Abstract
Each year there are now more than 30% fewer deaths from breast cancer than there would have been had screening not been initiated. Therapy has improved, but treatment saves lives when breast cancer is found earlier. Analysts who have claimed that excess cancer detection is indicative of overdiagnosis have ignored the fundamentals.
The effort to reduce access to mammography screening has been going on for decades and has intensified in the past few years. Using flawed science that included retrospective subgroup analysis of data that lacked statistical power [1], the National Cancer Institute dropped support for screening of women aged 40–49 in 1993. This position was reversed in 1997, when the National Cancer Advisory Board recognized that the randomized controlled trials (RCTs) did, indeed, show that mortality was decreased with screening beginning at the age of 40 [2]. It has been clear for years that none of the parameters of screening change abruptly at the age of 40 or at any other age [3]. The age of 50 was originally chosen as a surrogate for menopause, yet no data show that menopause has any major influence on the factors associated with mammography screening. More than 40% of the years of life lost to breast cancer are among women diagnosed in their 40s [4]. The parameters of screening change gradually with increasing age, with no abrupt change at age 50 or any other age [3]. There is no scientific or biological reason to delay the start of screening until the age of 50.
Ignoring the scientific facts [5], in 2009, the U.S. Preventive Services Task Force (USPSTF) issued new breast cancer screening guidelines that supported only routine screening beginning at age 50 and extended the interval to every 2 years. The USPSTF has been described as a “panel of experts,” yet only one member actually had any previous experience analyzing the breast cancer screening data. None of the members was involved in breast cancer care. The analysis that was provided to the USPSTF clearly shows that the most lives are saved by annual screening beginning at age 40 [6]. Most are unaware that, using the same models on which the USPSTF relied, Hendrick and Helvie showed that as many as 100,000 women in their 30s whose lives would be saved by annual screening beginning at age 40 would die, unnecessarily, from breast cancer if they followed the USPSTF guidelines [7]. The only way to prove that screening saves lives is through RCTs. By looking only at deaths from breast cancer, RCTs eliminate bias (e.g., lead time, length, selection). Everyone agrees, including the USPSTF and the American College of Physicians [8], that RCTs of screening have proven that lives are saved by screening beginning at age 40.
The most recent effort to reduce access has re-formed around the so-called harms of screening. Using this pejorative term and the misleading concern about false positives, the USPSTF was more concerned about women being recalled based on screening for additional evaluation than about saving lives. The USPSTF argued against screening, primarily, using the rate of false positives. They failed to inform women and their physicians what they meant by this term. In fact, they were referring to the rate of women who have a screening mammogram and are then, because of something seen on the study, recalled for additional evaluation. If these women were not found to have breast cancer, they were all called “false positives.” The USPSTF failed to provide any context. The task force could have explained, for example, that the approximately 10% rate of women recalled for additional evaluation is actually the same as the rate of women recalled based on a questionable cervical cancer (“Pap”) screen. I suspect that the USPSTF’s argument would not seem as reasonable if they explained that of the approximately 100 of 1,000 women who are recalled based on screening, approximately 56 of 100 will have nothing more than a few additional mammographic views or ultrasound showing that there is no cancer, and there will be no further action. Approximately 25 of 100 will be followed up in 6 months. Imaging-guided needle biopsy using local anesthesia will be recommended for 19 (1.9% of the 1,000), and 6 of 19 (32%) will be found to have breast cancer. This is actually a fairly high yield of cancers. If, for example, a woman waits until she has a lump that a clinician thinks should be biopsied, the yield of cancer is only 15% [9], and when a palpable lesion proves to be malignant, the cancers are usually larger, later stage, and less curable than those found by mammography. Recalls from screening cause anxiety and inconvenience, but they should not carry the pejorative connotation of a false-positive study. Failing to alert women and their physicians as to just what a “false positive” actually meant, the inexperienced USPSTF essentially concluded that avoiding recalls was more important than saving lives.
That there is a real effort to eliminate screening was clearly articulated by one of the leading opponents, who stated in his article, entitled “Time to Stop Mammography Screening?” [10] that “The best method we have to reduce the risk of breast cancer is to stop the screening program.” To try to bolster the “stop screening” effort, a number of articles have made their way into the medical literature suggesting that screening has had little or no effect when introduced into the general population [11–13]. Some of the authors have been severely criticized for their poor methodology [14]. These analyses have been based on registry reviews and not on direct patient data, making their conclusions specious.
In another paper, published in the New England Journal of Medicine, it was suggested that there was little screening in Norway prior to the government-sponsored screening program and that when screening was introduced, it had little influence on the death rate [15]. It turns out that there had been a considerable amount of screening in Norway prior to the introduction of the organized program [16], and the study had only 2 years of follow-up. It is clear that screening does not begin to save lives immediately [17]. Because of length bias, the death rate begins to decline 5–7 years after screening is initiated [18]. This often-quoted paper was scientifically questionable. In fact, in a paper that did not receive widespread attention, when direct patient data in Norway were reviewed, there was a clear decline in deaths associated with the introduction of screening [19]. The same is true in multiple other analyses using direct patient data [20–27]. Although not scientific proof, in the U.S., screening began in large enough numbers to cause a steep climb in national incidence statistics in the mid-1980s, and, as expected, the death rate from breast cancer, which had been unchanged since 1940, began a sharp decline in 1990 [28]. Each year there are now more than 30% fewer deaths from breast cancer than there would have been had screening not been initiated. Therapy has improved, but treatment saves lives when breast cancer is found earlier.
Numerous specious arguments have been made over the years to try to reduce access to screening. In the Canadian National Breast Screening Study, all the patients included underwent a clinical breast examination before randomization so that it was known, prior to allocation, who had palpable cancers and who had palpable axillary lymph nodes. The women were then assigned to be in the screening or control arm of the trial using open lists. Those assigning the women could simply skip a line to ensure that a woman with a mass got into the mammography arm. This resulted in a statistically significant excess of women with advanced cancers being allocated to the screening arm [29, 30]. It should have been no surprise that more women from the screening arm died in the early years of this, yet supposed experts have ignored the facts. Somewhat incredibly, in an argument against screening in 2000 [31], the leader of the antiscreening effort wrote that the Canadian National Breast Screening Study was one of “the two trials with adequate randomisation.” Ignoring the fact that their unblinded randomization process placed more women with advanced, incurable cancers in the screening arm of their trial, the Canadian investigators, trying to explain the excess of breast cancer deaths in the screening arm, claimed that mammography might be squeezing cancer cells into the blood, leading to early deaths. After raising a great deal of concern, they later recanted this ridiculous interpretation [32].
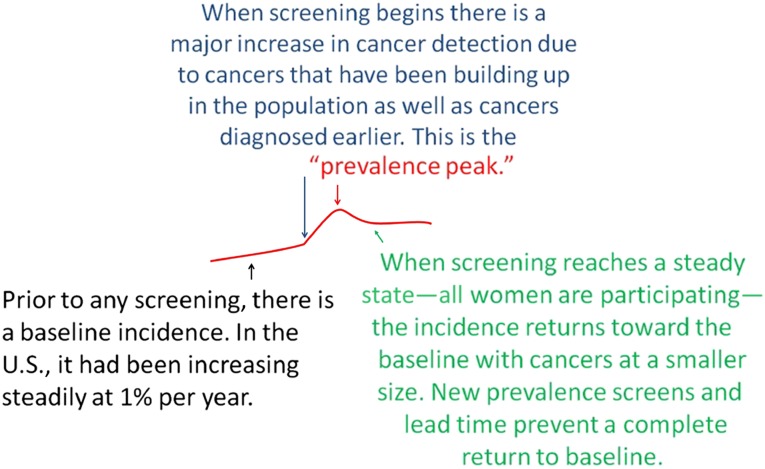
Much of the confusion, I believe, results from a failure of analysts to understand some of the fundamentals of screening. I think that we can assume that cancers are being initiated in the population every day. Breast cancers do not suddenly develop from a single cell to a 2-cm mass in 1 year. They may take years or even decades to reach a size that can be detected. There are many undiagnosed breast cancers in the population, some of which become clinically evident each year [33]. In the era before screening, most cancers were found by the woman herself. Screening tests are efficacious if they find cancers that have not yet reached a size at which they can be detected by the patient, or her physician, and if finding these cancers at a smaller size can lead to cure. What many seem to not understand is that when screening is initiated in a population, cancers that would have been detected that year are found, but palpable cancers that could have been detected by the patient or her physician (ignored or overlooked) are also detected. In addition, the screening test finds cancers 1 year, 2 years, or more before they would have been clinically evident. This results in a major jump in the number of cancers detected, which is well known as a “prevalence peak” (Fig. 1). This jump in cancers detected does not mean that the test is finding nonlethal cancers. It is expected with the initiation of any screening program. If screening continues and everyone who is going to participate complies, then the annual incidence will return toward the rate at which cancers were being detected before screening began, but the cancers will be at a smaller size, as determined by the threshold of the screening test. Because younger women eventually reach the age at which screening is recommended, they will bring their prevalence cancers and will keep the annual incidence from returning to the prescreening baseline.
Figure 1.
Schematic explaining that the baseline before screening begins is not necessarily horizontal. In the U.S., the incidence of invasive breast cancer was probably increasing by 1% per year prior to any screening. Initiating screening leads to a prevalence peak followed by a return toward the baseline if screening continues.
Another phenomenon that prevents the annual incidence from returning immediately to baseline while screening continues is the result of “lead time.” By detecting cancers years earlier, more cancers will be detected because the underlying incidence of breast cancer increases with age. If screening finds cancers 2 years earlier than before screening, women aged 50 years, for example, would have the higher incidence of 52-year-old women, and so on. This lead time means that screening will detect more cancers each year than would have been expected in the absence of screening. If screening is conducted for many years, there will eventually be some compensatory decline if screening is ended for older women (cancers have already been weeded out). Analysts who have claimed that excess cancer detection is indicative of overdiagnosis have ignored these fundamentals.
The most recent effort to reduce access to screening is based on this suggestion that screening leads to the detection of cancers that would never become clinically evident. This has been termed “overdiagnosis.” Scientific studies that have been based on direct patient data have shown that there is little if any overdiagnosis [16, 34–39], but these have been ignored by a few analysts who believe that thousands of breast cancers each year would not progress. Indeed, it has been stated that thousands would disappear on their own if left undiscovered [9]. The media have reported on these analyses, yet no one has ever addressed a fundamental question: Why, if there are thousands of these cancers being discovered each year, is there not one credible report of an invasive breast cancer “disappearing” on its own without some intervention? The answer is that there is no evidence that invasive cancers will regress and disappear on their own. This is pure fiction. The papers claiming massive overdiagnosis are scientifically flawed. They did not take into account lead time and variation in breast cancer risk between the populations [40] as well as new prevalence screening each year. The only way to directly measure overdiagnosis is from the RCTs. In the Malmö trial, overdiagnosis was estimated to be under 10% [41], whereas Duffy et al. found a rate of overdiagnosis of less than 1% [39].
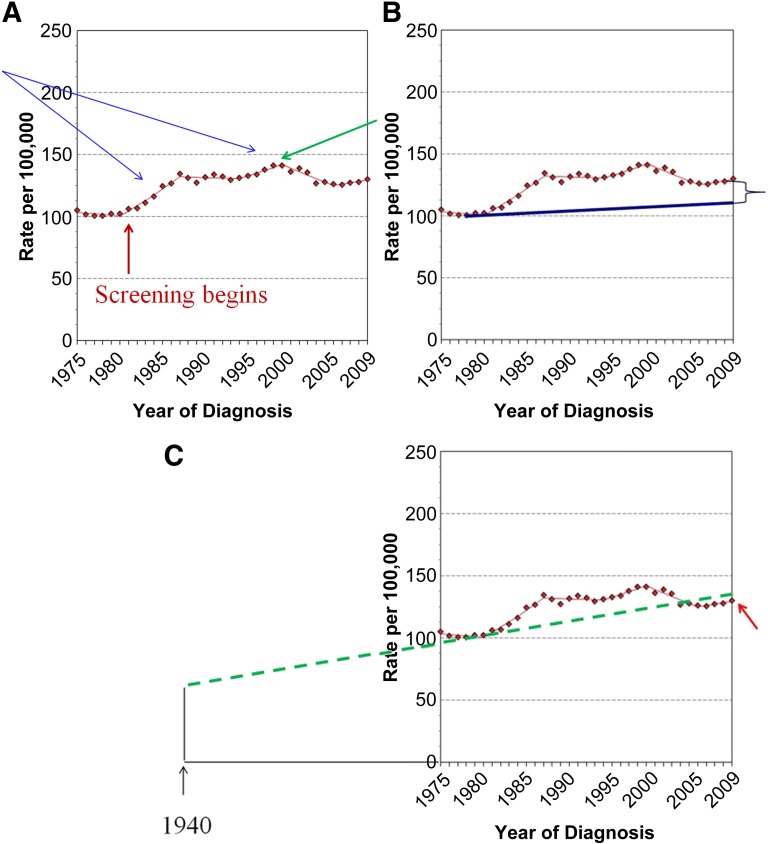
Perhaps one of the more egregious papers to suggest massive overdiagnosis was published recently in the New England Journal of Medicine [42]. The authors claimed that in 2008, as many as 70,000 cancers in the U.S., detected by screening, would have never become clinically evident. They concluded that mammography screening was leading to massive overdiagnosis of breast cancer. This paper is an excellent example of a failure of peer review. It should never have been published and should be withdrawn. Science is based on direct patient measures. This paper did not have direct patient information but used registry data from the Surveillance Epidemiology and End Results (SEER) database. Despite the fact that the authors had no data to determine who had mammograms and which cancers were found by mammography, they concluded that mammography screening was resulting in massive overdiagnosis. They based their conclusions on assumptions, estimates, and extrapolations that prove to be incorrect (Fig. 2).
Figure 2.
Age-adjusted incidence of female breast cancer, 1975–2009, adapted from the Surveillance Epidemiology and End Results database at http://seer.cancer.gov/faststats/selections.php?#Outputlastaccessed7/20/2010. (A): The rise in cancer incidence in the mid-1980s (blue arrows) was related to the, apparently annual, increase in participation in screening mammography. More and more women participated in screening until 1999, when participation appears to have leveled off (green arrow). This likely accounts for the decline in incidence from 1999 to 2006, as incidence returned toward the baseline. The baseline appears to have continued to increase at 1% per year from 2006 to 2009. (B): Bleyer and Welch estimated that had there been no screening, incidence would have increased from 1974 to 2008 by only 0.25% (blue line), leading them to conclude that the difference between the actual numbers (red dotted line) and their estimate must be the result of overdiagnosed cancers [42]. (C): If Bleyer and Welch had used the 40 years of data from the Connecticut Tumor Registry, which showed a baseline increase of 1% per year (green dotted line) from 60 per 100,000 in 1940 and confirmed by the fact that from 2006 to 2009 the incidence returned to 1% per year (red arrow), they would have found that the actual number of invasive breast cancers (red dotted line) fell below what would have been expected had there been no screening. Based on their methodology, screening actually led to fewer cancers than would be expected. There was certainly no overdiagnosis of invasive cancers.
The conclusions are based on the authors’ estimate of what the breast cancer incidence would have been had screening not been available from 1974 to 2008 [42]. They estimated this “baseline” by using SEER data from 1976 to 1978. The SEER database began in 1974. In 1974, the wife of the U.S. president and the wife of the U.S. vice president were both diagnosed with breast cancer. This coincidence initiated a period in which an indeterminate number of women underwent screening mammography, which likely resulted in a rise in annual incidence because of prevalence screening of these women. It is not clear how many women underwent screening. If no new women were starting screening, there would be a drop in the cancer rate back toward the prescreening incidence. If women stopped screening, then there would be a drop in incidence because screening had removed some of the cancers that would have appeared in subsequent years had they not been detected by screening. Regardless, given the absence of critical information and the lack of available data, it is impossible to correct for these factors, so the period 1976–1978 is very unlikely to be representative of the prescreening baseline.
The authors ignored far more robust data provided by the Connecticut Tumor Registry going back 40 years to 1940. This was a period prior to any general screening, and the data showed that the rate of invasive cancers had been increasing steadily at 1% per year [43]. This was four times the estimate used by Bleyer and Welch, who claimed that, in the absence of screening, the incidence would have increased at the rate of only 0.25% per year [42]. It has been argued that the Connecticut data may not be representative and that it is best to use numbers from the same database being studied. I would suggest not only that 40 years of data are far more reliable than 3 years but also that some data in the SEER database suggest that 1% per year is the correct rate. From the early 1980s to 1999, the incidence of breast cancer rose quickly and continued to rise (Fig. 2). This reflects prevalence screening, as women were participating in increasing numbers over time, as well as the underlying increasing baseline [44]. By 1999, the number of women participating in screening appears to have plateaued [45]. The prolonged prevalence peak that was maintained by more and more women starting screening began, for the first time, to decline after 1999. As expected once screening is at a steady rate, by 2006 the incidence had returned toward the underlying baseline [9]. Also as expected, SEER data from 2006 to 2009 show that the incidence of invasive cancers once again began to increase and at a rate of 1% per year. Once participation in screening plateaus, then any increase in annual incidence would reflect the background increase that is independent of screening. This is within the SEER database and is in complete agreement with the data from Connecticut. There is no other reasonable explanation for the sudden increase in incidence that began in 2006. Bleyer and Welch simply ignored this return to an increasing baseline that is unrelated to screening.
For unexplained reasons, the authors made another critical error. They combined the number of cases of ductal carcinoma in situ (DCIS) with the numbers of small invasive cancers, calling these, together, “early stage cancer.” To the uninformed, this might seem like a legitimate definition, but knowledgeable analysts would never combine the two. DCIS constitutes a series of lesions that are highly controversial, and their relation to invasive cancers is debated. Even the SEER database separates invasive and in situ cancers. The combining of DCIS with small invasive cancers was done either from a lack of understanding or intentionally to dilute and corrupt the estimates.
In 1980, SEER reported 102 invasive cancers per 100,000 women in the population. If we use a 1%-per-year increase in the incidence of invasive cancers, by 2008, in the absence of screening, there would have been 132 invasive breast cancers per 100,000 women in the population. Because of lead time and new women who begin screening for the first time, adding prevalence cancers each year, the number of invasive cancers should have been even higher than 132 per 100,000 without any overdiagnosis. In fact, with widespread screening in the U.S., the SEER data show that there were only 128 per 100,000 cases of invasive breast cancers in 2008 [46]. This amount is clearly less than should have been expected had there been no screening (Fig. 2), and it is much less than would have been expected when considering lead time and prevalence cancers. This means that, using the authors’ methods but the correct extrapolation, not only was there no overdiagnosis of invasive breast cancer but there were actually many fewer cases than would have been expected.
Bleyer and Welch admit that, had they used the 1%-per-year estimate, there would have still been 34,000 overdiagnosed cancers in 2008 [47]; however, they continued to use a figure inflated by adding DCIS to the invasive cancers. In 2008, 57,000 cases of DCIS were predicted [48]. If the authors remove DCIS from their estimate (as they should have done), then by their own calculation, there were 23,000 fewer invasive breast cancers than would have occurred in the absence of screening (34,000 − 57,000 = −23,000). By their own calculations, they have to agree that there is not only no overdiagnosis of invasive cancers but also thousands fewer invasive cancers than one might predict. Although it is not possible to determine in this fashion, we can wonder if the apparent decline in invasive breast cancer is because mammography screening has led to the removal of DCIS lesions over the preceding years, and perhaps this is the reason there are fewer invasive cancers than would have occurred in the absence of screening.
The authors also argue that in order for screening to be efficacious, it must reduce the number of “late-stage” cancers. In fact, a decline in late-stage cancers has been seen in the only rigorously scientific studies of screening—the RCTs [49]—but it is not a prerequisite. Women die from breast cancers diagnosed at all sizes and stages. Decreasing the size within stages also leads to a decline in mortality [50]. As with their fundamental approach, the methods Bleyer and Welch used to assess the numbers of late-stage cancers is highly questionable. Nevertheless, even using their methods, the authors also underestimated the decline in late-stage cancer. They used the change in the rate of late-stage cancers among women under age 40 (presumably not being screened) to estimate what the rate would have been among women aged 40 and older. Among the younger women, the number of late-stage cancers appears to have increased from 5 per 100,000 to 6 per 100,000 over the time period, a change of 20%. If this is applied to the data they used for older women, the rate of late-stage cancers would have gone from 102 to 122 per 100,000 had there not been any screening. They show that it actually declined to 94 per 100,000 over the time period. This is a drop of 28 per 100,000 (a decline of 23%), which is more than 3 times the decline that the authors suggest. Using their own questionable methods, the authors are also incorrect in their assessment of the rate of change for late-stage cancers.
It is clear that DCIS is found almost exclusively by mammography. There have been major efforts to try to address the issues that surround DCIS with regard to diagnosis and treatment. Questions about DCIS are far from new [51]. They are not worthy of publication to only try to confuse the issues. The scientifically unsupportable paper from the New England Journal of Medicine has been used to raise concerns among women and their physicians. A recent article in Consumer Reports was clearly influenced and placed emphasis on the issue of overdiagnosis in advising women to follow the USPSTF guidelines [52]. An op-ed piece by Welch in the New York Times [53] was coordinated with the publication of his paper, and a recent article in the Sunday Magazine section of the New York Times by a writer who voices doubts about the importance of early detection, referencing Welch and others [54] shows that these papers have been promoted to the public and will no doubt discourage some women from participating in screening. Even the Ladies' Home Journal is passing on advice based on the paper by Bleyer and Welch [42] and others from the Dartmouth Institute for Health Policy and Clinical Practice [55]. There are rumors that some European countries are using the paper by Bleyer and Welch to argue against any breast cancer screening. The analysis used in this paper is fundamentally flawed, and the conclusions are not scientifically supported. Nevertheless, using the authors’ methods but with more accurate extrapolation, there is no evidence of overdiagnosis of invasive breast cancer. Contrary to their assertion, the rate of late-stage cancer has dropped dramatically. The paper’s methods are questionable, and the conclusions are incorrect. The paper should be withdrawn by the New England Journal of Medicine.
Footnotes
Disclosures
Daniel B. Kopans: Uncompensated owner of patents for several mammographic- and biopsy-related devices (IP).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Kopans DB, Halpern E, Hulka CA. Statistical power in breast cancer screening trials and mortality reduction among women 40-49 years of age with particular emphasis on the National Breast Screening Study of Canada. Cancer. 1994;74:1196–1203. doi: 10.1002/1097-0142(19940815)74:4<1196::aid-cncr2820740403>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Hendrick RE, Smith RA, Rutledge JH, 3rd, et al. Benefit of screening mammography in women aged 40-49: A new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87–92. doi: 10.1093/jncimono/1997.22.87. [DOI] [PubMed] [Google Scholar]
- 3.Kopans DB, Moore RH, McCarthy KA, et al. Biasing the interpretation of mammography screening data by age grouping: Nothing changes abruptly at age 50. Breast J. 1998;4:139–145. [Google Scholar]
- 4.Shapiro S. Evidence on screening for breast cancer from a randomized trial. Cancer. 1977;39(Suppl):2772–2782. doi: 10.1002/1097-0142(197706)39:6<2772::aid-cncr2820390665>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Kopans DB. The 2009 U.S. Preventive Services Task Force guidelines ignore important scientific evidence and should be revised or withdrawn. Radiology. 2010;256:15–20. doi: 10.1148/radiol.10100057. [DOI] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Cronin KA, Bailey S, et al. Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network Effects of mammography screening under different screening schedules: Model estimates of potential benefits and harms. Ann Intern Med. 2009;151:738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: Science ignored. AJR Am J Roentgenol. 2011;196:W112-W116. doi: 10.2214/AJR.10.5609. [DOI] [PubMed] [Google Scholar]
- 8. Screening mammography in women aged 40–49: A report of the American College of Physicians and American College of Radiology consensus meeting. Available at http://www.acpinternist.org/archives/2012/05/policy.htm. Accessed September 9. 2012.
- 9.Spivey GH, Perry BW, Clark VA, et al. Predicting the risk of cancer at the time of breast biopsy. Variation in the benign to malignant ratio. Am Surg. 1982;48:326–332. [PubMed] [Google Scholar]
- 10.Gøtzsche PC. Time to stop mammography screening? CMAJ. 2011;183:1957–1958. doi: 10.1503/cmaj.111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zahl PH, Maehlen J, Welch HG. The natural history of invasive breast cancers detected by screening mammography. Arch Intern Med. 2008;168:2311–2316. doi: 10.1001/archinte.168.21.2311. [DOI] [PubMed] [Google Scholar]
- 12.Autier P, Boniol M, Gavin A, et al. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: Trend analysis of WHO mortality database. BMJ. 2011;343:d4411. doi: 10.1136/bmj.d4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jørgensen KJ, Gøtzsche PC. Overdiagnosis in publicly organised mammography screening programmes: Systematic review of incidence trends. BMJ. 2009;339:b2587. doi: 10.1136/bmj.b2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock K, Borisch B, Cawson J, et al. Effect of population-based screening on breast cancer mortality. Lancet. 2011;378:1775–1776. doi: 10.1016/S0140-6736(11)61766-2. [DOI] [PubMed] [Google Scholar]
- 15.Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203–1210. doi: 10.1056/NEJMoa1000727. [DOI] [PubMed] [Google Scholar]
- 16.Lynge E, Braaten T, Njor SH, et al. Mammography activity in Norway 1983 to 2008. Acta Oncol. 2011;50:1062–1067. doi: 10.3109/0284186X.2011.599339. [DOI] [PubMed] [Google Scholar]
- 17.Kopans DB. Screening for breast cancer and mortality reduction among women 40-49 years of age. Cancer. 1994;74(Suppl):311–322. doi: 10.1002/cncr.2820741316. [DOI] [PubMed] [Google Scholar]
- 18.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: Impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260:658–663. doi: 10.1148/radiol.11110469. [DOI] [PubMed] [Google Scholar]
- 19.Hofvind S, Ursin G, Tretli S, et al. Breast cancer mortality in participants of the Norwegian Breast Cancer Screening Program. Cancer. 2013;119:3106–3112. doi: 10.1002/cncr.28174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabár L, Vitak B, Chen HH, et al. Beyond randomized controlled trials: organized mammographic screening substantially reduces breast carcinoma mortality. Cancer. 2001;91:1724–1731. doi: 10.1002/1097-0142(20010501)91:9<1724::aid-cncr1190>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Duffy SW, Tabár L, Chen HH, et al. The impact of organized mammography service screening on breast carcinoma mortality in seven Swedish counties. Cancer. 2002;95:458–469. doi: 10.1002/cncr.10765. [DOI] [PubMed] [Google Scholar]
- 22.Otto SJ, Fracheboud J, Looman CWN, et al. National Evaluation Team for Breast Cancer Screening Initiation of population-based mammography screening in Dutch municipalities and effect on breast-cancer mortality: a systematic review. Lancet. 2003;361:1411–1417. doi: 10.1016/S0140-6736(03)13132-7. [DOI] [PubMed] [Google Scholar]
- 23.van Schoor G, Moss SM, Otten JD, et al. Increasingly strong reduction in breast cancer mortality due to screening. Br J Cancer. 2011;104:910–914. doi: 10.1038/bjc.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto SJ, Fracheboud J, Verbeek ALM, et al. National Evaluation Team for Breast Cancer Screening Mammography screening and risk of breast cancer death: A population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2012;21:66–73. doi: 10.1158/1055-9965.EPI-11-0476. [DOI] [PubMed] [Google Scholar]
- 25.Swedish Organised Service Screening Evaluation Group Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data. Cancer Epidemiol Biomarkers Prev. 2006;15:45–51. doi: 10.1158/1055-9965.EPI-05-0349. [DOI] [PubMed] [Google Scholar]
- 26.Hellquist BN, Duffy SW, Abdsaleh S, et al. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years: Evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2011;117:714–722. doi: 10.1002/cncr.25650. [DOI] [PubMed] [Google Scholar]
- 27.Coldman A, Phillips N, Warren L, et al. Breast cancer mortality after screening mammography in British Columbia women. Int J Cancer. 2007;120:1076–1080. doi: 10.1002/ijc.22249. [DOI] [PubMed] [Google Scholar]
- 28.Kopans DB. Beyond randomized controlled trials: Organized mammographic screening substantially reduces breast carcinoma mortality. Cancer. 2002;94:580–581; author reply 581–583. doi: 10.1002/cncr.10220. [DOI] [PubMed] [Google Scholar]
- 29.Kopans DB, Feig SA. The Canadian National Breast Screening Study: A critical review. AJR Am J Roentgenol. 1993;161:755–760. doi: 10.2214/ajr.161.4.8372752. [DOI] [PubMed] [Google Scholar]
- 30.Tarone RE. The excess of patients with advanced breast cancer in young women screened with mammography in the Canadian National Breast Screening Study. Cancer. 1995;75:997–1003. doi: 10.1002/1097-0142(19950215)75:4<997::aid-cncr2820750415>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Gøtzsche PC, Olsen O. Is screening for breast cancer with mammography justifiable? Lancet. 2000;355:129–134. doi: 10.1016/S0140-6736(99)06065-1. [DOI] [PubMed] [Google Scholar]
- 32.Merz B. Author of Canadian Breast Cancer Study retracts warnings. J Natl Cancer Inst. 1992;84:833–834. [PubMed] [Google Scholar]
- 33.Kopans DB, Rafferty E, Georgian-Smith D, et al. A simple model of breast carcinoma growth may provide explanations for observations of apparently complex phenomena. Cancer. 2003;97:2951–2959. doi: 10.1002/cncr.11434. [DOI] [PubMed] [Google Scholar]
- 34.Yen AM, Duffy SW, Chen TH, et al. Long-term incidence of breast cancer by trial arm in one county of the Swedish Two-County Trial of mammographic screening. Cancer. 2012;118:5728–5732. doi: 10.1002/cncr.27580. [DOI] [PubMed] [Google Scholar]
- 35.Njor SH, Olsen AH, Blichert-Toft M, et al. Overdiagnosis in screening mammography in Denmark: Population based cohort study. BMJ. 2013;346:f1064. doi: 10.1136/bmj.f1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen MF, Tabár L, Vitak B, et al. Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. Eur J Cancer. 2003;39:1746–1754. doi: 10.1016/s0959-8049(03)00260-0. [DOI] [PubMed] [Google Scholar]
- 37.Kopans DB, Smith RA, Duffy SW. Mammographic screening and “overdiagnosis”. Radiology. 2011;260:616–620. doi: 10.1148/radiol.11110716. [DOI] [PubMed] [Google Scholar]
- 38.Paci E, Warwick J, Falini P, et al. Overdiagnosis in screening: is the increase in breast cancer incidence rates a cause for concern? J Med Screen. 2004;11:23–27. doi: 10.1177/096914130301100106. [DOI] [PubMed] [Google Scholar]
- 39.Duffy SW, Agbaje O, Tabar L, et al. Overdiagnosis and overtreatment of breast cancer: Estimates of overdiagnosis from two trials of mammographic screening for breast cancer. Breast Cancer Res. 2005;7:258–265. doi: 10.1186/bcr1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puliti D, Duffy SW, Miccinesi G, et al. EUROSCREEN Working Group Overdiagnosis in mammographic screening for breast cancer in Europe: A literature review. J Med Screen. 2012;19(Suppl 1):42–56. doi: 10.1258/jms.2012.012082. [DOI] [PubMed] [Google Scholar]
- 41.Zackrisson S, Andersson I, Janzon L, et al. Rate of over-diagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: Follow-up study. BMJ. 2006;332:689–692. doi: 10.1136/bmj.38764.572569.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 43.Garfinkel L, Boring CC, Heath CW., Jr Changing trends. An overview of breast cancer incidence and mortality. Cancer. 1994;74(Suppl):222–227. doi: 10.1002/cncr.2820741304. [DOI] [PubMed] [Google Scholar]
- 44. Use of mammography among women 40 years of age and over. Available at http://www.cdc.gov/nchs/data/health_policy/mammography.pdf. Updated July 2009. Accessed April 17, 2013.
- 45. Use of mammography among women 40 years of age and over. Available at http://www.cdc.gov/nchs/health_policy/mammography.htm. Updated September 25, 2009. Accessed July 28, 2013.
- 46. Stat Fact Sheets SEER. Breast. Available at http://seer.cancer.gov/statfacts/html/breast.html. Accessed March 23, 2013.
- 47.Bleyer A, Welch HG. Effect of screening mammography on breast cancer incidence. N Engl J Med. 2013;368:679. doi: 10.1056/NEJMc1215494. [DOI] [PubMed] [Google Scholar]
- 48.American Cancer Society . Breast cancer facts & figures 2007-2008. Atlanta, GA: American Cancer Society; 2007. p. 9. [Google Scholar]
- 49.Smith RA, Duffy SW, Gabe R, et al. The randomized trials of breast cancer screening: What have we learned? Radiol Clin North Am. 2004;42:793–806, v. doi: 10.1016/j.rcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Connor RJ, Chu KC, Smart CR. Stage-shift cancer screening model. J Clin Epidemiol. 1989;42:1083–1095. doi: 10.1016/0895-4356(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 51.Ernster VL, Barclay J, Kerlikowske K, et al. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275:913–918. [PubMed] [Google Scholar]
- 52.Save your life: cancer screening is oversold. Know the tests to get—and those to skip. Consum Rep. 2013;78:28–33. [PubMed] [Google Scholar]
- 53. Welch HG. If you feel O.K., maybe you are O.K. Available at http://www.nytimes.com/2012/02/28/opinion/overdiagnosis-as-a-flaw-in-health-care.html?_r=0.
- 54. Orenstein P. Our feel-good war on breast cancer. Available at http://www.nytimes.com/2013/04/28/magazine/our-feel-good-war-on-breast-cancer.html?pagewanted=all. Accessed April 29, 2013.
- 55. Skolnik D. Go ahead, blow it off! 6 Health rules you can break. Available at http://www.lhj.com/health/news/blow-it-off-6-health-rules-you-can-break/?page=2. Accessed August 20, 2013.