Abstract
NrdH-redoxins shuffle electrons from the NADPH pool in the cell to Class Ib ribonucleotide reductases, which in turn provide the precursors for DNA replication and repair. NrdH-redoxins have a CVQC active site motif and belong to the thioredoxin-fold protein family. As for other thioredoxin-fold proteins, the pKa of the nucleophilic cysteine of NrdH-redoxins is of particular interest since it affects the catalytic reaction rate of the enzymes. Recently, the pKa value of this cysteine in Corynebacterium glutamicum and Mycobacterium tuberculosis NrdH-redoxins were determined, but structural insights explaining the relatively low pKa remained elusive. We subjected C. glutamicum NrdH-redoxin to an extensive molecular dynamics simulation to expose the factors regulating the pKa of the nucleophilic cysteine. We found that the nucleophilic cysteine receives three hydrogen bonds from residues within the CVQC active site motif. Additionally, a fourth hydrogen bond with a lysine located N-terminal of the active site further lowers the cysteine pKa. However, site-directed mutagenesis data show that the major contribution to the lowering of the cysteine pKa comes from the positive charge of the lysine and not from the additional Lys-Cys hydrogen bond. In 12% of the NrdH-redoxin family, this lysine is replaced by an arginine that also lowers the cysteine pKa. All together, the four hydrogen bonds and the electrostatic effect of a lysine or an arginine located N-terminally of the active site dynamically regulate the pKa of the nucleophilic cysteine in NrdH-redoxins.
Keywords: NrdH-redoxin, pKa, molecular dynamics, cysteine reactivity, redox, hydrogen bond
Introduction
NrdH-redoxins provide electrons to the Class Ib ribonucleotide reductases NrdEF and are, as such, important reductases in the cell.1 NrdH-redoxins have a thioredoxin-fold, a CVQC active site motif, and a glutaredoxin-like amino acid sequence, but receive electrons from thioredoxin reductase (TrxR).2–3 In the first step of the catalytic reduction of NrdEF by NrdH-redoxin, NrdH-redoxins use a thiol disulfide exchange reaction in which the N-terminal cysteine performs a nucleophilic attack on one of the cysteines of the disulfide substrate. This reaction depends, among other factors, on the pKa of the N-terminal cysteine.4 Recently, we determined a pKa of 6.2 and 6.3 for the N-terminal cysteine of Corynebacterium glutamicum (Cg) and Mycobacterium tuberculosis NrdH-redoxin, respectively.5 However, no structural information on the origin of this relatively low pKa is available since all the reported structures of NrdH-redoxins are in the oxidized state (i.e., the active site cysteines are disulfide bonded).2–7 In the other members of the thioredoxin family [e.g., thioredoxin (Trx), glutaredoxin (Grx), and mycoredoxin (Mrx)], the relatively low pKa of the nucleophilic cysteine is governed by the stabilizing effect of hydrogen bonds on the thiolate form of this cysteine.8–9 However, the contribution of hydrogen bonds to the lowering of the pKa of a cysteine is often not easily recognized from the crystal or solution structures. Extensive molecular dynamics (MD) simulations of Grx clearly showed this limitation.10 Foloppe et al. showed that during MD simulations, the active site of several Grxs converges to a consensus structure despite different starting conformations. In this consensus structure, the N-terminally located nucleophilic cysteine receives three hydrogen bonds from residues within the CXXC motif and these hydrogen bonds maintain the relatively low cysteine pKa. However, the hydrogen bonds were not always present in the experimentally obtained structures used to initiate these simulations. Moreover, in Mrx1 the N-terminal cysteine has an experimentally determined pKa of 6.8, while MD simulations revealed that this pKa could transiently decrease to a value as low as 5.11
We used a MD study to investigate the origin of the low pKa value of the N-terminal cysteine of Cg NrdH-redoxin. Starting from the oxidized structure of Cg NrdH-redoxin, we modeled the reduced protein and subjected it to a MD simulation. During this simulation, the active site of NrdH-redoxin adopts a similar conformation as the consensus structure observed in the MD studies of Grx.10 Furthermore, we observed that a lysine frequently hydrogen bonds the N-terminal cysteine. Site-directed mutagenesis of this lysine and the experimental determination of the cysteine pKa in these mutants showed that the positive charge of the lysine and not the Cys-Lys hydrogen bond is a major factor in regulating the low pKa of the N-terminal cysteine in NrdH-redoxins.
Results and Discussion
We used the crystal structure of oxidized Cg NrdH-redoxin (PDB: 4FIW) to construct a model of reduced NrdH-redoxin and submitted it to a 45 ns MD simulation. Briefly, we made a model of reduced Cg NrdH-redoxin in which the disulfide between the two active site cysteines (Cys11 and Cys14) has been removed and Cys11 was treated as thiolate based on the experimentally determined pKa.5 This model was energy minimized and subjected to a MD simulation in explicit solvent. No major conformational changes were observed during the MD simulation and the average r.m.s.d. for all snapshots was 1.06 Å on 77 Cα atoms. Furthermore, the structure of the reduced protein obtained from the simulation did not substantially differ from the oxidized crystal structure (maximal r.m.s.d. of 2.2 Å on 77 Cα atoms). This is in agreement with the observation that none of the Trx-fold proteins undergo a large conformational change upon oxidation or reduction.12 As such, we decided to focus on the active site cysteines of NrdH-redoxin. The simulation was started with the torsion angles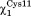 and
and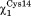 in trans and gauche minus (g−) respectively. These conformations were mostly maintained during the entire simulation and allowed the formation of a hydrogen bond between the side chain thiol of Cys14 and the thiolate of Cys11 (Figs. 1 and 2).
in trans and gauche minus (g−) respectively. These conformations were mostly maintained during the entire simulation and allowed the formation of a hydrogen bond between the side chain thiol of Cys14 and the thiolate of Cys11 (Figs. 1 and 2).
Figure 1.
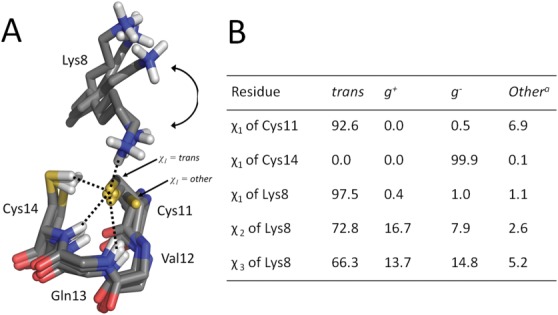
The four hydrogen bonds formed with Cys11 during the MD simulation. A: Five representative snapshots were selected and the overlay of the CXXC active site is shown. In two of the snapshots Lys8 is hydrogen bonded to Cys11. In the other three snapshots Lys8 is pointing away from Cys11. During the MD simulation Lys8 adopts all conformation between the conformations shown. One snapshot shows Cys11 with a χ1 angle outside the trans conformation. For clarity, only the side chain of Lys8 and the main chain of Val12 and Gln13 are shown. Hydrogen bonds are depicted as black dotted lines. B: A table summarizing the conformations of the active-site cysteines and Lys8 during the MD simulation is shown. The side-chains torsion angle χ is given in percent of the time during the simulation. g+: gauche plus; g−: gauche minus. othera: conformations outside the conventional ranges trans, g+ or g−. See experimental procedures for the criteria used to define the torsion angles.
Figure 2.
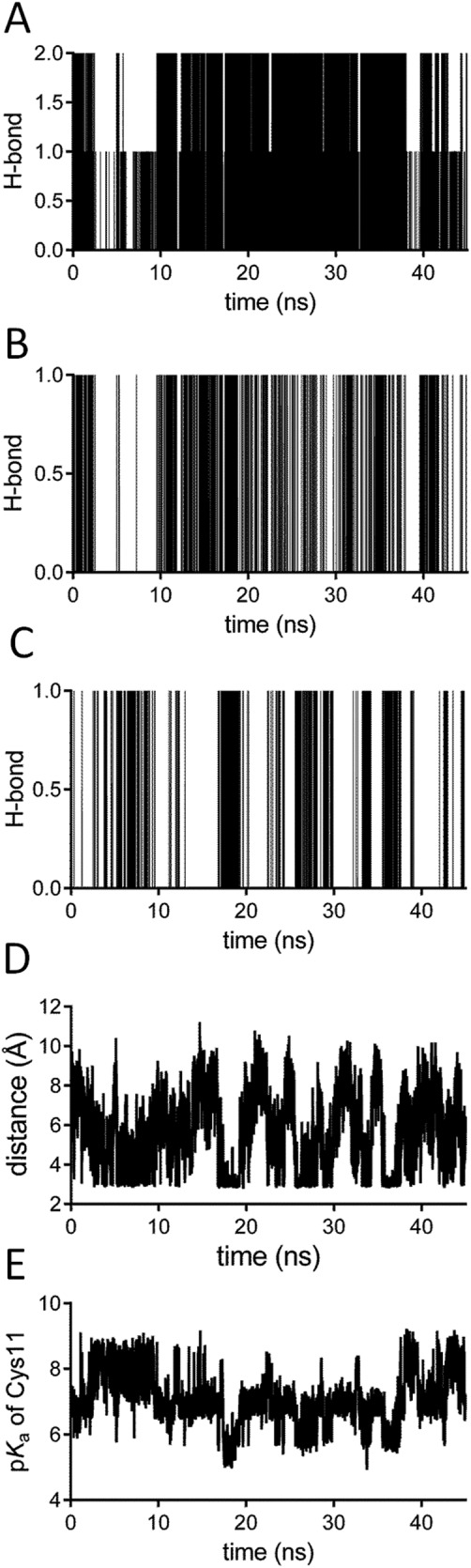
A dynamic view on the pKa of Cys11 and its interactions. A: The hydrogen bonds formed between Cys14 and the thiolate of Cys11. Note that both the side chain thiol and the main chain NH group of Cys14 form a hydrogen bond with Cys11. B: The hydrogen bond formed between the main chain NH group of Gln13 and Cys11. C: The hydrogen bond formed between the side chain of Lys8 and Cys11. D: The distance between the N atom of the side chain of Lys8 and the sulfur atom of Cys11 in function of the simulation time. E: The pKa of Cys11 in function of the simulation time. The cysteine pKa continuously changes due to the fluctuating number of hydrogen bonds and the variable electrostatic effect of Lys8. Furthermore, the effect of the hydrogen bonds on the lowering of the pKa depends on the distance between the hydrogen donor and acceptor and on the donor-hydrogen-acceptor angle. See experimental procedures for the criteria used to define a hydrogen bond.
In 7% of the time, the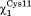 angle moves outside the trans conformation and the hydrogen bond between the side chain of Cys14 and Cys11 is not present. When
angle moves outside the trans conformation and the hydrogen bond between the side chain of Cys14 and Cys11 is not present. When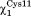 is in trans, two additional hydrogen bonds are formed with the backbone NH group of Cys14 and Gln13. This active site conformation (
is in trans, two additional hydrogen bonds are formed with the backbone NH group of Cys14 and Gln13. This active site conformation (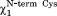 = trans and
= trans and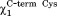 = g−) and the pattern of hydrogen bonds (Fig. 1) resemble the consensus structure observed during the MD runs of Grx and Mrx1.10–11
= g−) and the pattern of hydrogen bonds (Fig. 1) resemble the consensus structure observed during the MD runs of Grx and Mrx1.10–11
A fourth hydrogen bond formed between the thiolate of Cys11 and the side chain of Lys8 is occasionally forming during the simulation [Figs. 1 and 2(C)]. This hydrogen bond is more transient than the other three hydrogen bonds and is only 12.41% of the time present [Fig. 2(C)]. The solution structure of human Grx1 (PDB: 1JHB) shows a similar hydrogen bond with a lysine located N-terminal from the active site but mutagenesis of this lysine did not influence the pKa of the N-terminal cysteine.13–14 A MD simulation of human Grx1 revealed that although the hydrogen bond is formed in 4 of the 20 solution structures, it is only present 0.5% of the simulation time.10 In our simulation of Cg NrdH-redoxin, the hydrogen bond with Lys8 occurs 25 times more frequently and might thus influence the pKa of the N-terminal cysteine.
We mutated Lys8 of Cg NrdH-redoxin to an alanine and determined the pKa of the N-terminal cysteine (i.e., Cys11). The experimentally determined pKa in the K8A mutant is increased with 0.9 units relative to the wild type protein (Table1 and Supporting Information Fig. S1). Thus, Lys8 plays an important role in regulating the relatively low pKa of the N-terminal cysteine in Cg NrdH-redoxin. However, at this point, we cannot claim that the transient hydrogen bond observed during the MD simulations is responsible for the increased cysteine pKa of the K8A mutant. A cysteine pKa can also be influenced by its electrostatic environment.8 Thus, apart from the Lys8-Cys11 hydrogen bond, the positive charge of the Lys8 side chain could also stabilize the thiolate of the N-terminal cysteine and as such explain its relatively low pKa. To determine the influence of the positive charge of Lys8 on the cysteine pKa, we constructed a K8Q mutant of Cg NrdH-redoxin. Gln can act as a hydrogen bond donor at physiological pH but has no charge. The pKa of the N-terminal cysteine in the K8Q mutant is 6.93 (Table1 and Supporting Information Fig. S1). This is only 0.2 units lower than the cysteine pKa value observed for the K8A mutant and still 0.7 units higher than the cysteine pKa in wild-type NrdH-redoxin. To exclude the possibility that the K8Q mutant is compromised in its ability to form a Gln8-Cys11 hydrogen bond, we subjected the K8Q mutant to a 10 ns MD simulation. In this simulation, the Gln8-Cys11 hydrogen bond is even more frequently formed (46.04% of the total simulation time; Supporting Information Fig. S2) than the Lys8-Cys11 hydrogen bond in the wild type enzyme [12.41% of the total simulation time; Fig. 2(C)]. These data indicate that the positive charge of Lys8 and not the Lys8-Cys11 hydrogen bond is the major factor determining the relatively low pKa of the N-terminal cysteine in Cg NrdH-redoxin.
Table 1.
The Experimentally Determined pKa of Cys11 in the Wild Type, the K8A Mutant, the K8Q Mutant, and the K8R Mutant of Cg NrdH-redoxin
| Mutation | pKa of Cys11 | Hydrogen bond donor at position 8 | Positive charge at position 8 | Reference |
|---|---|---|---|---|
| WT | 6.21 ± 0.03 | Yes | Yes | Van Laer et al., 2013 |
| K8A | 7.11 ± 0.12 | No | No | This work |
| K8Q | 6.93 ± 0.06 | Yes | No | This work |
| K8R | 5.77 ± 0.07 | Yes | Yes | This work |
Next, we investigated whether Lys8 is conserved in other NrdH-redoxins. A survey of all NrdH-redoxins found in the UniRef90 database shows that Lys8 is conserved in 84.7% of the NrdH-redoxin clusters. In 12.4% of the NrdH-redoxins, an arginine replaces the lys8. Noteworthy, both residues are positively charged and can act as hydrogen bond donors at physiological pH. In the remaining 2.9% of the NrdH-redoxin clusters, a threonine (1.5%), serine (0.7%), or alanine (0.7%) is found at the corresponding position.
We speculated that Arg could, similarly to Lys8, stabilize the thiolate of the N-terminal cysteine. To test this hypothesis, we mutated Lys8 of Cg NrdH-redoxin to arginine and determined the pKa of the N-terminal cysteine. The pKa of the N-terminal cysteine in the K8R mutant is 5.77 (Table1 and Supporting Information Fig. S1). Thus, the arginine or lysine residue at position 8 contributes in 97.1% of all NrdH-redoxins to the relatively low pKa value of the N-terminal cysteine.
Although the experimental data on the Lys8 mutants clearly show the influence of Lys8 or Arg8 on the cysteine pKa, they do not reveal how the pKa changes in function of time. Indeed, the distance between Lys8 and Cys11 (i.e., the N-terminal cysteine) is continuously changing during the MD simulation and will strengthen or weaken the influence of Lys8 on the pKa of Cys11 [Fig. 2(D)]. Furthermore, the four hydrogen bonds formed with Cys11 are frequently broken which will increase the cysteine pKa for short periods of time (Fig. 2). To obtain a more dynamic picture of how the pKa changes during the MD simulation, we used PROPKA2.0 to calculate the pKa of Cys11 every 5 fs of the simulation [Fig. 2(E)].15 The four hydrogen bonds (Figs. 1 and 2) and the electrostatic effect of Lys8 have a different influence on the pKa of Cys11 which yields a complex picture in which the cysteine pKa fluctuates between 4.9 and 9.2. The MD simulation gives an average pKa of 7.0 for Cys11 whereas the experimentally determined cysteine pKa is 6.2. This discrepancy is presumably a result of the limited accuracy of PROPKA2.0. Roos et al. noted an average deviation of 0.88 pKa units between the calculated cysteine pKa (using PROPKA) and the experimentally determined pKa in several Trx and arsenate reductase proteins.8
In conclusion, we found that the active site of NrdH-redoxins adopts a similar consensus conformation as Grxs. In this conformation, the nucleophilic cysteine receives three hydrogen bonds from residues within the CVQC active site motif. These hydrogen bonds stabilize the thiolate of the nucleophilic cysteine and, as such, lower the cysteine pKa. The pKa of the nucleophilic cysteine is further decreased by a transient hydrogen bond with and the electrostatic effect of a lysine or arginine located N-terminally of the active site in 97.1% of all NrdH-redoxins. We propose that this positively charged hydrogen-donating residue, located N-terminally of the active site, is as common feature within the family of NrdH-redoxins and is required to facilitate the relative low pKa of the nucleophilic cysteine in this protein family.
Experimental Procedures
The experimental procedures are described in the electronic Supporting Information. The Supporting Information contains the setup of the MD simulations of the wild type and K8Q mutant, the computational pKa calculation, the site-directed mutagenesis protocol used to mutate the K8A, K8Q, and K8R mutants, and the experimental pKa determination.
For the analysis of the MD simulations, we defined the gauche minus (g−), gauche plus (g+), and trans torsion angles as −60° ± 30; 60° ± 30, and 180° ± 30, respectively.16 The criteria to define a hydrogen bond was a maximal distance of 3.6 Å between the sulfur and the hydrogen bond donor D and the angle (S-H-D) between 150 and 210°.
Acknowledgments
Authors are grateful to Mike Sleutel for experimental and computational support. KVL thanks the IWT for a doctoral fellowship. JM is a group leader at the VIB. No conflict of interest.
Glossary
- Cg
Corynebacterium glutamicum
- Grx
glutaredoxin
- LWM
low molecular weight
- MD
molecular dynamics
- Mrx1
mycoredoxin-1
- RNR
ribonucleotide reductase
- Trx
thioredoxin
- TrxR
thioredoxin reductase
Additional Supporting Information may be found in the online version of this article.
References
- Rabinovitch I, Yanku M, Yeheskel A, Cohen G, Borovok I, Aharonowitz Y. Staphylococcus aureus NrdH redoxin is a reductant of the class Ib ribonucleotide reductase. J Bacteriol. 2010;192:4963–4972. doi: 10.1128/JB.00539-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehr M, Schneider G, Aslund F, Holmgren A, Lindqvist Y. Structural basis for the thioredoxin-like activity profile of the glutaredoxin-like NrdH-redoxin from Escherichia coli. J Biol Chem. 2001;276:35836–35841. doi: 10.1074/jbc.M105094200. [DOI] [PubMed] [Google Scholar]
- Jordan A, Aslund F, Pontis E, Reichard P, Holmgren A. J Biol Chem. 1997;272:18044–18050. doi: 10.1074/jbc.272.29.18044. Characterization of Escherichia coli NrdH. A glutaredoxin-like protein with a thioredoxin-like activity profile. [DOI] [PubMed] [Google Scholar]
- Jensen KS, Hansen RE, Winther JR. Kinetic and thermodynamic aspects of cellular thiol-disulfide redox regulation. Antioxid Redox Signal. 2009;11:1047–1058. doi: 10.1089/ars.2008.2297. [DOI] [PubMed] [Google Scholar]
- Van Laer K, Dziewulska AM, Fislage M, Wahni K, Hbeddou A, Collet JF, Versees W, Mateos LM, Tamu Dufe V, Messens J. NrdH-redoxin of Mycobacterium tuberculosis and Corynebacterium glutamicum dimerizes at high protein concentration and exclusively receives electrons from thioredoxin reductase. J Biol Chem. 2013;288:7942–7955. doi: 10.1074/jbc.M112.392688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehr M, Lindqvist Y. NrdH-redoxin of Corynebacterium ammoniagenes forms a domain-swapped dimer. Proteins. 2004;55:613–619. doi: 10.1002/prot.20126. [DOI] [PubMed] [Google Scholar]
- Phulera S, Mande SC. The crystal structure of Mycobacterium tuberculosis NrdH at 0.87 A suggests a possible mode of its activity. Biochemistry. 2013;52:4056–4065. doi: 10.1021/bi400191z. [DOI] [PubMed] [Google Scholar]
- Roos G, Foloppe N, Messens J. Understanding the pK(a) of redox cysteines: the key role of hydrogen bonding. Antioxid Redox Signal. 2013;18:94–127. doi: 10.1089/ars.2012.4521. [DOI] [PubMed] [Google Scholar]
- Roos G, Loverix S, Geerlings P. Origin of the pKa perturbation of N-terminal cysteine in alpha-and 3(10)-helices: a computational DFT study. J Phys Chem B. 2006;110:557–562. doi: 10.1021/jp0549780. [DOI] [PubMed] [Google Scholar]
- Foloppe N, Vlamis-Gardikas A, Nilsson L. The -Cys-X1-X2-Cys-motif of reduced glutaredoxins adopts a consensus structure that explains the low pK(a) of its catalytic cysteine. Biochemistry. 2012;51:8189–8207. doi: 10.1021/bi3006576. [DOI] [PubMed] [Google Scholar]
- Van Laer K, Buts L, Foloppe N, Vertommen D, Van Belle K, Wahni K, Roos G, Nilsson L, Mateos LM, Rawat M, Van Nuland NA, Messens J. Mycoredoxin-1 is one of the missing links in the oxidative stress defence mechanism of Mycobacteria. Mol Microbiol. 2012;86:787–804. doi: 10.1111/mmi.12030. [DOI] [PubMed] [Google Scholar]
- Xia TH, Bushweller JH, Sodano P, Billeter M, Bjornberg O, Holmgren A, Wuthrich K. NMR structure of oxidized Escherichia coli glutaredoxin: comparison with reduced E. coli glutaredoxin and functionally related proteins. Protein Sci. 1992;1:310–321. doi: 10.1002/pro.5560010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Berardi MJ, Bushweller JH. The NMR solution structure of human glutaredoxin in the fully reduced form. J Mol Biol. 1998;280:687–701. doi: 10.1006/jmbi.1998.1913. [DOI] [PubMed] [Google Scholar]
- Jao SC, English Ospina SM, Berdis AJ, Starke DW, Post CB, Mieyal JJ. Computational and mutational analysis of human glutaredoxin (thioltransferase): probing the molecular basis of the low pKa of cysteine 22 and its role in catalysis. Biochemistry. 2006;45:4785–4796. doi: 10.1021/bi0516327. [DOI] [PubMed] [Google Scholar]
- Olsson MH. Protein electrostatics and pKa blind predictions; contribution from empirical predictions of internal ionizable residues. Proteins. 2011;79:3333–3345. doi: 10.1002/prot.23113. [DOI] [PubMed] [Google Scholar]
- Markley JL, Bax A, Arata Y, Hilbers CW, Kaptein R, Sykes BD, Wright PE, Wuthrich K. Recommendations for the presentation of NMR structures of proteins and nucleic acids. IUPAC-IUBMB-IUPAB Inter-Union Task Group on the Standardization of Data Bases of Protein and Nucleic Acid Structures Determined by NMR Spectroscopy. J Biomol NMR. 1998;12:1–23. doi: 10.1023/a:1008290618449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


