Abstract
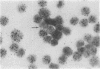
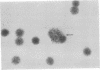
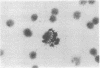
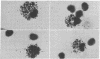
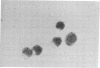
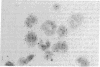
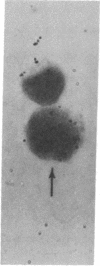
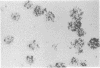
Mouse thymuses with more than 99% T cells have been reported to contain immunoglobulin κ mRNA-like molecules (κ RNA) in relatively large quantities. The present study was undertaken to rule out the possibility that the κ RNA was mainly a product of a few contaminating B cells of the thymus and to determine whether all T-cell subpopulations contained κ RNA. By in situ hybridization with DNA complementary to κ mRNA (κ cDNA) the following observations were made: 98.5% of thymus cell preparations hybridized with κ cDNA; the 1.5% unlabeled cells were generally larger and paler staining than the majority of thymus cells. Only 0.015% of thymus cells were intensely labeled and appeared to be plasma cells. Also, 87% of spleen cells hybridized with κ cDNA; most of these showed similar labeling intensity to the majority of thymus cells. The number of unlabeled cells corresponded to the percentage of hemopoietic cells and macrophages in the spleen. Spleen cells in the range of 0.37-0.85% were intensely labeled and appeared to be plasma cells. The following controls supported the conclusion that the results with thymus and spleen were due to specific hybridization: most of the κ mRNA-deficient tissue culture cells of the plasmocytoid tumor ABPL-4 did not hybridize with κ cDNA. The κ mRNA-producing cells from myeloma PC 3741 hybridized in situ with κ cDNA. Furthermore, all cells from this tumor and all spleen cells hybridized uniformly with a cDNA probe complementary to most of the total cellular poly(A)-containing RNA species of these cells. These results indicate that T cells of all types in the thymus as well as in the periphery contain substantial quantities of κ RNA.
Keywords: T and B cells, complementary DNA
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Feigelson P., Schutz G. Analysis of the complexity and diversity of mRNA from chicken liver and oviduct. Cell. 1976 Feb;7(2):247–254. doi: 10.1016/0092-8674(76)90024-6. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Conkie D., Affara N., Paul J. In situ localization of globin messenger RNA formation. I. During mouse fetal liver development. J Cell Biol. 1974 Nov;63(2 Pt 1):402–413. doi: 10.1083/jcb.63.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. R., Conkie D., Paul J., Jones K. Localisation of cellular globin messenger RNA by in situ hybridisation to complementary DNA. FEBS Lett. 1973 May 15;32(1):109–112. doi: 10.1016/0014-5793(73)80749-5. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Huber B., Cantor H., Shen F. W., Boyse E. A. Independent differentiative pathways of Ly1 and Ly23 subclasses of T cells. Experimental production of mice deprived of selected T-cell subclasses. J Exp Med. 1976 Oct 1;144(4):1128–1133. doi: 10.1084/jem.144.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U., Hager L., Putnam D., Buck L., Farin F., Clagett J. Sequences related to immunoglobulin kappa chain messenger RNA in T cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2467–2471. doi: 10.1073/pnas.73.7.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U., Hager L., Wilson R., Putnam D. Expression of immunoglobulin and globin genes in B and T lymphocytes and other cells. Biochemistry. 1977 Dec 13;16(25):5432–5438. doi: 10.1021/bi00644a005. [DOI] [PubMed] [Google Scholar]
- Storb U., Weiser R. S. Kinetics of mouse spleen cell populations during the immune response. J Reticuloendothel Soc. 1968 Apr;5(2):81–106. [PubMed] [Google Scholar]
- Szenberg A., Marchalonis J. J., Warner N. L. Direct demonstration of murine thymus-dependent cell surface endogenous immunoglobin. Proc Natl Acad Sci U S A. 1977 May;74(5):2113–2117. doi: 10.1073/pnas.74.5.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M., Riblet R. Genetic control of antibody variable regions. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):837–846. doi: 10.1101/sqb.1977.041.01.093. [DOI] [PubMed] [Google Scholar]
- Young B. D., Birnie G. D. Complexity and specificity of polysomal poly(A+) RNA in mouse tissues. Biochemistry. 1976 Jun 29;15(13):2823–2829. doi: 10.1021/bi00658a019. [DOI] [PubMed] [Google Scholar]