcher.
Statistics
The data are expressed as mean ± standard deviation and analysed using one-way ANOVA followed by Tukey’s post hoc test. The differences between groups were considered to be statistically significant when the probability of a type I error was less than 0.05.
Results
Clot formation in normal and thrombocytopenic blood
Normal and reconstituted thrombocytopenic blood samples were supplemented with fibrinogen, FXIII or both coagulation factors together.
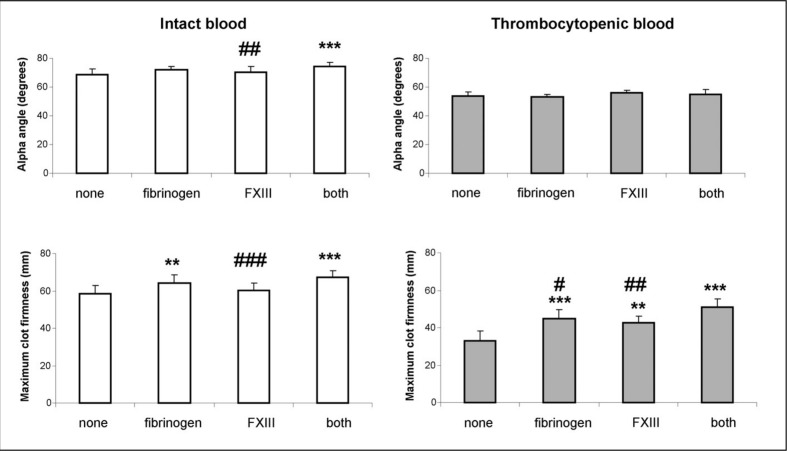
The results of the experiments presented in Figure 1 show that the α-angle was not changed while the MCF value was two times shorter in thrombocytopenic blood than in normal blood (33.1±5.3 versus 58.3±4.5 mm, respectively). In normal blood samples, the α-angle and MCF were not changed by addition of either fibrinogen or FXIII although their values were higher when both agents were added in combination (74.5±2.6 versus 68.8±3.6 degrees and 67.4±3.3 versus 58.3±4.5 mm). In thrombocytopenic blood, the α-angle was not affected by the introduction of fibrinogen and FXIII. In contrast, the MCF was augmented by both fibrinogen and FXIII and to a greater extent by both agents together (44.8±4.9, 42.6±3.7 and 51.1±4.1 versus 33.1±5.3 mm, respectively).
Figure 1.
Effect of fibrinogen and factor XIII on clot formation.
FXIII: factor XIII. Native and reconstituted thrombocytopenic (platelet count [16± 4]×109 mL−1) whole blood samples were treated with fibrinogen (3 g/L), factor XIII (2 U/mL) or the both agents. Blood samples were subjected to clotting by CaCl2 (20 mM) and EXTEM-reagent (final dilution 1:1,700) and assayed in a rotation thromboelastometry device. The α-angle and maximum clot firmness (MCF) were measured. The data are expressed as means ± standard deviation (SD). **p<0.01, ***p<0.001 statistically significant differences between treated and non-treated whole blood. ##p<0.01, ###p<0.001 statistically significant differences between treatment with both and single agents (n=8). The data were analysed using one-way repeated measures ANOVA followed by Tukey’s post hoc test.
Clot formation and resistance to fibrinolysis in thrombocytopenia blood
In these experiments we assayed the effect of fibrinogen, FXIII and TAFI on clot formation in the presence of tPA and susceptibility to tPA-induced fibrinolysis. In the presence of tPA, all ROTEM variables were lower in thrombocytopenic blood than in normal blood (α-angle and MCF, both p<0.001 and LOT p<0.01; Table I). Reduction of the LOT value means higher sensitivity of the clot to tPA-induced fibrinolysis.
Table I.
Parameters of clot formation and fibrinolysis in thrombocytopenia.
| Normal | Thrombocytopenia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Buffer | Buffer | Fib | FXIII | TAFI | Fib+FXIII | Fib+TAFI | FXIII+TAFI | Fib+FXIII+TAFI | ||
| α-angle | Mean | 66.1 | 55.1 | 73.4 | 61 | 56.8 | 79.3 | 75.4 | 62.8 | 70.5 |
| SD | 6.7 | 2.4 | 2.4 | 4.2 | 3.0 | 3.4 | 4.9 | 2.7 | 4.3 | |
|
| ||||||||||
| MCF | Mean | 45.6 | 28.5 | 41.6 | 36.6 | 42.4 | 47.9 | 52.1 | 44.4 | 50.5 |
| SD | 6.8 | 3.4 | 4.2 | 2.3 | 3.7 | 4.6 | 3.6 | 7.0 | 2.9 | |
|
| ||||||||||
| LOT | Mean | 25.6 | 17.6 | 17.8 | 22.4 | 31.4 | 24.5 | 35.4 | 39.2 | 37.1 |
| SD | 5.2 | 5.2 | 6.9 | 6.4 | 4.2 | 7.8 | 8.3 | 9.7 | 4.1 | |
Fib: fibrinogen; FXIII: factor XIII; TAFI: thrombin-activatable fibrinolysis inhibitor. Reconstituted thrombocytopenic whole blood (platelet count [16±4]×109/L) was treated with fibrinogen (3 g/L), factor XIII (3 U/mL), or thrombin activatable fibrinolysis inhibitor (0.25 U/mL) as well as with these agents in combinations. Blood samples were subjected to clotting by CaCl2 (20 mM) and EXTEM-reagent (final dilution 1:1,700) in the presence of tPA (160 ng/mL) and assayed in a rotation thromboelastometry device. Alpha-angle (α-angle in degrees), maximum clot firmness (MCF, mm), and lysis onset time (LOT, min) were measured (n=8). The data were analysed using one-way repeated measures ANOVA followed by Tukey’s post hoc test.
Alpha-angle
Augmentation of the α-angle was documented by spiking thrombocytopenic blood with fibrinogen and FXIII comparing the results with those at baseline (p<0.001, p<0.05, respectively). The combination of fibrinogen with FXIII led to a further increase of this variable which became significantly higher than with fibrinogen (p<0.05) or FXIII alone (p<0.001). TAFI did not affect the α-angle even in combinations with fibrinogen or FXIII. It is important to note that the introduction of fibrinogen by itself to thrombocytopenic blood was enough to overcome the level of the α-angle in normal blood. Thus, clotting propagation was stimulated by fibrinogen and FXIII and this effect was strengthened by combining the agents.
Maximum clot firmness
The MCF value was markedly higher in thrombocytopenic blood spiked with either fibrinogen, FXIII or TAFI compared to the baseline condition (p<0.001, p<0.05 and p<0.001, respectively). The MCF further increased when fibrinogen was added together with TAFI or FXIII. Of note, addition of each of the agents to thrombocytopenic blood was enough to achieve the MCF level in normal blood.
Lysis onset time
The onset of fibrinolysis was not affected by fibrinogen, FXIII or spiking with both agents together. In contrast, fibrinolysis was substantially inhibited by TAFI (p<0.001) but remained at the same level when TAFI was combined with fibrinogen or with FXIII. Thus, among the three agents, only TAFI increased the anti-fibrinolytic potential.
Clot formation and resistance to fibrinolysis in diluted thrombocytopenia blood
Thrombocytopenic blood was diluted by 40% with TRIS/saline buffer. There were substantial reductions in the α-angle (38.4±3.7 versus 55.1±2.4 degrees), MCF (4.0±4.1 versus 28.5±3.4 mm) and LOT (11.5±2.7 versus 17.6±5.2 min) values in diluted and non-diluted thrombocytopenic blood, i.e. decreases of clot propagation, clot strength and stability (see Tables I and II).
Table II.
Parameters of clot formation and fibrinolysis in 40% diluted thrombocytopenia.
| Normal (diluted) | Thrombocytopenia (diluted) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Buffer | Buffer | Fib | FXIII | TAFI | Fib+FXIII | Fibr+TAFI | FXIII+TAFI | Fib+FXIII+TAFI | ||
| α-angle | Mean | 58.3 | 38.4 | 44.8 | 41.4 | 39.4 | 46.4 | 52.3 | 44 | 57.9 |
| SD | 7.1 | 3.7 | 4.4 | 2.9 | 2.9 | 4.9 | 5.1 | 3 | 4.4 | |
|
| ||||||||||
| MCF | Mean | 19.3 | 4 | 24.9 | 18.1 | 20.9 | 26.5 | 32 | 24 | 44.5 |
| SD | 6.3 | 4.1 | 3.9 | 5.4 | 5 | 3.4 | 2.9 | 4 | 6 | |
|
| ||||||||||
| LOT | Mean | 20.5 | 11.5 | 13.7 | 19.4 | 25.3 | 20.8 | 27.5 | 26.1 | 31.1 |
| SD | 2.9 | 2.7 | 2.1 | 1.9 | 7.5 | 2.2 | 4.8 | 5.1 | 3.2 | |
Fib: fibrinogen; FXIII: factor XIII; TAFI: thrombin-activatable fibrinolysis inhibitor. Reconstituted thrombocytopenic whole blood [platelet count (16± 4)×109/L] was diluted by 40% with TRIS/saline solution. The blood samples were treated with fibrinogen (3 g/L), factor XIII (2 U/mL), or thrombin activatable fibrinolysis inhibitor (0.25 U/mL) as well as with these agents in combinations. Blood samples were subjected to clotting by CaCl2 (20 mM) and EXTEM-reagent (final dilution 1:1,700) in the presence of tPA (160 ng/mL) and assayed in a rotation thromboelastometry device. Alpha-angle (α-angle in degrees), maximum clot firmness (MCF, mm) and lysis onset time (LOT, min) were measured (n=8). The data were analysed using one-way repeated measures ANOVA followed by Tukey’s post hoc test.
Alpha-angle
Neither fibrinogen, FXIII nor TAFI altered the α-angle variable compared to that of non-treated thrombocytopenic blood. In contrast, combining fibrinogen with either FXIII or TAFI augmented the α-angle values (p<0.01 and p<0.001, Table II). The triple combination of fibrinogen, FXIII and TAFI produced a further increase of the α-angle, although this improvement did not reach statistical significance. Thus, clot propagation was not affected by fibrinogen, FXIII or TAFI but was stimulated by combining these agents.
Maximum clot firmness
Spiking blood with fibrinogen, FXIII and TAFI led to enlargement of clot strength (all p<0.001) reaching the value of MCF in normal diluted blood. Furthermore, fibrinogen and TAFI had a synergistic effect on MCF and the triple combination of all agents was followed by a greater augmentation of the MCF value which overcame the level of MCF in normal diluted blood.
Lysis onset time
Fourty percent dilution of both normal and thrombocytopenic blood led to increased susceptibility to tPA (shortening the LOT value) compared to that of non-diluted blood. Spiking thrombocytopenic blood with FXIII or TAFI led to prolongation of LOT compared to that of non-treated blood (both p<0.001) with TAFI exerting a stronger effect than FXIII. Fibrinogen did not change the LOT value. No synergistic effect was documented using TAFI in combination with FXIII or with fibrinogen and only a slight increase of LOT was observed when all three agents were used together (p<0.05 compared to TAFI alone).
Discussion
In thrombocytopenia patients, massive or even fatal bleeding usually occurs at platelets counts from 5×109/L to 20×109/L although in some studies a significant predictor of bleeding was thought to be a history of bleeding in the preceding several days32,33. The treatment options for patients with severe thrombocytopenia are limited and mainly restricted to blood or platelet transfusions. The intention of this study was to evaluate, in vitro, alternative therapeutic options for improving clot formation and stability in reconstituted thrombocytopenia (platelet count: [16±4]×109/L). For this purpose we used thromboelastometry based on the findings that this method is superior to other haemostatic tests in predicting bleeding in thrombocytopenia patients and that it offers the possibility of evaluating both clot formation and resistance to fibrinolysis20,34.
Thrombocytopenia was characterised by no change of clotting propagation (α-angle) and about a two-fold reduction of MCF compared to intact blood. Spiking the severely thrombocytopenic blood with fibrinogen (3 g/L) improved clot strength. This effect of fibrinogen has been found in other studies in both in vitro and ex vivo conditions14–17. However, in our experiments, supplementation of blood with fibrinogen was not enough to compensate the impaired blood clotting. Administration of FXIII (2 U/mL) stimulated blood clotting to a similar extent whereas a synergistic effect was observed when fibrinogen and FXIII were used in combination.
There are limited and conflicting data regarding the effect of FXIII on clot formation in thrombocytopenia and massive bleeding. It was recently shown that increasing concentrations of FXIII added to whole blood of healthy individuals and thrombocytopenic patients did not affect clot development but moderately increased the maximum amplitude value35. In contrast, FXIII had a slight or even no effect on clot formation in 30% and 60% diluted normal blood and in blood from patients with Glanzmann’s thrombasthenia20,36,37. Nevertheless, we show here that the effect of spiking blood with both fibrinogen and FXIII together is superior to that achieved with each of these haemostatic agents in compensation of disturbed clot formation in thrombocytopenia.
Improving clot stability is of importance in preventing and treating bleeding disorders. In the present study an attempt was undertaken to evaluate resistance to fibrinolysis in blood with severe thrombocytopenia and a possible protective effect of fibrinogen, FXIII and TAFI against tPA-induced fibrinolysis. The rationale for including TAFI in this study was that platelets contain and secrete TAFI upon activation26,38. A deficiency of platelet-mediated resistance against fibrinolysis may, therefore, occur in severe thrombocytopenia. Of interest, the values of MCF were lower in the presence of tPA than in its absence, both in normal and in thrombocytopenic blood apparently because of the initiation of fibrinolysis before the clot reached its maximal amplitude. As expected, in the presence of tPA, among fibrinogen, FXIII and TAFI, only fibrinogen stimulated the initial clotting process whereas each of these agents increased clotting strength. A synergistic effect was observed when fibrinogen was administered together with either FXIII or TAFI. Among these agents, TAFI strongly and FXIII slightly inhibited fibrinolysis which remained at the same extent when TAFI was combined with fibrinogen or FXIII. This was in accordance with earlier reports which showed that TAFI, and to a lesser extent FXIII attenuate tPA-induced fibrinolysis in human plasma39. In another study, FXIII strongly and rFVIIa modestly improved anti-fibrinolysis in whole blood from thrombocytopenic patients35.
To bring the thrombocytopenia model used closer to the situation that may occur in patients subjected to massive transfusion of blood substitutes, we assayed clot formation and protective potential against fibrinolysis in 40% diluted thrombocytopenia blood. As could be expected, haemodilution led to a further impairment of blood clotting but surprisingly was accompanied by increased susceptibility to tPA. In this condition, clotting propagation (α-angle) was not affected by spiking the blood with fibrinogen, FXIII or TAFI but was augmented when fibrinogen was combined with FXIII or TAFI. In contrast, clot strength (MCF) was substantially increased (4–6 times) by separate addition of each of the agents used. Combining fibrinogen and TAFI further improved clot strength. The order of stimulating blood clotting was: fibrinogen/FXIII/TAFI > fibrinogen/TAFI > fibrinogen > TAFI > FXIII. Blood clots treated with either TAFI or FXIII lysed significantly more slowly indicating a greater resistance to fibrinolysis. This effect was stronger when the blood was treated with the triple combination of fibrinogen, FXIII and TAFI.
Our in vitro study has some limitations. In fact, the attempt to characterise the coagulation alterations in thrombocytopenia was undertaken using a technological approach without taking into account the shear conditions upon blood flow or the modulation of haemostasis by endothelial cells. Furthermore, it should be taken into account that centrifugation steps may affect platelet activity, although it is unlikely that this is associated with essential changes in blood coagulation. The results obtained in vitro cannot, therefore, be automatically translated to the in vivo situation. Nevertheless, the model may help to understand alterations of clot formation and stability in severe thrombocytopenia better and to indicate new therapeutic approaches in critically ill patients who are usually treated with blood, platelet or fluid transfusions.
In summary, our observations highlight the effects of each of the haemostatic agents used and their combinations in thrombocytopenia. It is possible that augmentation of clot formation and anti-fibrinolysis through the combination of fibrinogen with FXIII or TAFI could be a beneficial treatment for patients with severe thrombocytopenia especially complicated by haemodilution following the administration of fluids to compensate for massive blood loss.
Footnotes
The Authors declare that they have no conflicts of interest regarding this research.
References
- 1.Zhou B, Zhao H, Yang RC, Han ZC. Multi-dysfunctional pathophysiology in ITP. Crit Rev Oncol/Hematol. 2005;54:107–16. doi: 10.1016/j.critrevonc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Stasi R, Evangelista ML, Stipa E, et al. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost. 2008;99:4–13. doi: 10.1160/TH07-08-0513. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–65. [PubMed] [Google Scholar]
- 4.Katori N, Tanaka KA, Szlam F, Levy JH. The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thromboelastography. Anesth Analg. 2005;100:1781–5. doi: 10.1213/01.ANE.0000149902.73689.64. [DOI] [PubMed] [Google Scholar]
- 5.Schochl H, Frietch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differrential diagnosis of lysis patterns and prognostic value of thrombioelastometry. J Trauma. 2009;67:125–31. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 6.Bolliger D, Szlam F, Levy JH, et al. Haemodilution-induced profibrinolytic state is mitigated by fresh-frozen plasma: implications for early haemostatic intervention in massive haemorrhage. Br J Anaesth. 2010;104:318–25. doi: 10.1093/bja/aeq001. [DOI] [PubMed] [Google Scholar]
- 7.Salley GP. Current therapies in primary immune thrombocytopenia. Semin Thromb Hemost. 2011;37:621–30. doi: 10.1055/s-0031-1291372. [DOI] [PubMed] [Google Scholar]
- 8.Spahr JE, Rodgers GM. Treatment of immune-mediated thrombocytopenia purpura with concurrent intravenous immunoglobulin and platelet transfusion: a retrospective review of 40 patients. Am J Hematol. 2008;83:122–5. doi: 10.1002/ajh.21060. [DOI] [PubMed] [Google Scholar]
- 9.Wrobel G, Dobaczewski G, Patkowski D, et al. Experiments with recombinant activated factor VII in the treatment of severe refractory thrombocytopenia. Pediatr Blood Cancer. 2006;47:729–30. doi: 10.1002/pbc.21013. [DOI] [PubMed] [Google Scholar]
- 10.Kristensen J, Killander A, Hippe E, et al. Clinical experience with recombinant factor VIIa in patients with thrombocytopenia. Haemostasis. 1996;26(Suppl 1):59–64. doi: 10.1159/000217260. [DOI] [PubMed] [Google Scholar]
- 11.Salama A, Rieke M, Kiesewetter H, et al. Experiences with recombinant FVIIa in the emergency treatment of patients with autoimmune thrombocytopenia: a review of the literature. Ann Hematol. 2009;88:11–5. doi: 10.1007/s00277-008-0608-3. [DOI] [PubMed] [Google Scholar]
- 12.Baxter MS, Schroeder WS, Cheng Y, Bernstein ZP. Diminished response to recombinant factor VIIa in a patient with idiopathic thrombocytopenic purpura. Ann Parmacother. 2006;40:2053–8. doi: 10.1345/aph.1H331. [DOI] [PubMed] [Google Scholar]
- 13.Scrutton MC, Ross-Murphy SB, Bennett GM, et al. Changes in clot deformability – a possible explanation for the epidemiologic association between plasma fibrinogen concentration and myocardial infarction. Blood Coagul Fibrinolysis. 1994;5:719–23. doi: 10.1097/00001721-199410000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Fries D, Innerhofer P, Reif C, et al. The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an in vitro model. Anesth Analg. 2006;102:347–51. doi: 10.1213/01.ane.0000194359.06286.d4. [DOI] [PubMed] [Google Scholar]
- 15.Velik-Salchner C, Haas T, Innerhofer P, et al. The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost. 2007;5:1019–25. doi: 10.1111/j.1538-7836.2007.02481.x. [DOI] [PubMed] [Google Scholar]
- 16.Lang T, Johanning K, Metzler H, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg. 2009;108:751–8. doi: 10.1213/ane.0b013e3181966675. [DOI] [PubMed] [Google Scholar]
- 17.Misgav M, Shenkman B, Budnik I, et al. Distinct effects of fibrinogen and von Willebrand factor on clot formation and platelet adhesion in reconstituted and immune thrombocytopenia. Anesth Analg. 2011;112:1034–40. doi: 10.1213/ANE.0b013e318212fffc. [DOI] [PubMed] [Google Scholar]
- 18.Aledort IM. Factor VIII inhibitor bypassing activity (FEIBA) - addressing safety issues. Haemophilia. 2008;14:39–43. doi: 10.1111/j.1365-2516.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 19.Mosesson MW, Siebenlist KR, Hernandez I, et al. Evidence that alpha2-antiplasmin becomes covalently ligated to plasma fibrinogen in the circulation: a new role for plasma factor XIII in fibrinolysis regulation. J Thromb Haemost. 2008;6:1565–70. doi: 10.1111/j.1538-7836.2008.03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenkman B, Livnat T, Misgav M, et al. The in vivo effect of fibrinogen and factor XIII on clot formation and fibrinolysis in Glanzmann’s Thrombasthenia blood. Platelets. 2012;23:604–10. doi: 10.3109/09537104.2011.642031. [DOI] [PubMed] [Google Scholar]
- 21.Shenkman B, Livnat T, Lubetski A, et al. The in vitro effect of fibrinogen, factor XIII and thrombin-activatable fibrinolysis inhibitor on clot formation and susceptibility to tissue plasminogen activator-induced fibrinolysis in hemodilution model. Blood Coagul Fibrinolysis. 2012;23:370–8. doi: 10.1097/MBC.0b013e328352cb3f. [DOI] [PubMed] [Google Scholar]
- 22.Mutch NJ, Koikkalainen JS, Fraser SR, et al. Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis. J Thromb Haemost. 2010;9:2017–24. doi: 10.1111/j.1538-7836.2010.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Roffa MB, Bajzar L, et al. A study of the mechanism of inhibition of fibrinolysis by activated thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1998;273:27176–81. doi: 10.1074/jbc.273.42.27176. [DOI] [PubMed] [Google Scholar]
- 24.Schneider M, Nesheim M. A study of the protection of plasmin from antiplasmin inhibition within an intact fibrin clot during the course of clot lysis. J Biol Chem. 2004;279:13333–9. doi: 10.1074/jbc.M313164200. [DOI] [PubMed] [Google Scholar]
- 25.Mosnier LO, Buijtenhuijus P, Marx PF, et al. Identification of thrombin activatable fibrinolysis inhibitor (TAFI) in human platelets. Blood. 2003;101:4844–6. doi: 10.1182/blood-2002-09-2944. [DOI] [PubMed] [Google Scholar]
- 26.Schadinger SL, Lin HH, Garand M, Boffa MB. Secretion and antifibrinolytic function of thrombin-activatable fibrinolysis inhibitor from human platelets. J Thromb Haemost. 2010;8:2523–9. doi: 10.1111/j.1538-7836.2010.04024.x. [DOI] [PubMed] [Google Scholar]
- 27.Martini WZ, Cortez DS, Dubick MA, et al. Thromboelastography is better than PT, aPTT, and activated clotting time in detection clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma. 2008;65:535–43. doi: 10.1097/TA.0b013e31818379a6. [DOI] [PubMed] [Google Scholar]
- 28.Chen A, Teruya J. Global hemostasis testing thromboelastography: old technology, new applications. Clin Lab Med. 2009;29:391–407. doi: 10.1016/j.cll.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Levrat A, Gros A, Rugeri L, et al. Evaluation of rotation thromboelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008;100:792–7. doi: 10.1093/bja/aen083. [DOI] [PubMed] [Google Scholar]
- 30.Dirkmann D, Görlinger K, Gisbertz C, et al. Factor XIII and tranexamic acid but not recombinant factor VIIa attenuate tissue plasminogen activator-induced hyperfibrinolysis in human whole blood. Anesth Analg. 2012;114:1182–8. doi: 10.1213/ANE.0b013e31823b6683. [DOI] [PubMed] [Google Scholar]
- 31.Theisinger OM, Baulig W, Asmis LM, et al. In vitro factor XIII supplementation increases clot firmness in rotation thromboelastometry (ROTEM) Thromb Haemost. 2010;104:385–91. doi: 10.1160/TH09-12-0858. [DOI] [PubMed] [Google Scholar]
- 32.Slichter SJ. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfus Med Rev. 2004;18:153–67. doi: 10.1016/j.tmrv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Stasi R. Pathophysiology and therapeutic options in primary immune thrombocytopenia. Blood Transfus. 2011;9:262–73. doi: 10.2450/2010.0080-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunduz E, Akay OM, Bal C, Gulbas Z. Can thromboelastography be a new tool to assess bleeding risk in patients with idiopathic thrombocytopenic purpura? Platelets. 2011;22:516–20. doi: 10.3109/09537104.2011.571317. [DOI] [PubMed] [Google Scholar]
- 35.Johansson PI, Jacobsen N, Viuff D, et al. Differential clot stabilizing effect of rFVIIa and rFXIII-A2 in whole blood from thrombocytopenic patients and healthy volunteers. Br J haematol. 2008;143:559–69. doi: 10.1111/j.1365-2141.2008.07379.x. [DOI] [PubMed] [Google Scholar]
- 36.Haas T, Fries D, Velik-Salchner C, et al. The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesth Analg. 2008;106:1360–5. doi: 10.1213/01.ane.0b013e3181684339. [DOI] [PubMed] [Google Scholar]
- 37.Schlimp CJ, Cadamuro J, Solomon C, Redl H, Schöchl H. The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33% diluted blood with albumin, gelatine, hydroxyethyl starch or saline in vitro. Blood Transfus. 2012;10:1–9. doi: 10.2450/2012.0171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrieri C, Galasso R, Semeraro F, et al. The role of thrombin activatable fibrinolysis inhibitor and factor XI in platelet-mediated fibrinolysis resistance: a thromboelastographic study in whole blood. J Thromb Haemost. 2011;8:154–62. doi: 10.1111/j.1538-7836.2010.04120.x. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen VG, Steenwyk BL, Gurley WQ. Contact activation prolongs clot lysis time in human plasma: role of thrombin-activatable fibrinolysis inhibitor and Factor XIII. J Heart Lung. 2006;25:1247–52. doi: 10.1016/j.healun.2006.06.009. [DOI] [PubMed] [Google Scholar]