Abstract
Purpose
New therapies for lysosomal storage diseases (LSDs) have generated interest in screening newborns for these conditions. We present performance validation data on a digital microfluidic platform that performs multiplex enzymatic assays for Pompe, Fabry, Hunter, Gaucher, and Hurler diseases.
Methods
We developed an investigational disposable digital microfluidic cartridge that uses a single dried blood spot (DBS) punch for performing a 5-plex fluorometric enzymatic assay on up to 44 DBS samples. Precision and linearity of the assays were determined by analyzing quality control DBS samples; clinical performance was determined by analyzing 600 presumed normal and known affected samples (12 for Pompe, 7 for Fabry and 10 each for Hunter, Gaucher and Hurler).
Results
Overall coefficient of variation (CV) values between cartridges, days, instruments, and operators ranged from 2 to 21%; linearity correlation coefficients were ≥ 0.98 for all assays. The multiplex enzymatic assay performed from a single DBS punch was able to discriminate presumed normal from known affected samples for 5 LSDs.
Conclusions
Digital microfluidic technology shows potential for rapid, high-throughput screening for 5 LSDs in a newborn screening laboratory environment. Sample preparation to enzymatic activity on each cartridge is less than 3 hours.
Keywords: Newborn screening, lysosomal storage disease, digital microfluidics, dried blood spot, high throughput, multiplex enzymatic assay
1. Introduction
Newborn screening (NBS) is generally performed for those diseases for which both an inexpensive screening test and treatment are available and the disease, if untreated, leads to profound morbidity or mortality. Nationally, newborn screening began with a screen for phenylketonuria [1], and has since expanded to include >50 conditions [2] due to available treatments and technological advances in screening, such us multiplex tandem mass spectrometry (MS/MS) [3]. Lysosomal storage diseases (LSDs) have been identified as diseases where patients can greatly benefit from newborn screening. Although individual LSDs are rare, as a group they can lead to devastating consequences, including infant mortality [4], and many can be screened using dried blood spots (DBS) [5–6]. The combined incidence rate of LSDs is reported to be as high as 1:2,315 live births [7]. Recent advances in treatment, including enzyme replacement therapy [8], for certain lysosomal storage diseases have generated renewed interest in newborn screening for individual LSDs [9–10]. Here, we focus on a multiplex method using a single DBS punch to screen for five LSDs with proven treatments: Pompe disease (glycogen storage disease type II, caused by acid α-glucosidase (GAA) deficiency), Fabry disease (α-galactosidase (GLA) deficiency), Hunter disease (mucopolysaccharidosis type II, iduronate-2-sulfatase (IDS) deficiency), Gaucher disease (glucocerebrosidase (GBA) deficiency), and Hurler disease (mucopolysaccharidosis type I, α-iduronidase (IDU) deficiency).
At present, only 5 states in the USA (including New York, Illinois, Missouri, New Mexico, and New Jersey) have mandated newborn screening for select LSDs. Currently, the technologies available to perform DBS assays for lysosomal storage diseases are tandem mass spectrometry [11], microplate fluorometry [12], immunoassays [13] and, more recently, digital microfluidic fluorometry [14]. As more states move to adopt LSD screening, there is a need to develop rapid, efficient and economical high-throughput methods to screen for multiple LSDs simultaneously [15–17]. Orsini et al. reported using a 4+1 multiplex tandem MS/MS platform to screen for Gaucher, Pompe, Krabbe, Fabry, and Niemann-Pick A/B that reduced processing time and test complexity [18]. Mechtler et al. reduced tandem MS/MS incubation times to less than 4 hours using a multiplex assay for Pompe, Fabry, Hunter, Gaucher, and Niemann-Pick A/B [15]. We have previously demonstrated that digital microfluidics can be used to screen for Hunter, Pompe and Fabry diseases using low throughput disposable cartridges [14,19–20]. A prototype cartridge designed by Advanced Liquid Logic, Inc. (ALL) that performed 3 assays (Pompe, Fabry and Gaucher) on up to 12 samples was recently pilot tested in Illinois Department of Public Health laboratories [21]. From a total of 8,012 DBS samples screened, seven cases of Fabry and two cases of Gaucher disease were confirmed.
In this report, we demonstrate the capabilities of a new digital microfluidic system to meet the high throughput and rapid turnaround requirements of a newborn screening laboratory by means of a 5-plex fluorometric enzymatic assay for Pompe, Fabry, Hunter, Gaucher and Hurler diseases on a cartridge that accepts 44 specimens. The platform utilizes a single disposable cartridge that automates all liquid handling steps and reduces the overall time to result to <3 h, using a single 3 mm DBS punch from each of the 44 specimens.
2. Materials and Methods
2.1 Dried Blood Spot Samples
We obtained presumed normal, de-identified dried blot spots (NBS cards) from the North Carolina Division of Public Health NBS laboratories under a material transfer agreement. These spots were 2–3 months old and were stored at −20 °C upon receipt. Duke University Biochemical Genetics Laboratory (Durham, NC), Shire Human Genetic Therapies, Inc. (Lexington, MA) and the Centers for Disease Control and Prevention (Atlanta, GA; CDC) laboratories provided deidentified affected DBS for Pompe (n=12), Fabry (n=7), Hunter (n=10), Gaucher (n=10) and Hurler (n=10) diseases under an IRB-approved protocol. These affected spotted specimens were from disease patients (not from newborns) that had not received enzyme replacement therapy.
2.2 Reagents
4-Methylumbelliferyl α-D-galactopyranoside- (4MU-α-Gal), N-acetyl-D-galactosamine (GalNac), 4-methylumbelliferyl-α-D-glucopyranoside (4-MU-α-Gluc), acarbose, 4-methylumbelliferyl-β-D-glucopyranoside (4-MU-β-Gluc), 4-methylumbelliferone sodium salt (4-MU), D-saccharic acid 1,4-lactone monohydrate (D-Sac), sodium acetate (99% pure), sodium bicarbonate, sodium taurocholate, ethylenediaminetetraacetic acid, dimethyl sulfoxide (DMSO), citrate phosphate, and Tween 20 were all from Sigma-Aldrich Corp. (St. Louis, MO; www.sigmaaldrich.com). 4-methylumbelliferyl-α-L-iduronide sodium salt (4-MU-α-IDU) was from Affymetrix (Santa Clara, CA; http://www.affymetrix.com). 4-methylumbelliferyl-α-L-iduronide-2-Sulfate (4-MU-IDS) was from Moscerdam Substrates (The Netherlands; www.moscerdam.com). Triton X-100 was obtained from Mallinckrodt (Hazelwood, MO; www.mallinckrodt.com). Molecular grade water and methyl β-cyclodextrin (β-MBCD) were from Fisher Scientific (Pittsburgh, PA; www.fishersci.com), and acetic acid (glacial) was from Fluka (Sigma-Aldrich). 5cSt silicone oil was from Gelest (Morrisville, PA; www.gelest.com).
2.3 Sample and Reagent Preparation
For each dried blood spot from normal newborns and affected newborns, a single 3 mm punch was obtained and stored in a separate 96-well plate at −20 °C. Formulations for Pompe, Fabry, and Hunter reagents and inhibitor solutions have been published previously [14,19]. Briefly, Pompe and Fabry reagents were prepared as described earlier [14] with the addition of 20.0 mmol/l methyl β–cyclodextrin (β-MBCD). Hunter reagent was prepared in assay buffer as described earlier [19] with the addition of 20.0 mmol/l β-MBCD. Gaucher reagent was prepared in assay buffer (0.1/0.2 mol/l citrate phosphate buffer pH 5.2 with 0.01% Tween 20 and 1.5% sodium taurocholate) in the absence of inhibitor to a final reagent concentration of 16 mmol/l of 4-MU-β-Gluc with 1.5% sodium taurocholate. Hurler reagent was prepared in assay buffer (0.04 mol/l pH 3.5 acetate buffer with 20 mmol/l β-MBCD and 300 mmol/l NaCl and 0.01% Tween 20) in the presence of 3.0 mmol/l of D-Saccharolactone inhibitor to a final concentration of 2 mmol/l 4-MU-α-IDU with 3.0 mmol/l D-Sac and 20 mmol/l β-MBCD. Ready-to-use reagent aliquots containing the substrate, assay buffer, inhibitors and other additives were prepared ahead of time and stored at −80 °C; working solutions were prepared just before use for each experiment. Other buffers, such as the assay extraction buffer (0.1% (w/v) Tween 20 in water, labeled as EXT) and stop buffer (0.2 mol/l NaHCO3, pH 10.0, 0.01% (w/v) Tween 20) solutions were prepared and stored at room temperature.
2.4 Description and Loading Protocol for a 48 Sample-Input Cartridge
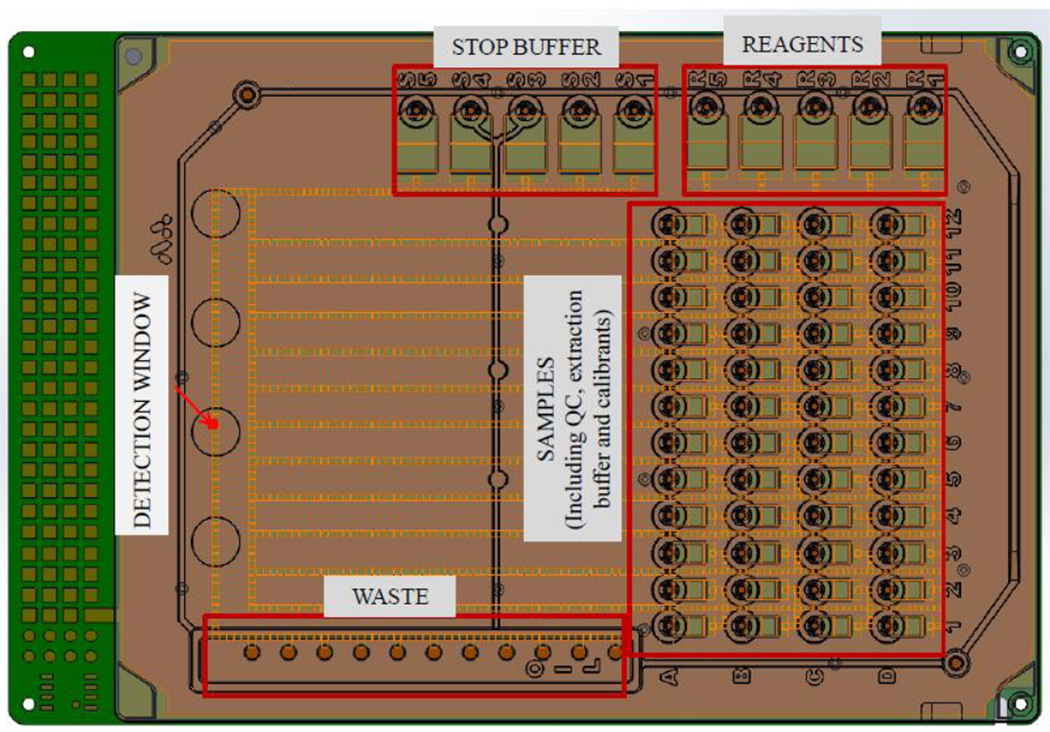
In order to achieve the sample throughput required by newborn screening laboratories, we developed a disposable, single use digital microfluidic cartridge that has 48 input reservoirs for DBS extracts, 4-Methyl Umbelliferone (4-MU) calibrants, extraction buffer and quality control (QC) spot extracts, 5 input reservoirs for enzymatic substrates in assay buffers, 5 input reservoirs for stop buffers, and one large output reservoir (waste reservoir) to collect all of the droplets after incubation and detection (Fig. 1, digital microfluidic cartridge). The sample reservoirs are labeled as per the 96-well format with rows (horizontal) labeled with letters and columns (vertical) labeled with numbers to assist in transferring the extracts from the 96-well plate. The entire space between the top plastic layer and the printed circuit board of the cartridge is filled with silicone oil prior to loading the reagents and DBS extracts to prevent evaporation of the droplets during the incubation process.
Figure 1.
Schematic of the digital microfluidic cartridge which conforms to a standard microtiter plate dimensions. Each cartridge has 48 sample wells (reservoirs 1–12, rows A–D), 5 reservoirs for reagents (R1–R5) and 5 reservoirs for stop buffer (S1–S5). Droplets dispensed from these reservoirs are 100 nl. All droplet manipulation is automated through a software program that performs dispensing, transport, mixing, incubation, and disposal of droplets as required. A builtin fluorometer reads the fluorescence at the detection window.
DBS punches (3 mm diameter) were eluted with extraction buffer (100 µl) in standard deep-well 96-well plates on an orbital shaker for 30 minutes at ambient temperature. Prior to sample loading, the cartridge was inserted into a deck on a desktop analyzer as described earlier in [22]. The analyzer houses all the electrical components required to perform microfluidic operations, a heated deck that can be programmed to the required temperature, and a fluorometer capable of detecting 4-MU at wavelengths of 370 nm for excitation and 460 nm for emission. The extracts from the dried blood spot samples (1.6 µl) were then transferred to the sample input wells on the digital microfluidic cartridge (Fig. 1) using a multi-channel pipette. The prepared reagents, calibrators and stop buffers were equilibrated to ambient temperature and transferred to the appropriate wells on the digital microfluidic cartridge. The protocol used to perform the enzymatic analysis on-cartridge is briefly described in the following section.
2.4.1 Five-Plex, 48 Sample Cartridge On-Chip Protocol
Once the cartridge had been loaded with the samples, buffers and reagents, all of the subsequent steps described in this protocol were performed entirely under software control and required no operator intervention. Before the start of the assay, a four-point 4-MU calibration curve was obtained in duplicate on every cartridge. From each calibrant reservoir labeled A1, B1, C1, and D1 in Figure 1, 3 unit-sized droplets (~100 nl each) were dispensed and merged to form a triple-sized droplet (~300 nl). This process was repeated to obtain 2 triple-sized droplets from each calibrant reservoir resulting in a total of eight triple-sized calibrant droplets. These droplets were routed to the detection window (Fig. 1) for fluorescence measurements. Duplicate 4-point calibration curves were constructed using linear regression. This calibration curve was used to convert the raw fluorescence values (RFU) into moles of 4-MU to report enzymatic activity.
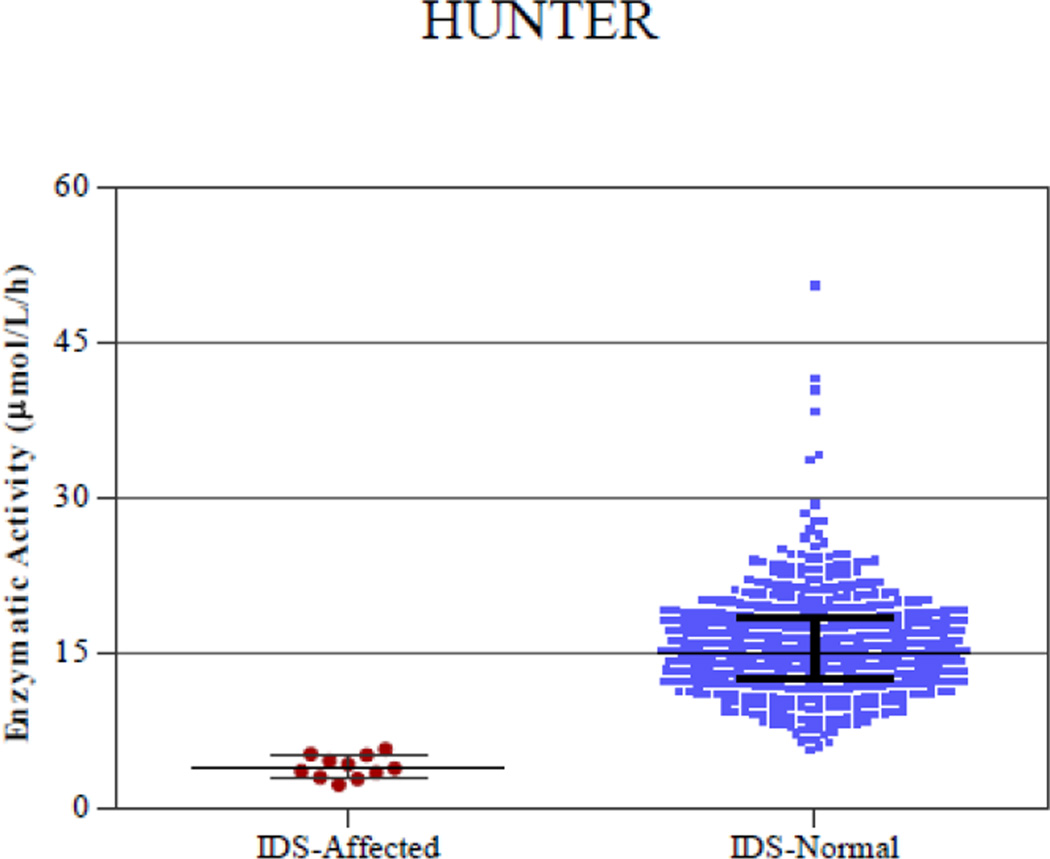
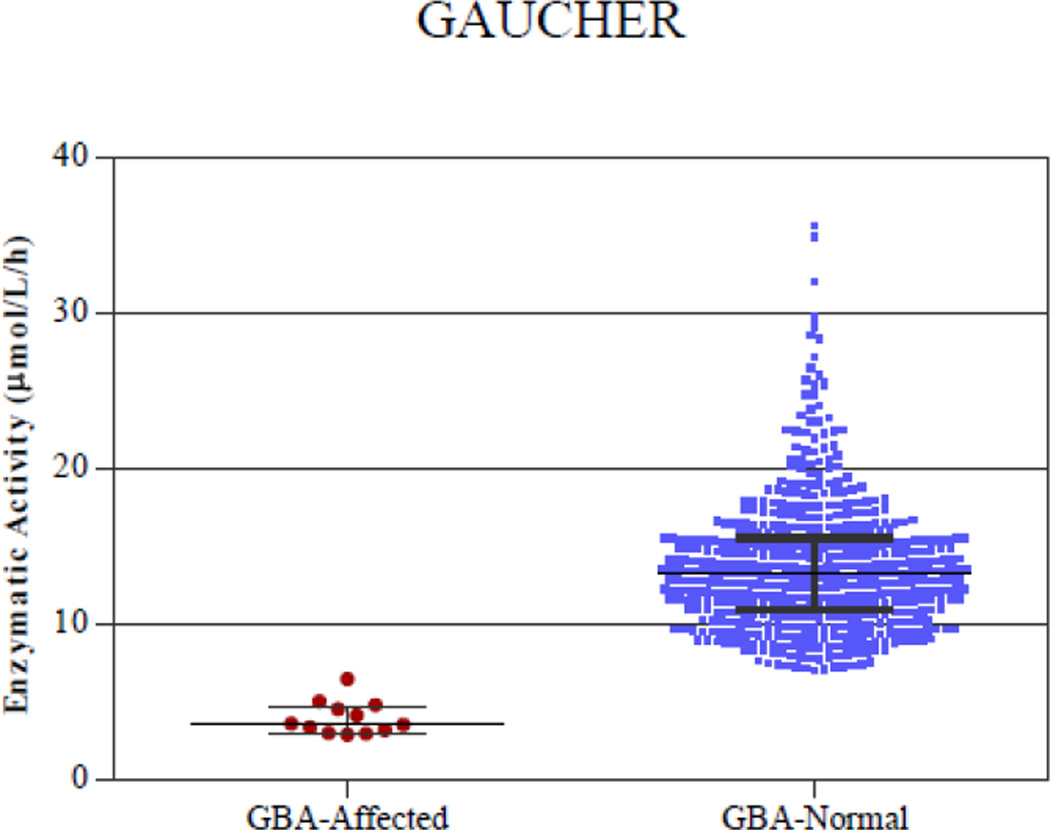
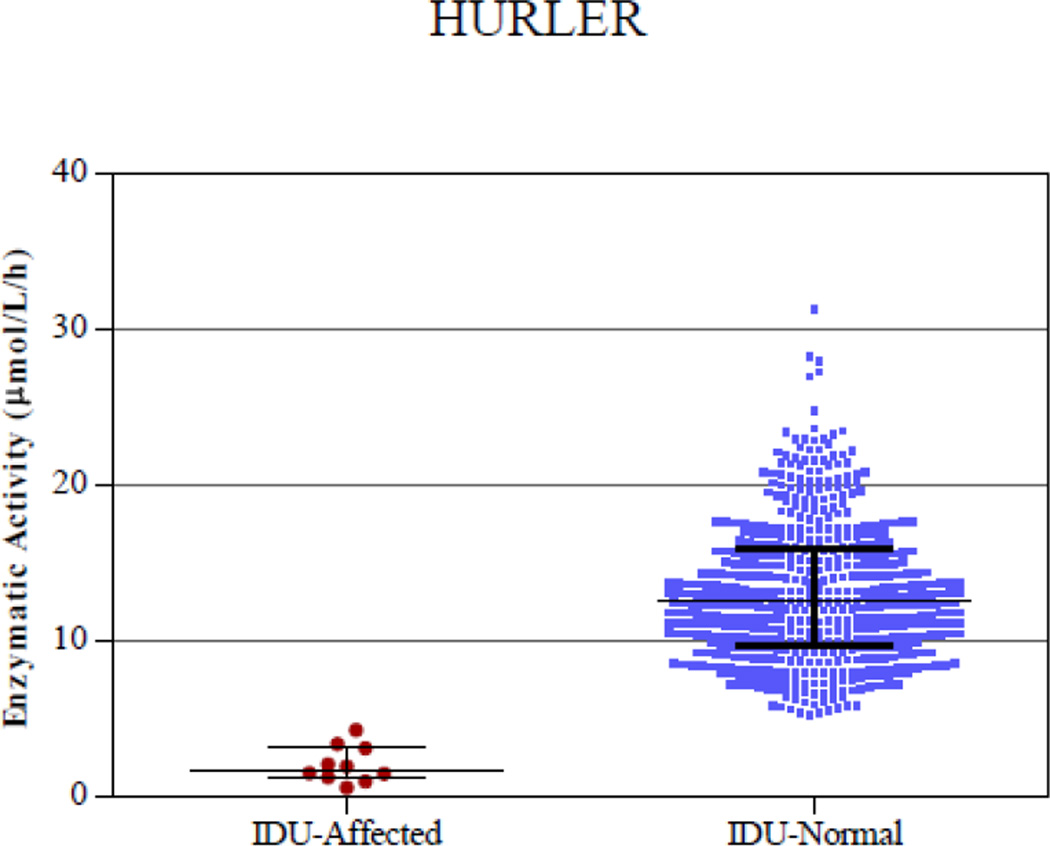
Figure 3.
Digital microfluidic analysis of normal and affected samples of all 5 enzymatic assays (Pompe, Fabry, Hunter, Gaucher, and Hurler). The line in the middle represents the median.
After calibration was complete, 44 unit-sized droplets were dispensed from the first reagent reservoir (R1, Fig. 1) and transported to the grid of sample reservoirs, where the unitsized reagent droplets were merged with unit-sized sample droplets to form 44 reaction mixture droplets. This process was repeated for the remaining 4 reagent reservoirs (R2–R5, Fig. 1), resulting in a total of 220 reaction mixture droplets. The reaction mixture droplets were incubated for 1 h at 37 °C. After incubation was complete, unit sized droplets were dispensed from the stop buffer reservoirs (S1–S5, Figure 1) and merged serially with the reaction mixture droplets to stop the enzymatic reactions. The fluorescence value was measured by transporting each droplet to the built-in fluorometer, with preset excitation and emission wavelengths of 370 nm and 460 nm, respectively (see indicated detection window in Fig. 1). Each fluorometer reading took approximately 6 sec, after which the droplets were transported to a waste reservoir. To obtain the fluorescence levels from the reagent background and non-enzymatic hydrolysis of the substrates, 2 of the 44 sample droplets were setup with extraction buffer alone (no enzyme) and incubated under the same conditions as the remaining DBS sample droplets. The net fluorescence value from the enzymatic reaction was obtained by subtracting the substrate background and non-enzymatic hydrolysis which was converted to enzymatic activity using the 4-MU calibration curve and reported as micro-moles of 4-MU produced per liter of blood per hour of incubation.
2.4.2 Protocols for Precision and Linearity Determination
To validate the performance of the cartridge to screen for all 5 LSDs (Pompe, Fabry, Hunter, Gaucher, and Hurler), we determined precision and linearity according to Clinical and Laboratory Standards Institute (CLSI) guidelines [23,24].
2.4.2.1 Precision Determination
Precision of enzymatic activity between instruments, between days, and between cartridges was determined according to the Clinical and Laboratory Standards Institute (CSLI) EP05-A2 [23]. All quality control (QC) spots were manufactured at ALL following a modification of the CDC protocol where a longer serum denaturation step was included [25, 26]. Samples from each QC level were tested on a total of 12 cartridges over a period of 3 days across 8 different instruments to determine variability between instruments and days. Each cartridge included 10 replicates of each QC level (base pool, low, medium, high) resulting in a total of 120 replicates for each QC. The QC samples were randomly distributed across the entire cartridge (layout shown in the Appendix Fig. A.1). Quality control low, medium, and high were only considered for precision analysis as these levels represent the range of expected activity values. Mean, SD, and CV of the results were calculated to analyze variability between days and instruments.
Figure A.1.
Sample layout of QC samples in the precision and linearity experiments. For the precision experiments, QCL, QCM, QCH, and QCBP represent different concentrations of cord blood, CAL is calibrant, and EXT is extraction buffer. For the linearity experiments, the numbers in each well represent different concentrations of QCH and QCL samples as detailed in Figure 2.
2.4.2.2 Linearity Determination
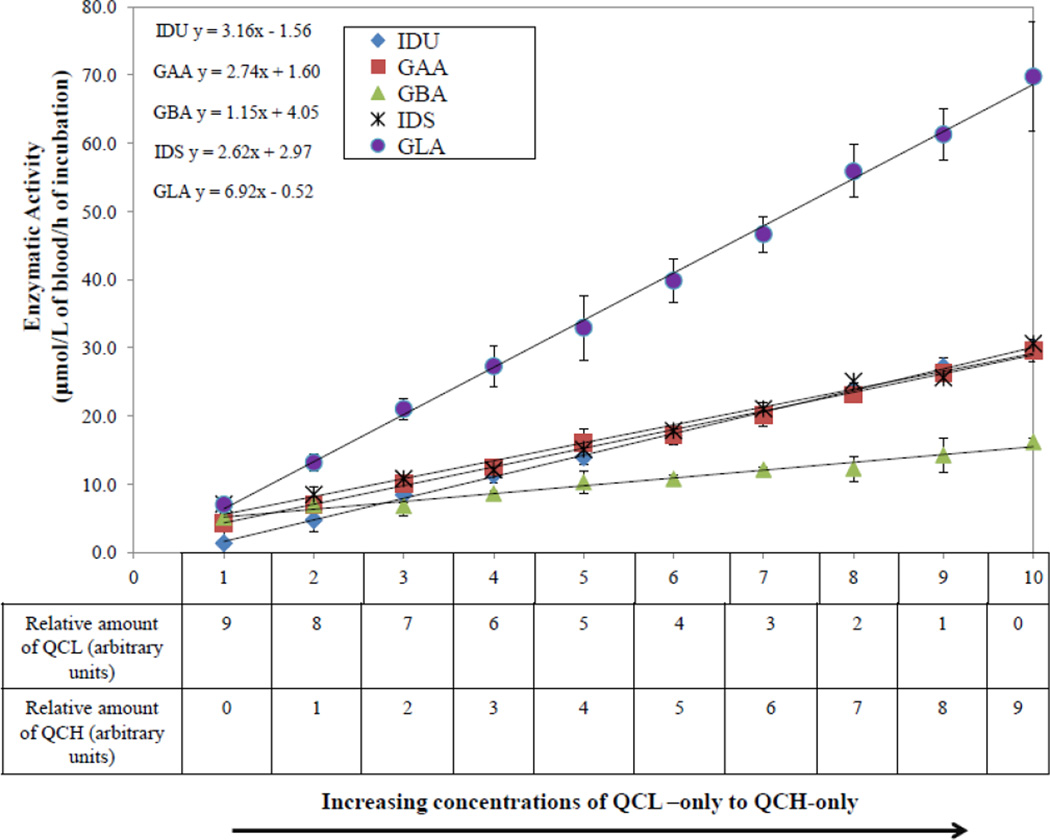
Linearity was determined according to CLSI EP06-A [24] using 4 replicates each of 10 different concentrations that ranged from QCL to the QCH levels. DBS extracts were obtained from QCL and QCH using the extraction protocol described in Section 2.4. Ten intermediate concentrations were prepared by mixing appropriate amounts of QCL and QCH as shown in Figure 2. The concentrations range from QCL-only, representing the axis label 1 in Figure 2, to QCH-only, representing the axis label 10. All 8 intermediate concentrations were distributed equally between these two QC levels, the relative proportions of which are shown in the table below the axis label in Figure 2. These 10 extracts were randomly distributed among the 44 sample reservoirs (layout shown in the Appendix Fig. A.1). Coefficients of determination (R2) values were calculated by performing a linear regression (in Microsoft Excel). All experiments were conducted on one instrument in one day.
Figure 2.
Linear regression analysis for all 5 enzymatic assays (Pompe, Fabry, Hunter, Gaucher, and Hurler). The enzyme concentration ranges from QCL to QCH.
2.4.3 Analysis with Presumed Normal Samples and Known Affected Samples
Extracts from 600 presumed normal and affected (12 Pompe, 7 Fabry, 10 Hunter, 10 Gaucher, and 10 Hurler) DBS samples were run on 8 instruments. Statistical analysis was performed to determine the mean, median, minimum and maximum enzymatic activity for affected and normal samples for all 5 enzymes.
3. Results and Discussion
3.1 Validation of 5-Plex Enzymatic Assays Using Digital Microfluidics
3.1.1 Precision
To determine day-to-day precision, mean enzymatic activities for each QC level for each instrument and each day were calculated. Overall mean, standard deviation, and % CV were calculated for each level. Enzymatic activity values presented in Table 1 are an average of the data points obtained over the eight instruments and three days. The instrument to instrument variation ranged between 6%–21.3% for QCH, 5.9%–23.6% for QCM and 13.2%–25.2% for QCL for all 5 assays. Similarly the day to day variation ranged between 1.5%–6.8% for QCH, 2.4%–12.4% for QCM and 1.2%–17.1% for QCL for all 5 assays. Day to day precision was determined by calculating the standard deviation between the means of enzymatic activities obtained over 3 different days (which includes enzymatic activity data from different instruments) whereas instrument to instrument was calculated by comparing the mean enzymatic activities obtained across different instruments. Although there were slight differences in the activities between instruments, they were consistent over different days. Hence, the coefficient of variation was slightly higher for instrument variability when compared to day to day variability. Previous studies with these enzymes using MS/MS have reported CV values in a similar range for all QC levels [15].
Table 1.
Precision performance (between instruments and different days) of 5-plex assays on the digital microfluidic cartridges.
| Parameter | Quality Control Sample |
Pompe (GAA) |
Fabry (GLA) |
Hunter (IDS) |
Gaucher (GBA) |
Hurler (IDU) |
|
|---|---|---|---|---|---|---|---|
| Instrument to Instrument | QCH | Meana | 28.92b | 65.34 | 18.97 | 15.47 | 25.11 |
| % CV | 6.0 | 9.1 | 21.3 | 8.2 | 9.0 | ||
| QCM | Mean | 18.21 | 36.08 | 11.00 | 10.12 | 12.26 | |
| % CV | 5.9 | 8.7 | 23.6 | 8.4 | 9.4 | ||
| QCL | Mean | 4.76 | 6.16 | 5.67 | 4.15 | 2.46 | |
| % CV | 13.2 | 16.4 | 22.5 | 13.3 | 25.2 | ||
| Day to Day | QCH | Mean | 28.26 | 64.93 | 20.61 | 15.29 | 25.44 |
| % CV | 4.2 | 1.5 | 6.8 | 4.5 | 5.5 | ||
| QCM | Mean | 17.03 | 33.61 | 12.00 | 9.44 | 12.31 | |
| % CV | 11.1 | 11.8 | 7.5 | 12.4 | 2.4 | ||
| QCL | Mean | 5.04 | 6.83 | 5.91 | 4.19 | 2.36 | |
| % CV | 12.2 | 11.8 | 11.9 | 1.2 | 17.1 | ||
Mean enzymatic activities across instruments or days
Enzymatic activity units are µmoles/l/h.
3.1.2 Linearity
The DBS extracts with different proportions of QCH and QCL as depicted in Figure 2 were analyzed for lysosomal enzymatic activity to determine if the assay response was linear within the measured range (QCL representing the affected samples to QCH representing the normal samples). Figure 2 illustrates linear regression plots for all five enzymatic assays. All results for Pompe, Fabry, Hunter, and Hurler assays demonstrated R2 values ≥ 0.99. The results for Gaucher showed a slightly less R2 value (0.98). Linear regression was the best fit and no statistically significant difference was observed between second order and third order polynomial fits when compared to the linear regression (data not shown).
3.1.3 Discrimination of Presumed Normal and Known Affected Samples
Samples from presumed normal DBS were analyzed along with DBS from patients affected with each disease. Figure 3 shows plots of enzymatic activity for presumed normal and confirmed affected samples for all five enzymes. Table 2 lists the mean, median, minimum and maximum enzymatic activity for both the affected and normal populations. There was clear separation between the affected and normal samples for IDU, GAA and GBA enzymes. The separation between the highest enzymatic activity in the affected samples and the lowest enzymatic activity in the normal samples was ~1 µmol/l/h. However, there was a slight overlap in the enzymatic activity values of 2 presumed normal samples for GLA and 1 presumed normal sample for IDS with the highest affected samples. Since the presumed normal samples were stored at room temperature for 2–3 months before analysis, there may have been some degradation of the lysosomal enzymes that could have resulted in slightly lower enzymatic activity in these samples. These particular samples were not re-tested using a confirmatory diagnostic method. Without considering these three presumed normal samples that were in the overlap region, the separation between the lowest activity in the normal sample and the highest activity in the affected samples is 1.2 µmol/l/h for IDS and 1.5 µmol/l/h for GLA enzyme.
Table 2.
Statistical analysis of enzymatic activity for normal and affected populations
| ENZYME | AFFECTED | NORMAL | ||||||
|---|---|---|---|---|---|---|---|---|
| MEAN | MEDIAN | MIN | MAX | MEAN | MEDIAN | MIN | MAX | |
| GAA | 5.27a | 4.82 | 4.05 | 7.46 | 26.25 | 25.58 | 8.41 | 75.62 |
| GLA | 4.24 | 3.91 | 2.76 | 6.69 | 26.65 | 24.06 | 5.24 | 106.07 |
| IDS | 4.07 | 3.91 | 2.38 | 5.79 | 15.76 | 15.11 | 5.71 | 50.54 |
| GBA | 4.01 | 3.62 | 2.95 | 6.50 | 13.83 | 13.28 | 7.1 | 35.69 |
| IDU | 2.00 | 1.67 | 0.50 | 4.23 | 13.10 | 12.45 | 5.23 | 31.2 |
Enzymatic activity units are µmoles/l/h.
4. Conclusions
The results presented here represent important steps towards the validation of a multiplex, automated digital microfluidic platform to screen for five lysosomal storage diseases on a single disposable cartridge. The assay uses a single 3 mm punch from each of 44 DBS samples to generate results within 3 hours on a single analyzer. In order to achieve a higher throughput that is required for a newborn screening laboratory, 4 analyzers are grouped together into a workstation for a throughput of up to 176 DBS samples processed in 3 hours on a single workstation. With as little as 2 h of total estimated personnel time, a state NBS lab can process up to 352 DBS samples in a single shift and a single workstation. Further work includes performance of inter-laboratory comparison of digital microfluidics with other methods. A pilot study was recently initiated using the digital microfluidic technology in the Missouri State Public Health Laboratory. We conclude that this new technology shows potential for a viable and costeffective option for newborn screening of LSDs.
Supplementary Material
Highlights.
Five-plex assay performed on a disposable cartridge capable of handling up to 44 samples.
Multiplex analysis validated for Pompe, Fabry, Hunter, Gaucher and Hurler.
All 5 enzymatic assays are completed from a single dried blood spot punch.
Punch to results within 3 hours for rapid and high throughput analysis.
Acknowledgements
We acknowledge Dr. Shu Chaing (NCDPH) for providing de-identified normal DBS, Duke University Biochemical Genetics Laboratory, Shire Human Genetic Therapies, Inc, and the Centers for Disease Control and Prevention for providing affected DBS, and Donovan Bort at Advanced Liquid Logic Inc. for technical assistance. Research reported in this publication was partly supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number R44HD057713. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Non Standard Abbreviations
- LSD
lysosomal storage diseases
- DBS
dried blood spot
- NBS
newborn screening
- GAA
acid α-glucosidase
- GLA
acid α-galactosidase
- IDS
acid α-L-iduronate-2-sulfatase
- GBA
acid β-D-glucosidase
- IDU
acid α-L-iduronidase
- ALL
advanced liquid logic Inc.
- CDC
Centers for Disease Control and Prevention
- 4-MU-α-Gal
4-methylumbelliferyl α-D-galactopyranoside
- GalNac
N-acetyl-D-galactosamine
- 4-MU-α-gluc
4-methylumbelliferyl α-D-glucopyranoside
- 4-MU-β-Gluc
4-methylumbelliferyl β-D-glucopyranoside
- 4-MU
4-methyl umbelliferone
- D-Sac
D-saccharic acid 1,4 lactone
- DMSO
dimethyl sulfoxide
- 4-MU-α-IDU
4-methylumbelliferyl α-L-iduronide
- 4-MU-α-IDS
4-methylumbelliferyl α-L-iduronate-2-sulfate
- β-MBCD
methyl-β-cyclodextrin
- RFU
relative fluorescence units
- CLSI
Clinical and Laboratory Standards Institute
- QCH
quality control high, cord blood adjusted to 50.5% hematocrit on filter paper
- QCM
quality control medium 50% cord blood + 50% leukoreduced adult blood adjusted to 50.5% hematocrit on filter paper
- QCL
quality control low, 5% cord blood + 95% leukoreduced adult blood adjusted to 50.5% hematocrit on filter paper
- QCBP
quality control base pool 0% cord blood + 100% leukoreduced adult blood adjusted to 50.5% hematocrit on filter paper
Appendix Material
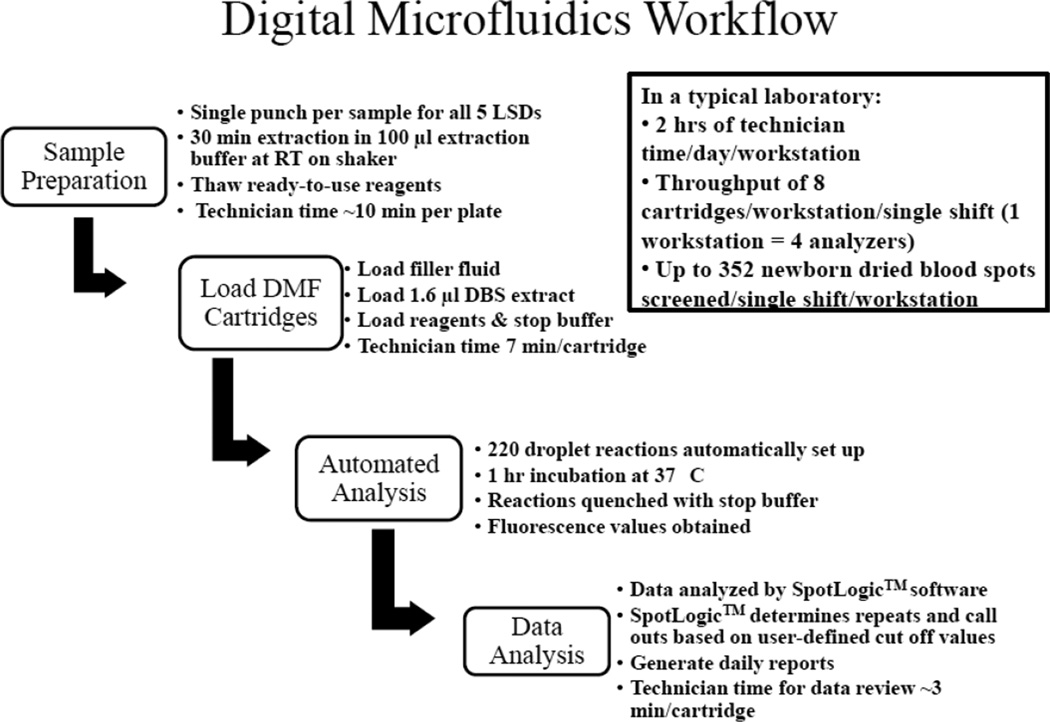
A sample layout of the precision and linearity experiments is presented in Figure A.1. The diagram indicates placement of the QC spots to determine precision and linearity of the assays. A workflow of the digital microfluidic fluorometric assay is presented in Figure A.2. The overall experimental procedure consists of sample preparation, sample and reagent loading onto disposable cartridges, automated fluorometric analysis, and data analysis. The attached video represents an abridged and annotated simulation of the entire multiplex enzymatic assay protocol on the digital microfluidic cartridge.
Figure A.2.
Workflow of the digital microfluidic fluorometric enzyme assays for 5 LSDs. The workflow provides a brief overview of the sample preparation, sample loading onto the disposable cartridge, automated analysis, and data analysis using digital microfluidics for LSD screening.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- 2.National Newborn Screening and Genetics Resource Center. National newborn screening status report. [Accessed on 1/11/13]; Available at: http://genes-r-us.uthscsa.edu/sites/genes-r-us/files/nbsdisorders.pdf. [Google Scholar]
- 3.Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J. Inherit. Metab. Dis. 1990;13:321–324. doi: 10.1007/BF01799385. [DOI] [PubMed] [Google Scholar]
- 4.Staretz-Chacham O, Lang TC, LaMarca ME, Krasnewich D, Sidransky E. Lysosomal storage disorders in the newborn. Pediatrics. 2009;123(4):1191–1207. doi: 10.1542/peds.2008-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millington DS. Rapid and effective screening for lysosomal storage disease: how close are we? Clin. Chem. 2008;54:1592–1594. doi: 10.1373/clinchem.2008.112110. [DOI] [PubMed] [Google Scholar]
- 6.Zhou H, Fernhoff P, Vogt RF. Newborn bloodspot screening for lysosomal storage disorders. J Pediatr. 2011;159(1):7–13. doi: 10.1016/j.jpeds.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Mechtler TP, Stary S, Metz TF, de Jesus VR, Greber-Platzer S, Pollak A, Herkner KR, Streubel B, Kasper DC. Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. The Lancet. 2012;379(9813):335–341. doi: 10.1016/S0140-6736(11)61266-X. [DOI] [PubMed] [Google Scholar]
- 8.Lim-Melia ER, Kronn DF. Current enzyme replacement therapy for the treatment of lysosomal storage diseases. Pediatr. Ann. 2009;38:448–455. doi: 10.3928/00904481-20090723-09. [DOI] [PubMed] [Google Scholar]
- 9.Hwu WL, Chien YH, Lee NC. Newborn screening for neuropathic lysosomal storage disorders. J Inherit. Metab. Dis. 2010;33:381–386. doi: 10.1007/s10545-010-9130-6. [DOI] [PubMed] [Google Scholar]
- 10.Marsden D, Levy H. Newborn screening of lysosomal storage disorders. Clin. Chem. 2010;56:1071–1079. doi: 10.1373/clinchem.2009.141622. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XK, Elbin CS, Chuang WL, Cooper SK, Marashio CA, Beauregard C, Keutzer JM. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin Chem. 2008;54:1725–1728. doi: 10.1373/clinchem.2008.104711. [DOI] [PubMed] [Google Scholar]
- 12.Hwu WL, Chien YH, Lee NC, Chiang SC, Dobrovolny R, Huang AC, Yeh HY, Chao MC, Lin SJ, Kitagawa T, Desnick RJ, Hsu LW. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset mutation c.936+919G>A (IVS4+919G>A) Hum. Mutat. 2009;30(10):1397–1405. doi: 10.1002/humu.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meikle PJ, Grasby DJ, Dean CJ, Lang DL, Bockmann M, Whittle AM, Fietz MJ, Simonsen H, Fuller M, Brooks DA, Hopwood JJ. Newborn screening for lysosomal storage disorders. Molecular Genetics and Metabolism. 2006;88:307–314. doi: 10.1016/j.ymgme.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Sista RS, Eckhardt AE, Wang T, Graham C, Rouse JL, Norton SM, Srinivasan V, Pollack MG, Tolun AA, Bali D, Millington DS, Pamula VK. Digital microfluidic platform for multiplexing enzyme assays: implications for lysosomal storage disease screening in newborns. Clin. Chem. 2011;57(10):1444–1451. doi: 10.1373/clinchem.2011.163139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mechtler TP, Metz TF, Muller HG, Ostermann K, Ratschmann R, De Jesus VR, Shushan B, Di Bussolo JM, Herman JL, Herkner KR, Kasper DC. Short-incubation mass spectrometry assay for lysosomal storage disorders in newborn and high-risk population screening. J. Chromat B. 2012;908:9–17. doi: 10.1016/j.jchromb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffey TA, Bellamy G, Elliott S, Fox AC, Glass M, Turecek F, Gelb MH, Scott CR. A tandem mass spectrometry triplex assay for the detection of Fabry, Pompe, and mucopolysaccharidosis-I (Hurler) Clin Chem. 2010;56:1854–1861. doi: 10.1373/clinchem.2010.152009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metz TF, Mechtler TP, Orsini JJ, Martin M, Shushan B, Herman JL, Ratschmann R, Item CB, Streubel B, Herkner KR, Kasper DC. Simplified newborn screening protocol for lysosomal storage disorders. Clin. Chem. 2011;57:1286–1294. doi: 10.1373/clinchem.2011.164640. [DOI] [PubMed] [Google Scholar]
- 18.Orsini JJ, Martin MM, Showers AL, Bodamer OA, Zhang XK, Gelb MH, Caggana M. Lysosomal storage disorder 4+1 multiplex assay for newborn screening using tandem mass spectrometry: application to a small-scale population study for five lysosomal storage disorders. Clin. Chim. Acta. 2012;413:1270–1273. doi: 10.1016/j.cca.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sista R, Eckhardt AE, Wang T, Sellos-Moura M, Pamula VK. Rapid, single-step assay for Hunter syndrome in dried blood spots using digital microfluidics. Clin. Chim. Acta. 2011;412(19–20):1895–1897. doi: 10.1016/j.cca.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Tolun AA, Graham C, Shi Q, Sista RS, Wang T, Eckhardt AE, Pamula VK, Millington DS, Bali DS. A novel fluorometric enzyme analysis method for Hunter syndrome using dried blood spots. Mol. Gen. and Metab. 2012;105:519–521. doi: 10.1016/j.ymgme.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Burton B, Charrow J, Angle B, Widera S, Waggoner D. A pilot newborn screening program for lysosomal storage disorders (LSD) in Illinois. Molecular Genetics and Metabolism. 2012;105(2):S23–S24. [Google Scholar]
- 22.Millington D, Sista R, Eckhardt A, Rouse J, Bali D, Goldberg R, Cotten M, Buckley R, Pamula V. Digital microfluidics: a future technology in the newborn screening laboratory. Seminars in Perinatology. 2010;34(2):163–169. doi: 10.1053/j.semperi.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCCLS. Evaluation of Precision Performance of Quantitative Measurement Methods; Approved Guideline—Second Edition. Wayne, Pennsylvania 19087- 1898 USA: NCCLS 940 West Valley Road, Suite 1400; 2004. NCCLS document EP5-A2 (ISBN 1-56238-542-9). [Google Scholar]
- NCCLS. Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. Wayne, Pennsylvania 19087-1898 USA: NCCLS 940 West Valley Road, Suite 1400; 2003. NCCLS document EP6-A (ISBN 1-56238-498-8). [Google Scholar]
- 25.De Jesus VR, Zhang XK, Keutzer J, Bodamer OA, Muhl A, Orsini J, Caggana M, Vogt RF, Hannon WH. Development and evaluation of quality control dried blood spot materials in newborn screening for lysosomal storage disorders. Pediatric Clinical Chemistry. 2009;55(1):158–164. doi: 10.1373/clinchem.2008.111864. [DOI] [PubMed] [Google Scholar]
- 26.Graham C, Eckhardt A, Sista R, Wong T, Wu N, Winger T, Xiong Y, Zhou H, Vogt R, Bali D, Millington D, Pamula V. Development of quality control spots for lysosomal storage disorders under cGMP. Poster presented at the 2011 APHL Annual Meeting.2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.