Although an improved understanding of breast cancer pathogenesis and the role of human epidermal growth factor receptor 2 (HER2) signaling has resulted in significant survival improvements in the past 20 years, resistance to HER2-targeted therapy remains a concern. This article reviews the role of HER2 signaling in breast cancer pathogenesis, potential therapeutic targets to overcome resistance to HER2-targeted therapy, and clinical trials evaluating agents targeting the HER family and downstream pathways.
Keywords: Epidermal growth factor receptor, Human epidermal growth factor receptor tyrosine kinase receptor family, Breast cancer, Tyrosine kinase inhibitor
Learning Objectives
Describe the role of HER2 in breast cancer pathogenesis.
List the approved and investigational agents targeting the HER receptor family and downstream signaling pathways with focus on overcoming resistance to HER2-targeted therapies.
Describe ongoing clinical trials evaluating the efficacy and safety of agents targeting HER and downstream pathways in breast cancer patients.
Abstract
Breast cancer characterized by overexpression of human epidermal growth factor receptor 2 (HER2) has been associated with more aggressive disease progression and a poorer prognosis. Although an improved understanding of breast cancer pathogenesis and the role of HER2 signaling has resulted in significant survival improvements in the past 20 years, resistance to HER2-targeted therapy remains a concern. A number of strategies to prevent or overcome resistance to HER2-targeted therapy in breast cancer are being evaluated. This article provides a comprehensive review of (a) the role of HER2 signaling in breast cancer pathogenesis, (b) potential receptor and downstream therapeutic targets in breast cancer to overcome resistance to HER2-targeted therapy, and (c) clinical trials evaluating agents targeting one or more members of the HER family and/or downstream pathways for the treatment of breast cancer, with a focus on metastatic disease.
Implications for Practice:
Although a greater understanding of the role of human epidermal growth factor receptor 2 (HER2) signaling in breast cancer pathogenesis has led to significant survival improvements, resistance to HER2-targeted therapy remains an important concern. Several treatment strategies are being evaluated to overcome resistance to HER2-targeted therapy, including targeting one or more members of the HER family and/or downstream signaling pathways. With the many ongoing investigations of HER family agents across various treatment settings in breast cancer, this is an exciting but challenging research area with rapidly evolving data.
Introduction
The human epidermal growth factor family of tyrosine kinase receptors includes human epidermal growth factor receptor 1 (HER1; also known as epidermal growth factor receptor [EGFR]), HER2, HER3, and HER4. Most of these receptors can be activated by ligand-dependent dimerization—the pairing of two of the same receptors (homodimerization) or two different receptors (heterodimerization)—within the plasma membrane. Although there are no known endogenous ligands for HER2, when HER2 is overexpressed, it can undergo ligand-independent activation via spontaneous homodimerization and autoactivation [1] and may facilitate heterodimerization with HER1 and HER3, resulting in the activation of multiple signal transduction pathways. HER2 overexpression (HER2 positivity) in breast cancer has been associated with poorer clinical outcomes compared with HER2-negative disease [2–6].
Trastuzumab
Trastuzumab (Herceptin; Genentech, South San Francisco, CA, http://www.gene.com) is an anti-HER2 monoclonal antibody initially approved as first-line treatment for metastatic breast cancer (MBC) in combination with paclitaxel in 1998 that is currently indicated by the U.S. Food and Drug Administration (FDA) for the treatment of HER2-positive breast cancer [7]. It appears to work by a variety of different mechanisms, including prevention of HER2 cleavage and downstream proliferative signaling, antiangiogenic effects, cellular cytotoxicity, and interference with DNA repair [8]. The original FDA approval of trastuzumab for MBC was based on a phase III trial comparing chemotherapy plus trastuzumab with chemotherapy alone in previously untreated HER2-positive MBC, with significantly greater time to progression (TTP), overall survival (OS), and overall response rate (RR) seen with trastuzumab plus chemotherapy [9]. However, an increase in heart failure rate was observed with the addition of trastuzumab (10% when given with chemotherapy vs. 2% with chemotherapy alone). Trastuzumab has also been investigated as a single agent in the metastatic setting, but RRs have been relatively low (approximately 20%–25%) [10, 11]. The combination of trastuzumab with antiestrogen therapies such as aromatase inhibitors has also been evaluated and was associated with improvement in progression-free survival (PFS) in two randomized trials (TAnDEM and eLEcTRA) of postmenopausal women with HER2-overexpressing and estrogen receptor (ER)-positive MBC (Table 1) [12, 13].
Table 1.
Completed phase III clinical trials of trastuzumab in HER2-positive breast cancer
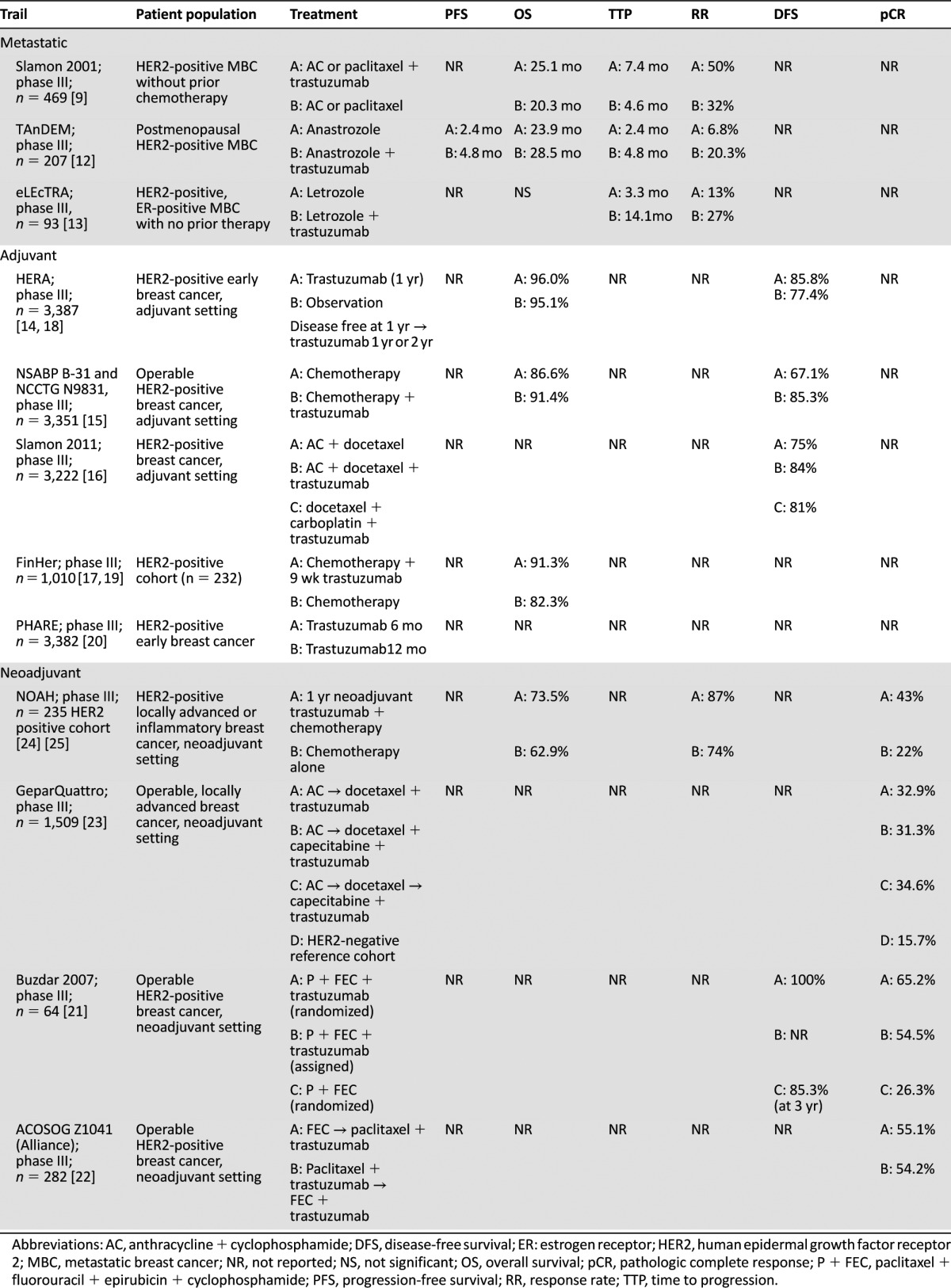
Trastuzumab was subsequently approved in the adjuvant setting, and its benefits have been demonstrated in multiple phase III trials including HERA, FinHer, Breast Cancer International Research Group 006, and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B31 [7, 14–17]. These randomized trials showed that trastuzumab therapy (in combination or subsequent to chemotherapy) was associated with improvements in disease-free survival (DFS) in patients with HER2-positive breast cancer [14–16]. The HERA trial demonstrated improvements in DFS and OS at a median follow-up of 8 years with both 1 and 2 years of trastuzumab maintenance; however, 2 years was not superior to 1 year of adjuvant trastuzumab [18]. Data on 9 weeks of maintenance trastuzumab are less substantiated [19], and the PHARE trial was inconclusive for noninferiority when comparing 6 versus 12 months of trastuzumab adjuvant maintenance therapy, suggesting that 12 months of adjuvant trastuzumab is to remain the standard [20].
Trastuzumab has also been evaluated in the neoadjuvant setting combined with chemotherapy, with pathologic complete response (pCR) rates of 60% when given with paclitaxel and subsequent fluorouracil/epirubicin/cyclophosphamide (FEC) [21] and approximately 55% in a study of different sequencing of paclitaxel and FEC [22]. In the phase III GeparQuattro trial, HER2-positive patients received neoadjuvant chemotherapy with trastuzumab, with a pCR rate of 31.7% compared with 15.7% in the HER2-negative reference group that received chemotherapy alone [23]. As in many similar trials, pCR rates were higher in hormone receptor-negative versus hormone receptor-positive HER2-positive cancers. The higher pCR rate following neoadjuvant trastuzumab-containing therapy seems to translate to better overall outcome: in the NOAH study of HER2-positive locally advanced or inflammatory breast cancer, patients treated with neoadjuvant/adjuvant trastuzumab-containing therapy had improved event-free survival at 3 years (71% vs. 56% for chemotherapy alone; p = .013) [24] and 5 years (57.5% vs. 43.3%; p = .016) [25], with a trend toward longer 5-year OS (73.5% vs. 62.9%; p = .055) [25].
EGFR Inhibitors
Limited clinical studies in breast cancer have assessed the efficacy and safety of erlotinib (Tarceva; Genentech), a reversible oral EGFR-targeted tyrosine kinase inhibitor (TKI) [26, 27]. A phase II study of erlotinib in 23 HER2-positive MBC patients showed only one partial response (PR) [28]. Combining erlotinib with trastuzumab as first-line therapy for HER2-positive MBC in a phase I/II trial revealed four PRs and TTP of 9.03 months in 12 patients [29]. This trial did not meet its accrual goal; hence, the efficacy of this regimen is unclear. Phase I/II studies also added gefitinib (Iressa; AstraZeneca, Wilmington, DE, http://www.astrazeneca.com), another EGFR-targeted TKI [27, 30], to trastuzumab in HER2-positive MBC. A study of trastuzumab and gefitinib resulted in 9% RR, and median TTP was 3 months in patients with no prior metastatic therapy and 5.3 months in those with prior therapy; median OS was 27 months [31]. A phase I/II study evaluating the addition of gefitinib to docetaxel and trastuzumab in MBC reported PFS of 12.7 months and RR of 64% [32].
Dual EGFR and HER2 Inhibitors
Lapatinib
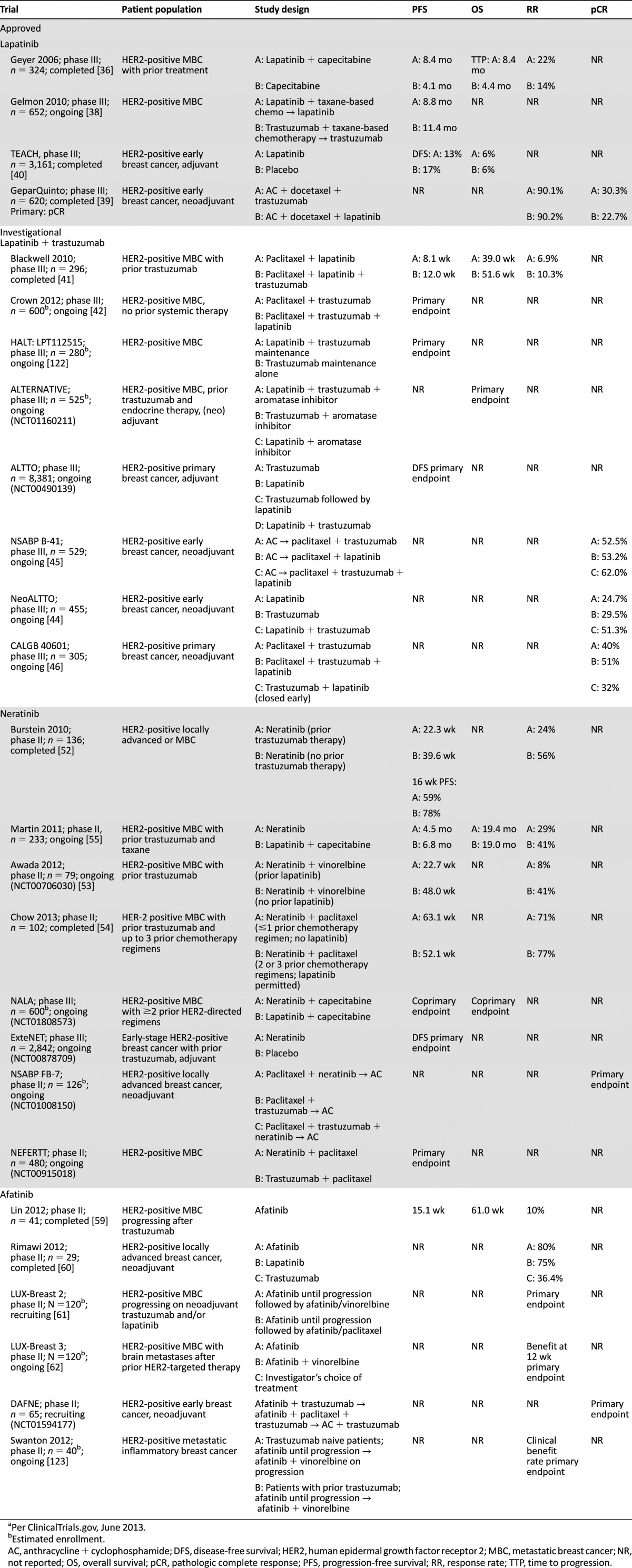
Dual TKIs that interact with several EGFR members have shown more promising results than EGFR inhibition alone. Lapatinib (Tykerb; GlaxoSmithKline, London, U.K., http://www.gsk.com/uk) is an oral small-molecule reversible inhibitor of both EGFR and HER2 [33]. Lapatinib was first approved by the FDA in 2007 in combination with capecitabine for the treatment of HER2-positive MBC after prior trastuzumab/chemotherapy [34, 35] (Table 2). Approval was based on a phase III study comparing lapatinib/capecitabine with capecitabine alone in patients with MBC progressing after chemotherapy/trastuzumab, as TTP improved from 4.4 to 8.4 months [36]. A phase III trial testing lapatinib in combination with antiestrogen therapy also showed improvement, with a median PFS of 3.0 and 8.2 months with letrozole/placebo versus lapatinib/letrozole, respectively, in hormone receptor-positive HER2-positive MBC [37]. However, a phase III study of HER2-positive MBC patients demonstrated longer PFS with trastuzumab compared with lapatinib (11.4 vs. 8.8 months) when combined with first-line taxane-based therapy; no significant difference in OS was observed, and more grade 3 to 4 events of diarrhea and rash were observed with lapatinib [38]. Trastuzumab was also superior to lapatinib in the neoadjuvant setting in the phase III GeparQuinto trial, with pCR of 30.3% versus 22.7%, respectively (p = .04) [39]. In the adjuvant setting, the TEACH phase III study evaluated lapatinib versus placebo, with improved DFS observed with lapatinib in patients with confirmed HER2-positive disease [40]. Thus, lapatinib may be an alternative in patients for whom adjuvant trastuzumab is contraindicated.
Table 2.
Completed and ongoing phase II and phase III clinical trials of approved and investigational multitargeted HER family tyrosine kinase inhibitors in HER2-positive breast cancera
Combination of Lapatinib and Trastuzumab
In a phase III study, lapatinib plus trastuzumab significantly prolonged median PFS compared with lapatinib alone in HER2-positive MBC patients progressing on prior trastuzumab, although RRs were not significantly different [41]. An international phase III study is evaluating lapatinib/trastuzumab plus chemotherapy in the first-line setting for MBC [42], and another phase III study is examining whether addition of lapatinib improves PFS in MBC patients receiving trastuzumab maintenance (NCT00968968). The ALTERNATIVE phase III study will randomize hormone receptor-positive HER2-positive MBC patients to letrozole and trastuzumab with and without lapatinib (NCT01160211) [43]. The phase III ALTTO study (NCT00490139) is evaluating lapatinib, trastuzumab, trastuzumab followed by lapatinib, and both together in the adjuvant setting. The phase III NeoALTTO trial evaluated neoadjuvant/adjuvant lapatinib, trastuzumab, and the combination with chemotherapy in women with early-stage disease [44]. The combination of trastuzumab and lapatinib resulted in a higher pCR (51.3%) compared with trastuzumab (29.5%) or lapatinib (24.7%) alone [44]. NSABP B-41 involved 529 patients who received chemotherapy plus neoadjuvant trastuzumab (pCR, 52.5%), lapatinib (53.2%), or trastuzumab/lapatinib (62%; p = .075), followed by a year of adjuvant trastuzumab [45]. Based on presented results of CALGB 40601, there was no significant difference in the pCR rate between weekly paclitaxel plus trastuzumab (40%) versus weekly paclitaxel plus trastuzumab/lapatinib (51%; p = .11) [46]. A prior meta-analysis of neoadjuvant trastuzumab and lapatinib trials found that pCR was highest with trastuzumab plus lapatinib [47], suggesting that the optimal use of lapatinib may be in conjunction with trastuzumab through dual blockade of HER2 and EGFR, as lapatinib alone appears less effective than in combination with trastuzumab in the first-line setting. Neoadjuvant lapatinib and trastuzumab along with hormonal therapy were also administered to hormone receptor-positive/HER2-positive patients in a phase II study, with a substantial pCR of 21% despite no concurrent chemotherapy [48].
Neratinib
Neratinib (PB-272; Puma Biotechnology, Inc., Los Angeles, CA, http://www.pumabiotechnolog.com [licensed October 2011 from Pfizer, New London, CT, http://www.pfizer.com] [49]) is an oral small-molecule irreversible TKI of EGFR, HER2, and HER4 [50, 51]. In a phase II study, neratinib showed a median PFS of 22.3 and 39.6 weeks in 136 HER2-positive MBC patients with or without prior trastuzumab, respectively [52]. In another phase II MBC study with neratinib/vinorelbine, RRs were 41% (no prior lapatinib) and 8% (prior lapatinib) [53]. In a phase I/II trial of neratinib/paclitaxel, the RR among patients with HER2-positive MBC was 71% in those who had received up to one prior non-lapatinib-containing chemotherapy regimen and 77% in those pretreated with two or three chemotherapy regimens, which may have included lapatinib [54]. As with earlier trials, diarrhea was the most frequent toxicity observed with neratinib/vinorelbine and neratinib/paclitaxel, affecting >90% of patients [53, 54]. Another phase II study found no significant difference with neratinib versus lapatinib/capecitabine (median PFS was 4.5 and 6.8 months, and OS was 19.4 and 19 months, respectively) in 233 HER2-positive MBC patients [55]. Preliminary data from a phase I study (NSABP FB-8) of neratinib with trastuzumab and paclitaxel in HER2-positive MBC patients revealed one complete response (CR) and three PRs in 10 patients [56]. Multiple trials are ongoing examining the potential role of neratinib in the neoadjuvant (NCT01008150; NSABP FB-7), adjuvant (NCT00878709 [ExteNET]), and metastatic settings (NCT00915018 [NEFERTT]; NCT01808573 [NALA]).
Afatinib
Afatinib (BIBW 2992; Boehringer Ingelheim, Ingelheim, Germany; http://www.boehringer-ingelheim.com) is an oral irreversible ErbB family inhibitor (EGFR, HER2, and HER4; Table 2) [57, 58]. Four of 41 patients had PR and 15 had stable disease in a phase II study of afatinib in heavily pretreated HER2-positive MBC [59]. Afatinib compared favorably with lapatinib and trastuzumab in 29 patients in the neoadjuvant setting, with RRs of 80%, 75%, and 36.4%, respectively [60]. There is an ongoing phase II study evaluating afatinib combined with trastuzumab-based therapy in the neoadjuvant setting (NCT01594177; DAFNE). Other phase II/III trials in the MBC setting include LUX-Breast 2 (NCT01271725) [61] and LUX-Breast 3 (NCT01441596) [62] (Table 2).
Newer Anti-HER2 Antibodies
Pertuzumab
The monoclonal antibody pertuzumab (Perjeta; Genentech) binds to a different region of the HER2 extracellular domain than trastuzumab and can block dimerization of HER2 with other HER family receptors [63]. Pertuzumab was FDA approved in June 2012 in combination with docetaxel/trastuzumab as first-line therapy for HER2-overexpressing MBC [64]. This approval was based on the CLEOPATRA phase III study, in which 808 HER2-positive MBC patients were randomized to receive pertuzumab/trastuzumab/docetaxel or placebo/trastuzumab/docetaxel (Table 3) [65, 66]. At interim analysis, the addition of pertuzumab increased PFS from 12.4 months to 18.5 months (p < .001), and OS analysis favored pertuzumab, with 17.2% deaths with pertuzumab versus 23.6% with control [65]. After an additional year of follow-up, median OS was 37.6 months with placebo/trastuzumab/docetaxel and had not been reached with pertuzumab/trastuzumab/docetaxel [66]. The addition of pertuzumab resulted in more diarrhea and febrile neutropenia, but did not increase cardiac dysfunction [65].
Table 3.
Completed and ongoing phase II and phase III clinical trials of trastuzumab-DM1 and pertuzumab in HER2-positive breast cancera
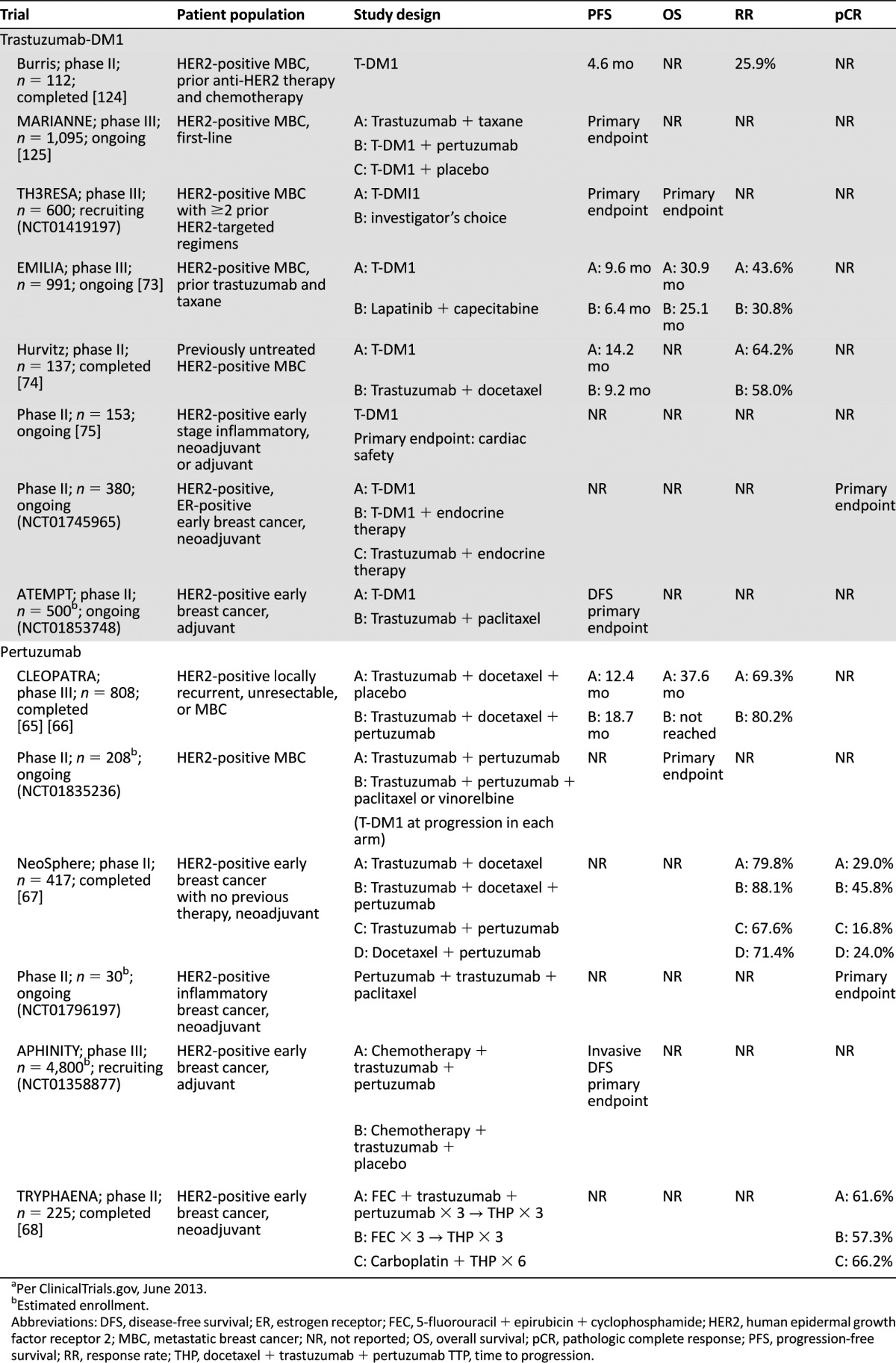
In the NeoSphere phase II trial, neoadjuvant pertuzumab/trastuzumab/docetaxel had the highest pCR rate in breast at 45.8%, as compared with trastuzumab/docetaxel (29%), pertuzumab/docetaxel (24%), and trastuzumab/pertuzumab (16.8%) [67]. pCR rates inclusive of breast and lymph nodes were also the highest with the triple combination (39.3%) versus trastuzumab/docetaxel (21.5%), pertuzumab/docetaxel (17.7%), and pertuzumab/trastuzumab (11.2%), with HER2-overexpressive hormone receptor-negative tumors responding best with 54.4% pCR inclusive of breast and lymph nodes [64]. The number of serious adverse events was similar across the three chemotherapy-containing arms but lower with pertuzumab/trastuzumab [67]. TRYPHAENA was a randomized phase II cardiac safety study of pertuzumab and trastuzumab as a component of neoadjuvant chemotherapy, reporting low incidences of left ventricular systolic dysfunction with pCR rates of 55% in breast and lymph nodes in patients with locally advanced stage II-III breast cancer treated with pertuzumab, trastuzumab, and docetaxel following FEC, and 64% with a nonanthracycline containing regimen of pertuzumab, trastuzumab, carboplatin, and docetaxel [68]. As a result of these trials, pertuzumab has recently been FDA approved for use as part of neoadjuvant therapy for patients with HER2-positive locally advanced, inflammatory, or early-stage breast cancer [64]. A phase III study (APHINITY) of trastuzumab and chemotherapy with or without pertuzumab in the adjuvant setting is ongoing (NCT01358877), as are phase II trials of pertuzumab with trastuzumab/paclitaxel as neoadjuvant therapy for inflammatory breast cancer (NCT01796197) and of pertuzumab and trastuzumab with or without chemotherapy in MBC (NCT01835236).
MM-111
MM-111 (Merrimack Pharmaceuticals, Cambridge, MA, http://merrimackpharma.com) is a monoclonal antibody that targets HER2 and HER3, preventing their dimerization. In preclinical studies, MM-111 was shown to decrease HER2/HER3-phosphatidylinositol 3-kinase (PI3K) signaling and was synergistic with trastuzumab and lapatinib in HER2-positive cancers [69]. A phase I study is investigating MM-111 in combination with trastuzumab in HER2-positive MBC progressing on previous therapies (NCT01097460), and results are awaited from a phase I study of MM-111 as monotherapy in HER2-positive solid tumors (NCT00911898).
Trastuzumab-DM1
Trastuzumab-DM1 (T-DM1; Kadcyla; Genentech) is an antibody-drug conjugate, with trastuzumab delivering the antimicrotubule agent DM1 (emtansine) to HER2-positive tumor cells [70, 71]. In preclinical models, T-DM1 has demonstrated inhibition of cellular proliferation and promotion of cell death in trastuzumab-resistant breast cancer cells [70]. Data from two separate single-arm phase II trials in MBC patients progressing on prior HER2-targeted therapy reported RRs of 33.6% and 26.9% with T-DM1 [72]. A phase III trial (EMILIA) in HER2-positive locally advanced or MBC patients previously treated with trastuzumab and a taxane demonstrated a PFS and OS benefit for TDM-1 (9.6 and 30.9 months, respectively) compared with lapatinib/capecitabine (6.4 and 25.1 months) [73]. The T-DM1 arm also had fewer grade ≥3 adverse events. This led to FDA approval of T-DM1 for HER2-positive MBC patients previously treated with trastuzumab and a taxane. T-DM1 also showed PFS improvement as first-line therapy in MBC patients versus trastuzumab/docetaxel in a phase II study [74]. MARIANNE (NCT01120184) is an ongoing phase III study of HER2-positive MBC patients randomized to receive first-line trastuzumab/taxane, T-DM1/placebo, or T-DM1/pertuzumab; T-DM1 is also being studied in the adjuvant and neoadjuvant settings (NCT01196052 [75]; NCT01745965; NCT01853748).
Targeting HER Family Downstream Signaling Pathways
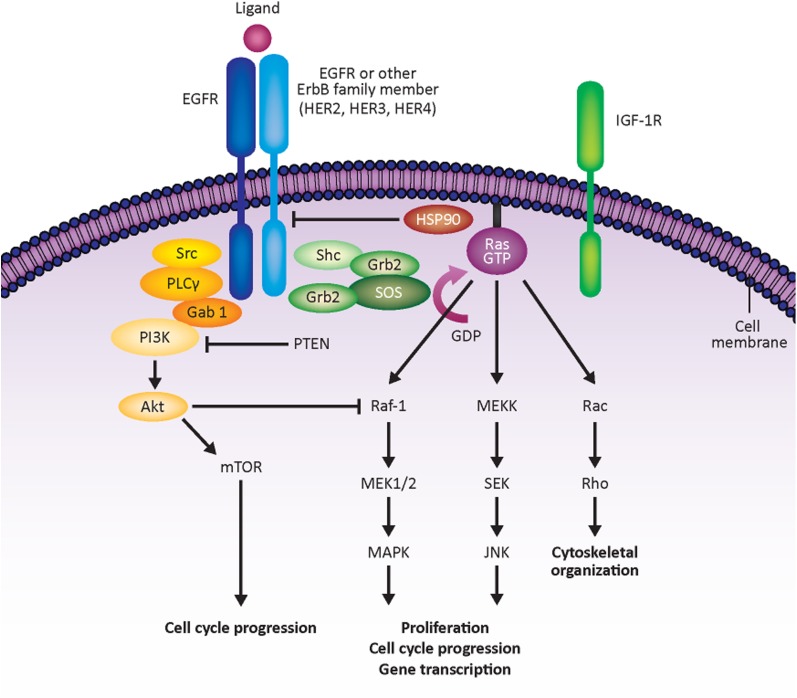
Targeting cellular signaling pathways downstream of HER family receptors is an emerging strategy for potential breast cancer therapies (Fig. 1).
Figure 1.
Downstream effectors of HER signaling. Figure adapted from Atalay et al. [77] by permission of Oxford University Press. Abbreviations: Akt, protein kinase B; EGFR, epidermal growth factor receptor; Gab 1, GRB2-associated binding protein 1; GDP, guanosine 5′-diphosphate; Grb2, growth factor receptor-bound protein 2; GTP, guanosine-5′-triphosphate; HER, human epidermal growth factor receptor; HSP90, heat shock protein 90kDa α; IGF-1R, insulin-like growth factor-1 receptor; JNK, mitogen-activated protein kinase 8; MAPK, mitogen-activated protein kinase 1; MEK1, mitogen-activated protein kinase kinase 1; MEKK, mitogen-activated protein kinase kinase kinase 1; mTOR, mechanistic target of rapamycin; PI3K, phosphatidylinositol 3-kinase; PLCγ, phospholipase C γ; PTEN, phosphatase and tensin homolog; Rac, v-akt murine thymoma viral oncogene homolog 1; Raf1, v-raf-1 murine leukemia viral oncogene homolog 1; Ras, Ras kinase family; Rho, rhodopsin; SEK, simple epithelial keratin; Shc, SHC (Src homology 2 domain containing) transforming protein 1; SOS, son of sevenless homolog; Src, sarcoma virus oncogene.
The PI3K/Akt Pathway
The PI3K/protein kinase B (Akt) pathway is associated with cellular proliferation/survival, motility, angiogenesis, inhibition of apoptosis, and metastasis [76–79]. Phosphorylated PI3K and Akt have been detected in circulating breast cancer cells from approximately 80% of patients with metastatic disease [80], and Akt is associated with overexpression of HER2 in breast tumors [81]. A biomarker analysis of the phase III CLEOPATRA study showed that patients with mutated PIK3CA had shorter PFS in both arms, suggesting that PI3K inhibitors could be combined with HER2-targeted therapy in these patients [82].
Phosphorylated PI3K and Akt have been detected in circulating breast cancer cells from approximately 80% of patients with metastatic disease, and Akt is associated with overexpression of HER2 in breast tumors. A biomarker analysis of the phase III CLEOPATRA study showed that patients with mutated PIK3CA had shorter PFS in both arms, suggesting that PI3K inhibitors could be combined with HER2-targeted therapy in these patients
GDC-0941 (Genentech), XL147 (SAR245408; Sanofi, Paris, France, http://en.sanofi.com), and BKM120 (NVP-BKM120; Novartis, Basel, Switzerland, http://www.novartis.com) are PI3K inhibitors, and BEZ235 (NVP-BEZ235; Novartis) is a dual PI3K/mammalian target of rapamycin (mTOR) inhibitor [83–85] that are currently being evaluated in phase I/II clinical trials in combination with trastuzumab with or without chemotherapy. Results from a phase Ib/II study of BKM120 plus trastuzumab in trastuzumab-resistant metastatic HER2-positive patients suggested preliminary activity [84], and the phase Ib/II PIKHER2 trial of BKM120 plus lapatinib in HER2-positive, PI3K-activated, trastuzumab-resistant disease is ongoing [86]. In MBC patients who progressed on prior trastuzumab, a phase I trial is ongoing to investigate GDC-0941 plus trastuzumab or T-DM1 (NCT00928330), and XL147 was given in combination with trastuzumab or trastuzumab/paclitaxel in another phase I/II study (NCT01042925). However, these trials do not limit patients to those with PIK3CA mutations.
In a phase I study, BEZ235 was evaluated in HER2-positive MBC patients with molecular alterations of PI3KCA or PTEN who progressed on prior trastuzumab; 1 PR and 4 stable disease were seen in 15 patients [87]. In phase I studies, the Akt inhibitor MK-2206 (Merck & Company, Inc.; Whitehouse Station, NJ, http://www.merck.com) was combined with trastuzumab and lapatinib in refractory HER2-positive breast and gastroesophageal tumors (one CR and one PR in 24 patients) [88] and with paclitaxel and trastuzumab in patients with HER2-positive solid tumors, including but not limited to breast cancer (two CRs and seven PRs in 14 patients) [89]. In the ongoing neoadjuvant I-SPY 2 phase II trial, HER2-positive patients receive trastuzumab/paclitaxel with MK-2206; other arms include neratinib or the insulin-like growth factor 1 receptor (IGF-1R) antibody, ganitumab (AMG 479; Amgen, Thousand Oaks, CA, http://www.amgen.com; NCT01042379).
The mTOR Pathway
mTOR is a key component of the PI3K/Akt pathway and is involved in the control of cell cycle progression [77]. The mTOR inhibitors temsirolimus (Torisel; Pfizer), everolimus (Afinitor; Novartis), and ridaforolimus (Merck & Company, Inc.) have been evaluated in breast cancer, and everolimus was approved by the FDA for postmenopausal women with advanced hormone receptor-positive HER2-negative breast cancer in combination with exemestane after failure of letrozole or anastrozole [90]. Pooled data from two phase I/II studies in HER2-positive MBC patients who progressed on trastuzumab reported an RR of 15% and median PFS of 4.1 months with everolimus plus trastuzumab [91]. Two phase Ib studies of MBC patients with prior trastuzumab therapy showed PFS of 30.1 and 34 weeks with everolimus in combination with trastuzumab/vinorelbine or trastuzumab/paclitaxel, respectively [92, 93]. BOLERO-1 (NCT00876395) and BOLERO-3 (NCT01007942) are phase III studies of everolimus in HER2-positive MBC (Table 4). BOLERO-3 compared the combination of trastuzumab, vinorelbine, and everolimus versus placebo in trastuzumab-resistant stage IV breast cancer, resulting in a small clinical PFS benefit of 7.00 versus 5.78 months, respectively (p = .0067) [94].
Table 4.
Completed and ongoing clinical trials of everolimus, pazopanib, and bevacizumab in HER2-positive breast cancera
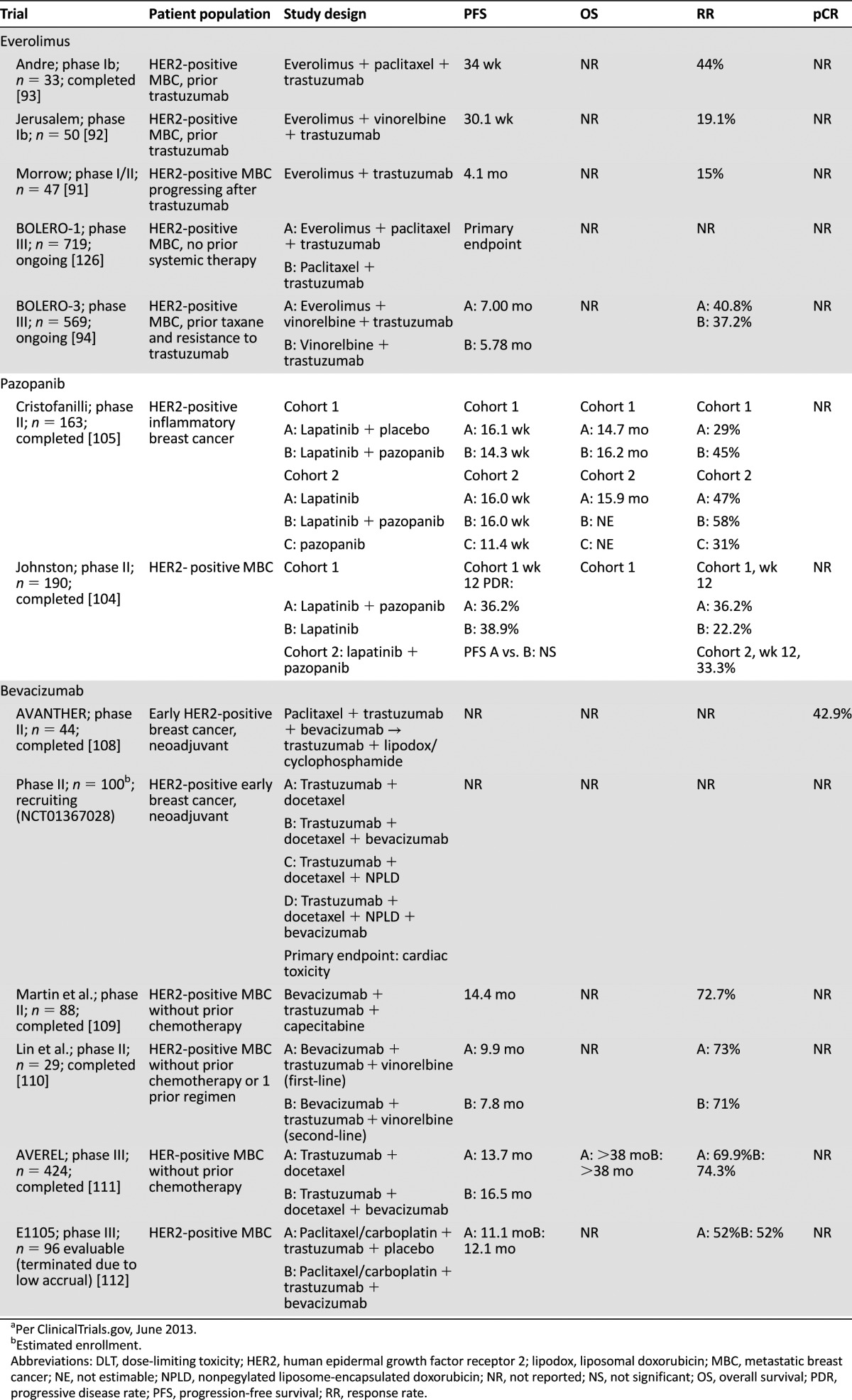
A phase II study evaluating two doses of temsirolimus in patients with heavily pretreated MBC showed an RR of only 9.2% [95]. A phase I/II study (NCT01111825) is ongoing that assesses temsirolimus and neratinib in trastuzumab-refractory HER2-amplified MBC. Ridaforolimus was combined with trastuzumab in the same patient population, with two PRs in 14 patients [96]. MLN0128 (INK128; Millennium Pharmaceuticals, Inc., Cambridge, MA, http://www.millennium.com) is another mTOR inhibitor in phase I study, with an expansion cohort with paclitaxel and weekly trastuzumab in HER2-positive patients (NCT01351350).
Other Targets
Overexpression of IGF-1R can lead to trastuzumab resistance [97], and results are forthcoming from phase II trials of the IGF-1R inhibitors BMS-754807 (Bristol-Myers Squibb, Princeton, NJ, http://www.bms.com) in combination with trastuzumab (NCT00788333) and cixutumumab (IMC-A12; Eli Lilly and Company, Indianapolis, IN, http://www.lilly.com) in combination with lapatinib/capecitabine in MBC (NCT00684983). Heat shock protein 90 (HSP90) inhibitors have also been tested in HER2-positive trastuzumab-refractory MBC. In a phase II trial of tanespimycin (Bristol-Myers Squibb) and trastuzumab, 6 of 27 patients had PR and a median PFS of 6 months [98]; however, there are no ongoing studies with tanespimycin in breast cancer. In a phase II study of AUY922 (Novartis) with trastuzumab in trastuzumab-refractory HER2-positive MBC, 5 PRs were seen in 22 patients [99].
Telomerase inhibitors have shown synergy with trastuzumab in inhibiting HER2-positive cancer cell growth and restoring trastuzumab sensitivity in trastuzumab-resistant cell lines [100]. Preliminary results with imetelstat (GRN163L; Geron Corporation, Menlo Park, CA, http://www.geron.com) and trastuzumab in women with trastuzumab-resistant breast cancer showed no significant toxicity, although no responses were seen [101].
Vascular endothelial growth factor (VEGF) inhibitors have also been combined with HER2-targeted therapy, given the possible association between HER2 and VEGF expression [102]. Pazopanib (Votrient; GlaxoSmithKline) is a multiple receptor TKI against VEGF receptor (VEGFR) 1-3, platelet-derived growth factor receptor, and stem cell factor receptor [103]. Two phase II studies of pazopanib and lapatinib in HER2-positive advanced or MBC did not show improvement in 12-week disease progression rates or PFS compared with lapatinib alone, while demonstrating increased toxicity (Table 4) [104, 105]. Sunitinib (Sutent; Pfizer) is another multiple receptor TKI of VEGFR 1-3, platelet-derived growth factor receptor, stem cell factor receptor, and colony-stimulating factor-1 receptor [106]. A single-arm phase II study of sunitinib, docetaxel, and trastuzumab in HER2-positive MBC reported 16 PRs in 22 patients [107]. In the phase II AVANTHER study of neoadjuvant bevacizumab (Avastin; Genentech), a monoclonal anti-VEGF antibody, with trastuzumab and paclitaxel, pCR was achieved in 18 of 44 HER2-positive patients [108]. Another phase II study is evaluating neoadjuvant trastuzumab and chemotherapy with or without bevacizumab (NCT01367028). In the locally recurrent and MBC setting, a single-arm phase II study combined bevacizumab with trastuzumab/capecitabine as first-line therapy, with an RR of 73% and median PFS of 14.4 months [109]. In a phase II study of bevacizumab with trastuzumab/vinorelbine in HER2-positive MBC, the RR was 73% as first-line therapy and 71% as second-line therapy; median PFS durations were 9.9 and 7.8 months, respectively [110]. The phase III AVEREL study combined trastuzumab/docetaxel with or without bevacizumab as first-line therapy. RRs were similar; improved median PFS with bevacizumab (from 13.7 to 16.5 months per investigator assessment; p = .0775) did not reach statistical significance [111]. Another phase III trial (E1105) compared first-line chemotherapy/trastuzumab with or without bevacizumab, with no significant differences in PFS or RR observed between treatments [112].
Future Directions
Defining Who May Benefit From HER2-Targeted Therapy
The Cancer Genome Atlas Network molecular analysis based on mRNA expression revealed the most frequent subtypes of human breast tumors are basal-like, luminal A and B, and HER2 enriched (HER2E) [113]. Luminal subtypes include ER-positive tumors, and the HER2E subtype has frequent HER2 amplification with a strong signal of EGFR, HER2, and phosphorylated HER2/EGFR. However, only approximately 50% of clinically HER2-positive tumors fell into this HER2E-mRNA subtype, and the rest were predominantly luminal mRNA subtypes. Several trials, including NeoALTTO, NOAH, NeoSphere, and NSABP B-41, have shown lower pCR rates with HER2-targeted therapy in patients with both ER and HER2 positivity compared with HER2-positive-only patients. It may be that some of these ER-positive/HER2-positive tumors are luminal and not of the HER2E mRNA subtype. In the neoadjuvant I-SPY 1 trial, pCR was highest in the HER2-enriched subset and lowest in the luminal A subset, and pCR was not as prognostic for ER-positive/HER2-positive tumors as for ER-negative/HER2-positive tumors [114]. There may be interaction between the ER and HER2 receptors, as inhibition of HER2 may be associated with increased activity of ER [115, 116]. Another study of stage I to III HER2-positive patients showed that hormone receptor-negative patients presented with higher stage and grade of disease were more likely to recur first in the central nervous system and had worse survival than hormone receptor-positive/HER2-positive patients [117]. Molecular analysis may help identify this subtype of HER2-positive tumors that may behave more similarly to ER-positive tumors.
Several trials, including NeoALTTO, NOAH, NeoSphere, and NSABP B-41, have shown lower pCR rates with HER2-targeted therapy in patients with both ER and HER2 positivity compared with HER2-positive-only patients. It may be that some of these ER-positive/HER2-positive tumors are luminal and not of the HER2E mRNA subtype.
In addition, except for early trastuzumab trials, which defined HER2 positivity criteria as immunohistochemistry (IHC) scores of 2+ or 3+, subsequent trials define positivity as 3+ by IHC or ≥2.0 by fluorescence in situ hybridization (FISH), or IHC 2+/FISH ≥2.0. In the NSABP B-31 trial, for example, there was no direct correlation between HER2 copy number by FISH and benefit from adjuvant trastuzumab in HER2-positive patients [118]. However, it is not known whether HER2-positive tumors that have lower activation of HER2/EGFR would be less likely to respond to HER2- and EGFR-targeted treatments than those with higher levels of activation.
There is also a small proportion of the HER2E mRNA subtype that is clinically considered triple-negative breast cancer [113]. It is not yet known whether HER2E triple-negative breast cancer benefits from HER2-targeted agents. There is also the possibility that HER2 expression level in a primary tumor may not be predictive of HER2 expression level in circulating tumor cells (CTCs). Several studies have shown HER2-positive CTCs in patients with HER2-negative tumor biopsy specimens and vice versa, with discordance rates of approximately 30% [119, 120]. In an ongoing phase III study (DEFECT III), patients with HER2-negative MBC and HER2-positive CTCs are randomized to receive standard therapy alone with or without lapatinib (NCT01619111).
Studies have also shown that patients can have different hormone receptor and HER2 status throughout tumor progression [121], although patients are not always rebiopsied at relapse. When tumor tissue from the NSABP B-31 trial was re-examined, 9.7% of women had breast cancer that should have been classified as HER2-negative [118]. The mRNA levels of HER2 in tumors that were HER2-negative were significantly lower than those in HER2-positive tumors. However, patients with these “HER2-negative/low” tumors, defined as IHC 1+ or 2+, benefited from trastuzumab. These observations prompted an ongoing phase III NSABP study (NCT01275677) randomizing patients with “HER2-low” invasive breast cancer (IHC 1+ or 2+) to receive chemotherapy alone or with trastuzumab.
Ongoing and future studies will help answer questions regarding different combinations of targeted agents in HER2-positive breast cancer, how they may be combined with specific chemotherapeutics or endocrine therapy, and the use of biomarkers to assist in determining trastuzumab resistance and treatments that may offer maximum benefit in individual patients.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
Editorial assistance was provided by Melissa Brunckhorst of MedErgy, which was contracted by Boehringer Ingelheim Pharmaceuticals for these services. Financial support for editorial assistance was provided by Boehringer Ingelheim Pharmaceuticals. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE), were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development. The authors received no compensation related to the development of the manuscript.
Footnotes
For Further Reading: Francisco J. Esteva, Sandra X. Franco, Maura K. Hagan et al. An Open-Label Safety Study of Lapatinib Plus Trastuzumab Plus Paclitaxel in First-Line HER2-Positive Metastatic Breast Cancer. The Oncologist 2013;18:661–666.
Implications for Practice: Dual targeting of the HER2 receptor using trastuzumab and lapatinib has been shown to be effective in HER2-positive metastatic breast cancer. In this study, we evaluated the safety of paclitaxel in combination with trastuzumab and lapatinib. The main side effect was diarrhea, which occurred in the majority of patients at the standard dosing of all three drugs. A pharmacokinetic interaction was found between paclitaxel and lapatinib, resulting in increased exposure of both drugs. We evaluated three dose levels of lapatinib and paclitaxel (all patients received standard trastuzumab dosing). A dose of lapatinib 750 mg/day had the lowest incidence of diarrhea in combination with paclitaxel 80 mg/m2 per week and trastuzumab 2 mg/kg per week. These doses should be used if the triplet is considered for further development in patients with HER2-positive metastatic breast cancer.
Author Contributions
Conception/Design: Zeynep Eroglu, Tomoko Tagawa, George Somlo
Provision of study material or patients: Zeynep Eroglu, Tomoko Tagawa, George Somlo
Collection and/or assembly of data: Zeynep Eroglu, Tomoko Tagawa, George Somlo
Data analysis and interpretation: Zeynep Eroglu, Tomoko Tagawa, George Somlo
Manuscript writing: Zeynep Eroglu, Tomoko Tagawa, George Somlo
Final approval of manuscript: Zeynep Eroglu, Tomoko Tagawa, George Somlo
Disclosures
George Somlo: Celgene, Genentech, Genoptix, National Comprehensive Cancer Network (C/A); Celgene, Genentech (H); National Institutes of Health, American Bioscience (Celgene) (RF). The other authors indicated no financial relationships.
Section Editors: Gabriel Hortobagyi: Antigen Express, Galena Biopharma, Novartis, Rockpointe, Pfizer (C/A); Novartis (RF); Taivex (O); founder and member of the board of directors for Citizen’s Oncology Foundation; Kathleen Pritchard: Novartis, Roche, AstraZeneca, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline, Sanofi, Ortho-Biotech, Amgen, Bristol-Myers Squibb (C/A); (H)
Reviewer “A”: Genentech (C/A); (RF)
Reviewer “B”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Flynn JF, Wong C, Wu JM. Anti-EGFR therapy: Mechanism and advances in clinical efficacy in breast cancer. J Oncol 2009;2009:526963. [DOI] [PMC free article] [PubMed]
- 2.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Sassen A, Rochon J, Wild P, et al. Cytogenetic analysis of HER1/EGFR, HER2, HER3 and HER4 in 278 breast cancer patients. Breast Cancer Res. 2008;10:R2. doi: 10.1186/bcr1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witton CJ, Reeves JR, Going JJ, et al. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 5.Rimawi MF, Shetty PB, Weiss HL, et al. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116:1234–1242. doi: 10.1002/cncr.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herceptin (trastuzumab) [package insert]. South San Francisco, CA: Genentech, Inc.; 2010.
- 8.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 11.Baselga J, Carbonell X, Castañeda-Soto NJ, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–2171. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 13.Huober J, Fasching PA, Barsoum M, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer - results of the eLEcTRA trial. Breast. 2012;21:27–33. doi: 10.1016/j.breast.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 15.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 16.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 18.Goldhirsch A, Piccart M, Procter M, et al. HERA TRIAL: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with HER2-positive early breast cancer at 8 years of median follow up [Abstract LBA6] Ann Oncol. 2012;23 (suppl 9):ixe2a. [Google Scholar]
- 19.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 20.Pivot X, Romieu G, Bonnefoi H et al. PHARE trial results comparing 6 to 12 months of trastuzumab in adjuvant early breast cancer [Abstract LBA5]. Ann Oncol 2012;23(suppl 9):ixe2a.
- 21.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 22.Buzdar A, Suman VJ, Meric-Bernstam F, et al. ACOSOG Z1041 (Alliance): Definitive analysis of randomized neoadjuvant trial comparing FEC followed by paclitaxel plus trastuzumab (FEC ? P+T) with paclitaxel plus trastuzumab followed by FEC plus trastuzumab (P+T ? FEC+T) in HER2+ operable breast cancer [Abstract 502] J Clin Oncol. 2013;31 (suppl) [Google Scholar]
- 23.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 24.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 25.Gianni L, Eiermann W, Semiglazov V, et al. Follow-up results of NOAH, a randomized phase III trial evaluating neoadjuvant chemotherapy with trastuzumab (CT+H) followed by adjuvant H versus CT alone, in patients with HER2-positive locally advanced breast cancer [Abstract 503] J Clin Oncol. 2013;31(suppl) [Google Scholar]
- 26.Tarceva (erlotinib tablets) [package insert]. South San Francisco, CA: Genentech, Inc.; 2013.
- 27.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci USA. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickler MN, Cobleigh MA, Miller KD, et al. Efficacy and safety of erlotinib in patients with locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2009;115:115–121. doi: 10.1007/s10549-008-0055-9. [DOI] [PubMed] [Google Scholar]
- 29.Britten CD, Finn RS, Bosserman LD, et al. A phase I/II trial of trastuzumab plus erlotinib in metastatic HER2-positive breast cancer: a dual ErbB targeted approach. Clin Breast Cancer. 2009;9:16–22. doi: 10.3816/CBC.2009.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iressa (gefitinib tablets) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2005.
- 31.Arteaga CL, O’Neill A, Moulder SL, et al. A phase I-II study of combined blockade of the ErbB receptor network with trastuzumab and gefitinib in patients with HER2 (ErbB2)-overexpressing metastatic breast cancer. Clin Cancer Res. 2008;14:6277–6283. doi: 10.1158/1078-0432.CCR-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somlo G, Martel CL, Lau SK, et al. A phase I/II prospective, single arm trial of gefitinib, trastuzumab, and docetaxel in patients with stage IV HER-2 positive metastatic breast cancer. Breast Cancer Res Treat. 2012;131:899–906. doi: 10.1007/s10549-011-1850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burris HA, 3rd, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 34.Ryan Q, Ibrahim A, Cohen MH, et al. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. The Oncologist. 2008;13:1114–1119. doi: 10.1634/theoncologist.2008-0816. [DOI] [PubMed] [Google Scholar]
- 35.TYKERB (lapatinib) tablets [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2012.
- 36.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 37.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 38. Gelmon KA, Boyle F, Kaufman B et al. Open-label phase III randomized controlled trial comparing taxane-based chemotherapy (Tax) with lapatinib (L) or trastuzumab (T) as first-line therapy for women with HER2+ metastatic breast cancer: Interim analysis (IA) of NCIC CTG MA.31/GSK EGF 108919 [Abstract LBA671]. J Clin Oncol 2012;30(suppl).
- 39.Untch M, Loibl S, Bischoff J, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): A randomised phase 3 trial. Lancet Oncol. 2012;13:135–144. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- 40.Goss PE, Smith IE, O’Shaughnessy J, et al. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: A randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:88–96. doi: 10.1016/S1470-2045(12)70508-9. [DOI] [PubMed] [Google Scholar]
- 41.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 42.Crown JP, Moulton B, O’Donovan N. A phase III randomized study of paclitaxel and trastuzumab versus paclitaxel, trastuzumab and lapatinib in first line treatment of HER2 positive metastatic breast cancer [Abstract OT1-1-06]. Cancer Res 2012;72(suppl 3). Paper presented at: San Antonio Breast Cancer Symposium (CTRC-AACR); December 4–8, 2012; San Antonio, TX. [Google Scholar]
- 43.Johnston S, Wroblewski S, Huang Y et al. ALTERNATIVE: Safety and efficacy of lapatinib (L), trastuzumab (T), or both in combination with an aromatase inhibitor (AI) for the treatment of hormone receptor-positive (HR+), human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer [Abstract OT1-1-04]. Cancer Res 2012;72(suppl 3). Paper presented at: San Antonio Breast Cancer Symposium (CTRC-AACR); December 4–8, 2012; San Antonio, TX. [Google Scholar]
- 44.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robidoux A, Tang G, Rastogi P, et al. Evaluation of lapatinib as a component of neoadjuvant therapy for HER2+ operable breast cancer: NSABP protocol B-41 [Abstract LBA506] J Clin Oncol. 2012;30(suppl) doi: 10.1016/S1470-2045(13)70411-X. [DOI] [PubMed] [Google Scholar]
- 46.Carey LA, Berry DA, Ollila D, et al. Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer [Abstract 500] J Clin Oncol. 2013;31(suppl) [Google Scholar]
- 47.Valachis A, Nearchou A, Lind P, et al. Lapatinib, trastuzumab or the combination added to preoperative chemotherapy for breast cancer: a meta-analysis of randomized evidence. Breast Cancer Res Treat. 2012;135:655–662. doi: 10.1007/s10549-012-2189-z. [DOI] [PubMed] [Google Scholar]
- 48.Rimawi MF, Mayer IA, Forero A, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol. 2013;31:1726–1731. doi: 10.1200/JCO.2012.44.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puma Biotechnology Inc. Puma Biotechnology announces licensing agreement with Pfizer for the development and commercialization of neratinib, an investigational PanHER inhibitor; closes $55 million private placement and completes merger. Available at http://www.pumabiotechnology.com/pr20111005.html Accessed February 16, 2012.
- 50.Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 51.Tsou HR, Overbeek-Klumpers EG, Hallett WA, et al. Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J Med Chem. 2005;48:1107–1131. doi: 10.1021/jm040159c. [DOI] [PubMed] [Google Scholar]
- 52.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 53.Awada A, Dirix L, Manso Sanchez L, et al. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol. 2013;24:109–116. doi: 10.1093/annonc/mds284. [DOI] [PubMed] [Google Scholar]
- 54.Chow LW, Xu B, Gupta S, et al. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br J Cancer. 2013;108:1985–1993. doi: 10.1038/bjc.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin M, Bonneterre J, Geyer CE, Jr. et al. A phase 2, randomized, open-label, study of neratinib (HKI-272) vs. lapatinib plus capecitabine for 2nd/3rd-line treatment of HER2+ locally advanced or metastatic breast cancer [Abstract S5-7]. Cancer Res 2011;71(suppl):113sa.
- 56.Jankowitz RC, Abraham J, Tan AR, et al. A phase I dose-escalation study evaluating weekly paclitaxel with neratinib and trastuzumab in women with metastatic HER2-positive breast cancer, NSABP FB-8 [Abstract 611] J Clin Oncol. 2012;30(suppl) [Google Scholar]
- 57.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343:342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 59.Lin NU, Winer EP, Wheatley D, et al. A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab. Breast Cancer Res Treat. 2012;133:1057–1065. doi: 10.1007/s10549-012-2003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rimawi MF, Aleixo SB, Rozas AA, et al. A neoadjuvant, randomized, open-label phase II trial of afatinib (A) versus trastuzumab (T) versus lapatinib (L) in patients (pts) with locally advanced HER2-positive breast cancer (BC) [Abstract 606] J Clin Oncol. 2012;30(suppl) doi: 10.1016/j.clbc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Hickish T, Tseng L-M, Mehta AO et al. LUX-breast 2: Phase II, open-label study of oral afatinib in HER2-overexpressing metastatic breast cancer (MBC) patients (pts) who progressed on prior trastuzumab (T) and/or lapatanib (L) [Abstract TPS651]. J Clin Oncol 2012;30(suppl).
- 62.Joensuu H, Kaci MO. LUX-breast 3: Randomized phase II study of afatinib alone or with vinorelbine versus investigator's choice of treatment in patients (pts) with HER2-positive breast cancer (BC) with progressive brain metastases (BM) after trastuzumab or lapatinib-based therapy [Abstract TPS647]. J Clin Oncol 2012;30(suppl).
- 63.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 64.PERJETA (pertuzumab) for injection, for intravenous use [package insert]. South San Francisco, CA: Genentech, Inc.; 2012.
- 65.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 68.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 69.McDonagh CF, Huhalov A, Harms BD, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11:582–593. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 70.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 71.KADCYLA (ado-trastuzumab emtansine) for injection, for intravenous use [package insert]. South San Francisco, CA: Genentech, Inc.; 2013.
- 72.LoRusso P, Krop IE, Burris HA, III, et al. Quantitative assessment of diagnostic markers and correlations with efficacy in two phase II studies of trastuzumab-DM1 (T-DM1) for patients (pts) with metastatic breast cancer (MBC) who had progressed on prior HER2-directed therapy [Abstract 1016] J Clin Oncol. 2010;28(suppl) [Google Scholar]
- 73.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–1163. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- 75. Dang CT, Gianni L, Romieu G et al. Cardiac safety in a phase II study of trastuzumab emtansine (T-DM1) following anthracycline-based chemotherapy as adjuvant or neoadjuvant therapy for early-stage HER2-positive breast cancer [Abstract 532J]. J Clin Oncol 2012;30(suppl).
- 76.Diermeier-Daucher S, Breindl S, Buchholz S, et al. Modular anti-EGFR and anti-Her2 targeting of SK-BR-3 and BT474 breast cancer cell lines in the presence of ErbB receptor-specific growth factors. Cytometry A. 2011;79:684–693. doi: 10.1002/cyto.a.21107. [DOI] [PubMed] [Google Scholar]
- 77.Atalay G, Cardoso F, Awada A, et al. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann Oncol. 2003;14:1346–1363. doi: 10.1093/annonc/mdg365. [DOI] [PubMed] [Google Scholar]
- 78.Dua R, Zhang J, Nhonthachit P, et al. EGFR over-expression and activation in high HER2, ER negative breast cancer cell line induces trastuzumab resistance. Breast Cancer Res Treat. 2010;122:685–697. doi: 10.1007/s10549-009-0592-x. [DOI] [PubMed] [Google Scholar]
- 79.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kallergi G, Agelaki S, Kalykaki A, et al. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2008;10:R80. doi: 10.1186/bcr2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tokunaga E, Kimura Y, Oki E, et al. Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. Int J Cancer. 2006;118:284–289. doi: 10.1002/ijc.21358. [DOI] [PubMed] [Google Scholar]
- 82.Baselga J, Cortes J, Im S-A et al. Biomarker analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in HER2-positive, first-line metastatic breast cancer (MBC) [Abstract S5-1]. Cancer Res 2012;72(suppl 3). Paper presented at: San Antonio Breast Cancer Symposium (CTRC-AACR); December 4–8, 2012; San Antonio, TX. [Google Scholar]
- 83.Von Hoff DD, Lo RP, Tibes R, et al. A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors [Abstract 2541] J Clin Oncol. 2010;28:7sa. [Google Scholar]
- 84.Pistilli B, Urruticoechea A, Chan S, et al. Ph IB/II study of BKM120 plus trastuzumab (T) in patients with T-resistant HER2+ advanced breast cancer (BC) [Abstract 3180] Ann Oncol. 2012;23(suppl 9):ix116a. [Google Scholar]
- 85.Brachmann SM, Hofmann I, Schnell C, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci USA. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goncalves A, Isambert N, Campone M, et al. PIKHER2: A phase Ib/II study evaluating safety and efficacy of oral BKM120 in combination with lapatinib in HER2-positive, PI3K-activated, trastuzumab-resistant advanced breast cancer [Abstract TPS663] J Clin Oncol. 2013;31(suppl) [Google Scholar]
- 87.Krop IE, Saura C, Rodon Ahnert J, et al. A phase I/IB dose-escalation study of BEZ235 in combination with trastuzumab in patients with PI3-kinase or PTEN altered HER2+ metastatic breast cancer [Abstract 508] J Clin Oncol. 2012;30(suppl) [Google Scholar]
- 88.Han HS, Swanton C, Janjigian YY, et al. A phase I study of the AKT inhibitor (MK-2206) with concurrent trastuzumab and lapatinib in patients with HER2-positive solid tumors [Abstract 3028] J Clin Oncol. 2011;29(suppl) [Google Scholar]
- 89.Chien AJ, Truong TG, Melisko ME, et al. Phase Ib dose-escalation trial of the AKT inhibitor (AKTi) MK2206 in combination with paclitaxel (P) and trastuzumab (H) in patients (pts) with HER2-overexpressing (HER2+) cancer [Abstract 2605] J Clin Oncol. 2013;31(suppl) [Google Scholar]
- 90.Afinitor (everolimus) tablets, for oral administration [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2012.
- 91.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125:447–455. doi: 10.1007/s10549-010-1260-x. [DOI] [PubMed] [Google Scholar]
- 93.Andre F, Campone M, O’Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 94.O’Regan R, Ozguroglu M, Andre F, et al. Phase III, randomized, double-blind, placebo-controlled multicenter trial of daily everolimus plus weekly trastuzumab and vinorelbine in trastuzumab-resistant, advanced breast cancer (BOLERO-3) [Abstract 505] J Clin Oncol. 2013;31(suppl) [Google Scholar]
- 95.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 96.Yardley DA, Seiler M, Ray-Coquard I, et al. Ridaforolimus (AP23573; MK-8669) in combination with trastuzumab for patients with HER2-positive trastuzumab-refractory metastatic breast cancer: a multicenter phase 2 clinical trial [Abstract 3091] Cancer Res. 2009;69(suppl) [Google Scholar]
- 97.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 98.Modi S, Stopeck AT, Linden HM, et al. HSP90 inhibition is effective in breast cancer: a phase 2 trial of tanespimycin (17aag) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab Clin Cancer Res 2011;17:5132–5139 [DOI] [PubMed] [Google Scholar]
- 99.Kong A, Rea D, Ahmed S, et al. Phase IB/II study of the HSP90 inhibitor AUY922, in combination with trastuzumab, in patients with HER2+ advanced breast cancer [Abstract 530] J Clin Oncol. 2012;30(suppl) [Google Scholar]
- 100.Goldblatt EM, Erickson PA, Gentry ER, et al. Lipid-conjugated telomerase template antagonists sensitize resistant HER2-positive breast cancer cells to trastuzumab. Breast Cancer Res Treat. 2009;118:21–32. doi: 10.1007/s10549-008-0201-4. [DOI] [PubMed] [Google Scholar]
- 101.Miller KD, Stedin CE, Prasad N et al. Inhibiting telomerase to reverse trastuzumab (T) resistance in HER2+ breast cancer [Abstract P5-18-13]. Cancer Res 2012;72(suppl 3). Paper presented at: San Antonio Breast Cancer Symposium (CTRC-AACR); December 4–8, 2012; San Antonio, TX. [Google Scholar]
- 102.Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–1716. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 103.Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 104.Johnston SR, Gómez H, Stemmer SM, et al. A randomized and open-label trial evaluating the addition of pazopanib to lapatinib as first-line therapy in patients with HER2-positive advanced breast cancer. Breast Cancer Res Treat. 2013;137:755–766. doi: 10.1007/s10549-012-2399-4. [DOI] [PubMed] [Google Scholar]
- 105.Cristofanilli M, Johnston SR, Manikhas A, et al. A randomized phase II study of lapatinib + pazopanib versus lapatinib in patients with HER2+ inflammatory breast cancer. Breast Cancer Res Treat. 2013;137:471–482. doi: 10.1007/s10549-012-2369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.SUTENT (sunitinib malate) capsules, oral [package insert]. New York, NY: Pfizer Inc.; 2011.
- 107.Cardoso F, Canon JL, Amadori D, et al. An exploratory study of sunitinib in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive metastatic breast cancer. Breast. 2012;21:716–723. doi: 10.1016/j.breast.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Fernandez M, Calvo I, Martinez N et al. Final results of neoadjuvant trial of bevacizumab (B) and trastuzumab (T) in combination with weekly paclitaxel (P) as neoadjuvant treatment in HER2-positive breast cancer: A phase II trial (AVANTHER) [Abstract P1-14-10]. Cancer Res 2012;72(suppl 3). Paper presented at: San Antonio Breast Cancer Symposium (CTRC-AACR); December 4–8, 2012; San Antonio, TX. [Google Scholar]
- 109.Martín M, Makhson A, Gligorov J, et al. Phase II study of bevacizumab in combination with trastuzumab and capecitabine as first-line treatment for HER-2-positive locally recurrent or metastatic breast cancer. The Oncologist. 2012;17:469–475. doi: 10.1634/theoncologist.2011-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin NU, Seah DS, Gelman R, et al. A phase II study of bevacizumab in combination with vinorelbine and trastuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;139:403–410. doi: 10.1007/s10549-013-2551-9. [DOI] [PubMed] [Google Scholar]
- 111.Gianni L, Romieu GH, Lichinitser M, et al. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 112.Arteaga CL, Mayer IA, O'Neill AM, et al. A randomized phase III double-blinded placebo-controlled trial of first-line chemotherapy and trastuzumab with or without bevacizumab for patients with HER2/neu-overexpressing metastatic breast cancer (HER2+ MBC): A trial of the Eastern Cooperative Oncology Group (E1105) J Clin Oncol. 2012;30:605a. [Google Scholar]
- 113.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2012;132:1049–1062. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nahta R, O’Regan RM. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat. 2012;135:39–48. doi: 10.1007/s10549-012-2067-8. [DOI] [PubMed] [Google Scholar]
- 117.Vaz Duarte Luis IM, Ottesen RA, Hughes ME, et al. Impact of hormone receptor (HR) status on clinicopathological features, patterns of recurrence, and clinical outcomes among patients (pts) with human epidermal growth factor receptor-2 positive (HER2) breast cancer (BC) in the National Comprehensive Cancer Network (NCCN) [Abstract 599] J Clin Oncol. 2012;30(suupl) doi: 10.1186/bcr3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–1411. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 119.Pestrin M, Bessi S, Galardi F, et al. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat. 2009;118:523–530. doi: 10.1007/s10549-009-0461-7. [DOI] [PubMed] [Google Scholar]
- 120.Somlo G, Lau SK, Frankel P, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128:155–163. doi: 10.1007/s10549-011-1508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 122.Lin NU, Danso MA, David AK, et al. Human epidermal growth factor receptor 2 (HER2) suppression with the addition of lapatinib to trastuzumab in HER2-positive metastatic breast cancer (HALT: LPT112515) [Abstract TPS664599] J Clin Oncol. 2013;31(suppl) [Google Scholar]
- 123.Swanton C, Cromer J. Open-label, phase II trial of afatinib, with or without vinorelbine (V), for the treatment of HER2-overexpressing inflammatory breast cancer (IBC) [Abstract TPS650] J Clin Oncol. 2012;30(suppl) [Google Scholar]
- 124.Burris HA, 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 125.Ellis PA, Barrios CH, Im Y, et al. MARIANNE: A phase III, randomized study of trastuzumab-DM1 (T-DM1) with or without pertuzumab (P) compared with trastuzumab (H) plus taxane for first-line treatment of HER2-positive, progressive, or recurrent locally advanced or metastatic breast cancer (MBC) [Abstract TPS102] J Clin Oncol. 2011;29(suppl) [Google Scholar]
- 126.Hurvitz SA, Andre F, Burris HA, et al. BOLERO-1: A randomized, phase III, double-blind, placebo-controlled multicenter trial of everolimus in combination with trastuzumab and paclitaxel as first-line therapy in women with HER2-positive (HER2+), locally advanced or metastatic breast cancer (BC) [Abstract TPS648] J Clin Oncol. 2012;30(suppl) [Google Scholar]