The authors review the history, causes, and regulatory context of oncology drug shortages in the U.S., as well as the literature, policy analyses, and other relevant sources to provide oncologists with ethical guidance when faced with oncology drug shortages. Practical recommendations are discussed.
Keywords: Oncology, Drug, Shortage, Policy, Ethics, Recommendations
Abstract
Shortages of injectable drugs affect many cancer patients and providers in the U.S. today. Scholars and policymakers have recently begun to devote increased attention to these issues, but only a few tangible resources exist to guide clinical oncologists in developing strategies for dealing with drug shortages on a recurring basis. This article discusses existing information from the scholarly literature, policy analyses, and other relevant sources and seeks to provide practical ethical guidance to the broad audience of oncology professionals who are increasingly confronted with such cases in their practice. We begin by providing a brief overview of the history, causes, and regulatory context of oncology drug shortages in the U.S., followed by a discussion of ethical frameworks that have been proposed in this setting. We conclude with practical recommendations for ethical professional behavior in these increasingly common and challenging situations.
Implications for Practice:
Shortages of injectable drugs affect many cancer patients and providers in the U.S. today. When a drug shortage occurs, oncologists face an acute moral dilemma: how to fairly manage or ration care in a situation of scarcity. Unfortunately, oncologists typically receive little or no training regarding the appropriate ethical frameworks and considerations in such circumstances. Therefore, this article seeks to provide an overview of existing information from the scholarly literature, policy analyses, and other relevant sources, in order to provide practical ethical guidance to the broad audience of oncology professionals who are increasingly confronted with such cases in their practice.
Consider the following cases:
J.W. is an 8-year-old girl diagnosed with acute lymphoblastic leukemia with intermediate risk features and an overall favorable prognosis. She receives induction therapy with vincristine, corticosteroids, and L-asparaginase and attains a complete remission. During consolidation therapy that includes systemic chemotherapy and intensive intrathecal therapy for central nervous system prophylaxis, her physicians are informed that there is a critical shortage of preservative-free methotrexate that is necessary for the administration of both the high-dose systemic methotrexate infusions and the intrathecal doses of methotrexate that are scheduled during her consolidation therapy.
A.L. is a 55-year-old woman diagnosed with stage III ovarian cancer. She receives debulking surgery and undergoes chemotherapy with paclitaxel and carboplatin. Within 4 months of her last cycle of paclitaxel and carboplatin, she develops recurrent disease. Her physicians start monthly therapy with pegylated liposomal doxorubicin. After two cycles of therapy, she has shrinkage of her disease. However, her physicians are informed that there is a critical shortage of liposomal doxorubicin and that alternative plans should be considered.
Should these patients’ physicians change regimens, lower doses, wait until the supply returns, or hold off starting chemotherapy on a different patient to give these patients access? What features of each case are relevant and appropriate to consider when weighing each of these patients’ needs against those of others? How can an oncologist best determine what behavior in these circumstances is most consistent with ethical principles?
Introduction
These cases demonstrate just two of the diverse situations in which oncology drug shortages can affect cancer patients today, which include shortages of drugs prescribed with curative intent, palliative treatment options, and essential supportive care medications. As a result of these shortages, difficult decisions have to be made, and care for cancer patients may suffer significantly. Shortages may force rationing of care or in some cases alternative drugs must be prescribed, which may be less efficacious, more toxic, and/or more expensive. For example, the recent shortage of mechlorethamine forced oncologists to substitute cyclophosphamide for treatment of pediatric Hodgkin’s lymphoma, and a retrospective comparison of these two regimens, while limited by small numbers of patients, revealed that the substitution was significantly less effective [1] and resulted in higher rates of disease recurrence.
Scholars and policymakers have recently devoted increased attention to these issues, but opinions continue to diverge about how oncology practices and providers should act when faced with these difficult circumstances at the bedside. Although this highly charged topic is becoming a familiar issue, only a few resources exist to guide strategy in dealing with drug shortages on a recurring basis [2]. This article discusses the existing scholarly work regarding these issues, as well as information from policy analyses and other sources, in order to provide practical and ethical guidance to the broad audience of oncology professionals who are increasingly confronted with such cases in their practice. In this article, we begin by providing a brief overview of the history, causes, and regulatory context of oncology drug shortages in the U.S., followed by a discussion of ethical frameworks that are available for decision making in this setting. We conclude with practical recommendations for oncologists in these increasingly common and challenging situations.
The History, Causes, and Regulatory Context of Oncology Drug Shortages in the United States
Drug shortages are not a new problem in U.S. oncology practice. Concerns about scarcity of paclitaxel date to its first demonstration of value in ovarian cancer in the late 1980s when the sole source of production was from the bark of the Pacific Yew tree [3]. Of note, there has been no shortage of taxol since it was approved because taxol is now made by semi-synthesis. Concerns about wider drug shortages in oncology are evidenced by a stakeholders’ meeting on drug shortages convened more than a decade ago by the American Medical Association and the American Society of Health-System Pharmacists (ASHP) after increasing member contacts about concerns in this area [4]. Mechanisms underlying shortages are complex, with no single cause, but were found to include material shortages, recalls, business decisions, and stockpiling, along with limitations on communication between manufacturers related to antitrust concerns.
Although drug shortage problems have existed for many years, as Michael Link, former president of the American Society of Clinical Oncology, succinctly noted, “We have had shortages before, but they have been intermittent, and never anything as extensive both in terms of the breadth of drugs affected and the depths of shortages and how long they lasted” [5]. Current data regarding drug shortages can be found on the website http://www.fda.gov/Drugs/drugsafety/DrugShortages/default.htm. Indeed, the Food and Drug Administration (FDA) reported 178 drug shortages in 2010, with 74% of shortages involving sterile injectable drugs, including chemotherapy [6]. In 2011, there were 251 drug shortages reported to the FDA, 183 involving sterile injectable drugs [6]. Of note, the FDA’s definition of drug shortage considers only medically necessary drugs for which there is no other source or alternative. The broader definition proposed by the ASHP includes all instances in which “a supply issue affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent.” Thus, the ASHP definition leads to the identification of even more shortages [7, 8]. A recent ASHP study found that nearly all hospitals surveyed had experienced at least one drug shortage in 2010, and most had experienced many shortages [9]. A more recent ASHP survey found that oncology drug shortages led to delays in chemotherapy, complicated the conduct of clinical research, increased the risks of medication errors, and were perceived to have had numerous other concerning downstream effects [10].
Fundamentally, the economic underpinnings of shortages in the market for medically necessary prescription drugs relate to a phenomenon economists dub the “price inelasticity” of both demand and supply [11]. In brief, demand is relatively unresponsive to changes in price because medically necessary drugs have few substitutes and because drug costs are typically covered by insurance. Supply is slow to respond to price, given the complexity of the drug production process and regulatory environment, which limit the ability for industry to increase capacity quickly. Analysts observe that there is little incentive for suppliers to carry reserve inventory, given limited shelf life and because oversupplied drugs cannot be marketed even at a very low price if there is insufficient demand [4]. Moreover, some have argued that price itself is fixed to some extent by Medicare payment policies that limit reimbursement for injectable generics to 6% over the average sales price and calculate the average sales price in a way that leads to a two-quarter lag in price updating and, therefore, a reduced price responsiveness to changed demand [12, 13]. For these reasons, shortages in oncology drugs may be more easily precipitated by unexpected or rapid changes in supply or demand compared with other commercial goods.
Demand is relatively unresponsive to changes in price because medically necessary drugs have few substitutes and because drug costs are typically covered by insurance. Supply is slow to respond to price, given the complexity of the drug production process and regulatory environment, which limit the ability for industry to increase capacity quickly.
The market for injectable oncology drugs is particularly complex. On the supply side, generic drugs currently constitute at least half the market, and that proportion is growing and will continue to grow as more drugs emerge from patent protections [12]. Individual generic oncology drugs are usually supplied by three or fewer manufacturers, and these manufacturers have limited facilities and lines on which cytotoxic drugs may be produced, limiting the ability to substitute production in the situation of a shortage [12]. A recurrent problem exists with regard to manufacturing capacity, caused by aging facilities, lack of redundancy of facilities, and ultimately the low-profit margin, which makes it impractical to devote industry resources to the construction of new facilities. In contrast, Europe, which lacks the rigid price control system on generics, has had no serious shortage problem. The Department of Health and Human Services reported in 2006–2010 an increase in both the overall quantity and range of drugs produced by generic oncology drug manufacturers (partly related to the timing of patent expirations) in the face of stable manufacturing capacity [6]. Given the increase in capacity utilization observed during that time period, it is not surprising to find shortages in drugs for which volume and prices had been declining, as manufacturers strategically diverted their resources toward growing sources of revenue. Regulatory barriers to entry of new suppliers further complicate the situation. Of note, shortages may vary from one hospital system to the next, depending on their ability to stockpile drug and to find black market sources.
Recently, legislators and government administrators have taken a number of steps to respond to these issues. In October 2011, along with the release of the Department of Health and Human Services analysis, President Obama issued an Executive Order that broadened reporting requirements regarding manufacturing discontinuances, ordered the FDA to expedite certain regulatory reviews, and required communication with the Department of Justice about collusion or price gouging related to shortages [14]. In February 2012, to address the shortage of liposomal doxorubicin, the FDA approved temporary importation of an FDA-unapproved replacement brand of the drug from foreign sources [15], recently approved a generic doxorubicin under expedited review [16], and also worked to maintain supplies of preservative-free methotrexate. In July 2012, President Obama signed bipartisan legislation that included provisions requiring manufacturers to notify the FDA 6 months before an anticipated shortage and instituting a generic drug user fee to provide resources for expedited review of generic drug applications [17].
Nevertheless, changes in the political and regulatory context have not successfully addressed the root causes of drug shortages, meaning providers will continue to be faced with shortages of critical chemotherapies and essential supportive care medicines. Therefore, ethical considerations regarding allocation of scarce resources—and the need for practical recommendations for dealing with oncology drug shortages—remain salient issues for clinical oncologists.
Ethical Frameworks
Ultimately, when an oncology drug shortage occurs and supply cannot be quickly increased to meet demand, oncologists face an acute moral dilemma: how to fairly manage or ration care in a situation of scarcity. Unfortunately, oncologists in the U.S. typically receive little or no training regarding the appropriate ethical frameworks and considerations in such circumstances. Moreover, because the U.S. health care system has generally relied on implicit mechanisms of resource allocation, based on insurance status or ability to pay, oncologists typically have had little direct experience with explicit prioritization. Therefore, oncologists must develop an understanding of the ethical frameworks that may help guide behavior in such circumstances.
Scholars have drawn from centuries of relevant philosophical deliberation to propose general frameworks for rationing health care interventions [18–21]. Of note, important lessons may be gleaned from experiences with explicit prioritization and rationing that have occurred in certain specific situations in the U.S., including allocation of organ transplants, hemodialysis, and proton therapy [22–26]. Just as in the case of drug shortages, these situations have been ones in which Americans have had to confront the uncomfortable reality that it is not possible to provide a health care intervention to all who might benefit—situations that have been described by Calabresi and Bobbitt as “tragic choices” [27].
In general, utilitarian frameworks for rationing prioritize on the basis of a principle of maximizing welfare at the societal level, often relying on cost-effectiveness analysis for guidance. However, such approaches conflict with the respect for individual human dignity that permeates the American political culture and consciousness. Therefore, scholars have sought alternative frameworks and have been particularly careful to avoid comparisons of the worthiness of different individuals to receive scarce health care resources. Moreover, a utilitarian approach may have appeal at the level of setting public health policy, but it is difficult to apply at the level of the individual clinical encounter in which oncologists are expected to have a professional fiduciary responsibility for each patient. How doctors can fulfill their dual obligations to individual patients and society is problematic in a number of settings [28, 29]. This well-described dilemma is magnified when an oncologist is forced to choose between the needs of an individual patient in clinic versus others in their practice or community in cases of oncology drug shortages. Importantly, these decisions should not be made on a case-by-case basis and not in isolation.
In general, utilitarian frameworks for rationing prioritize on the basis of a principle of maximizing welfare at the societal level, often relying on cost-effectiveness analysis for guidance. However, such approaches conflict with the respect for individual human dignity that permeates the American political culture and consciousness.
Recently, specific frameworks have been proposed for approaching situations of unanticipated shortages of medically necessary drugs such as those described in the introductory cases. Valgus and colleagues [2] endorse the “accountability for reasonableness” framework, initially described by Daniels and Sabin [30], for application to the context of oncology drug shortages specifically. Rosoff has provided more detailed specification and adaptation of this framework for guiding the equitable short-term rationing of drugs in short supply [31, 32]. This framework emphasizes a transparent process, consistently enforced, and relevant rules based on objective criteria; a mechanism for individual appeal and for revision of the allocation system over time; and consideration of fairness, particularly noting the importance of preventing powerful individuals or patients of influential providers from receiving preferential treatment. Rosoff focuses on large hospitals and envisions formation of a standing drug shortage allocation committee that includes a patient representative, along with other relevant hospital stakeholders. Based on these underlying principles, he proposes several specific steps for adoption when a drug shortage arises. The first involves using substitute therapies of comparable equivalence whenever possible. The second involves setting priorities for remaining cases in which the drug might be used, with the foremost criterion for allocation being the expected clinical benefit. Rosoff generally favors continuation of therapy for a patient on a drug over allocation to a patient newly starting a drug and advocates consideration of a shorter duration of therapy or lower doses to conserve the drug whenever this is clinically reasonable. In the setting of oncology treatment, however, this approach is unlikely to prove clinically reasonable very often, particularly for curative regimens, and is unappealing unless clinical trials have demonstrated equivalent activity with shorter courses or lower doses. Of note, Rosoff specifically articulates certain criteria that should not be considered in the allocation process: ability to pay, ethnicity or citizenship, age (unless directly related to the specific drug’s efficacy), or any notion of social worth.
Several scholars have provided comments critiquing or building on Rosoff’s proposal. Burda notes that for the allocation process to be truly fair, attention is necessary to decrease the influence of biases and conflicts of interest on the part of allocation committee members [33]. This includes recusal of committee members who have either current or past personal or professional relationships with patients being evaluated for receipt of a scarce drug, as well as removal of identifying information (including characteristics that ought not be considered in the allocation process, such as sex, race, age, marital and parental status, and other sociodemographic characteristics). She also emphasizes the importance of managing the emotional and psychological impact of these classic “tragic choices” on caregivers and allocation committee members, as well as patients. Goodman also emphasizes the need to consider the management of distress among providers confronted with this difficult situation [34]. Bamford and colleagues argue that clinical rather than cost effectiveness be clearly identified as the criterion on which allocation decisions should be based [35]. They also suggest greater clarification of the roles of need and efficacy in guiding allocation decisions. Finally, they note that greater clarification of the appropriate role of physicians in the allocation process is necessary.
Several other groups have articulated relatively similar frameworks for allocation of resources in other more specific circumstances of drug shortages [36, 37]. In the setting of a potential widespread shortage of injectable generics in Canada, a group of Ontario ethicists endorsed similar fair process principles, while highlighting six overarching ethical principles: beneficence (doing good), solidarity, utility, equity, stewardship, and trust [38]. They further specified those principles with a three-stage approach suitable for their cultural and regulatory environment. The first stage involves strategies to preserve the standard of care such as minimizing waste, substituting drugs of equivalent efficacy, and pursuing alternative sources. The second and third stages involve the application of primary allocation principles to optimize therapeutic benefit and secondary allocation principles that ensure fair access.
Peppercorn and colleagues provide more concrete guidance that is of specific relevance to oncologists in these circumstances. They discuss in detail one institution’s approach to rationing a promising novel oncology therapy in scarce supply [39]. After considering the classic principles of respect for patient autonomy, beneficence (and its corollary of nonmalfeasance), and justice, these scholars conclude that “objective medical criteria should guide our attempts to meet these sometimes competing ethical obligations.” They separate these medical criteria into two components: medical need (the risk of harm in the absence of the intervention—which depends on both prognosis and availability of alternative therapies) and chance of benefit (which incorporates evidence on the safety and efficacy of the intervention itself, the patient’s life expectancy from both the disease and any comorbid conditions, and the patient’s likelihood of receiving an adequate course of treatment, which in turn depends on both comorbidities and adherence). Of note, this group reported that while they chose to avoid consideration of social criteria, such as prior trial participation, they did consider likelihood of treatment compliance, including concerns about ability to keep appointments or other logistical concerns, which may in turn reflect disability or lack of social privilege in a way that may not be ideal. Beyond establishment of medical criteria, Peppercorn and colleagues recommended allocation on a first-come/first-served basis as most ethically appropriate, without subsequent consideration of other sociodemographic factors. Overall, the system articulated by this group provides clear guidelines that are specifically applicable to oncology shortages. However, just as with Rosoff's analysis, the generalizability of this approach may be limited in settings that are substantially different from the large academic medical center in which it was developed.
Of note, the frameworks discussed share many commonalities, including an emphasis on certain key principles, including beneficence and equity, as well as a recognition of the importance of establishing a fair, accountable, and transparent process. However, certain contrasts merit note. Specifically, as noted above, in the circumstances of the drug shortage of sipuleucel-T, Peppercorn and colleagues developed waiting lists with priority based on a first-come/first-served approach; they recognized the potential limitations of this approach and required that physicians act to minimize potential differences in patient awareness or ability to access the system [39]. Given concerns that such an approach might nevertheless favor more well-informed, privileged members of society, Rosoff and colleagues have instead proposed a coin toss for a decision between two clinically equivalent patients [32]. Gibson notes that either of these approaches constitutes reasonable secondary allocation principles that can be developed and sanctioned by affected stakeholders [38]. Ultimately, despite these differences regarding the potential operationalization of secondary allocation principles to follow consideration of clinical benefit, all of these scholars agree that the process should take care not to disadvantage those from underprivileged groups. `
Conclusion And Recommendations for the Clinical Oncologist
Drug shortages like those presented in the introductory cases are likely to recur and pose difficult challenges for the practicing oncologist. Shortages of drugs for which alternatives may be substituted can be addressed in a relatively straightforward fashion. Unfortunately, although existing regulations may provide greater advance notice and opportunity for planning, shortages of oncology drugs are likely to continue to occur and to persist for unpredictable durations. Existing guidelines focus primarily on the role of pharmacists [8] and fail to provide clear guidance for practical decision making for the practicing oncologist. Therefore, after careful consideration of the existing literature on oncology drug shortages, we make several recommendations to the practicing oncologist facing a drug shortage, as summarized in Table 1.
Table 1.
Practical Recommendations for Preventing and Managing Oncology Drug Shortages
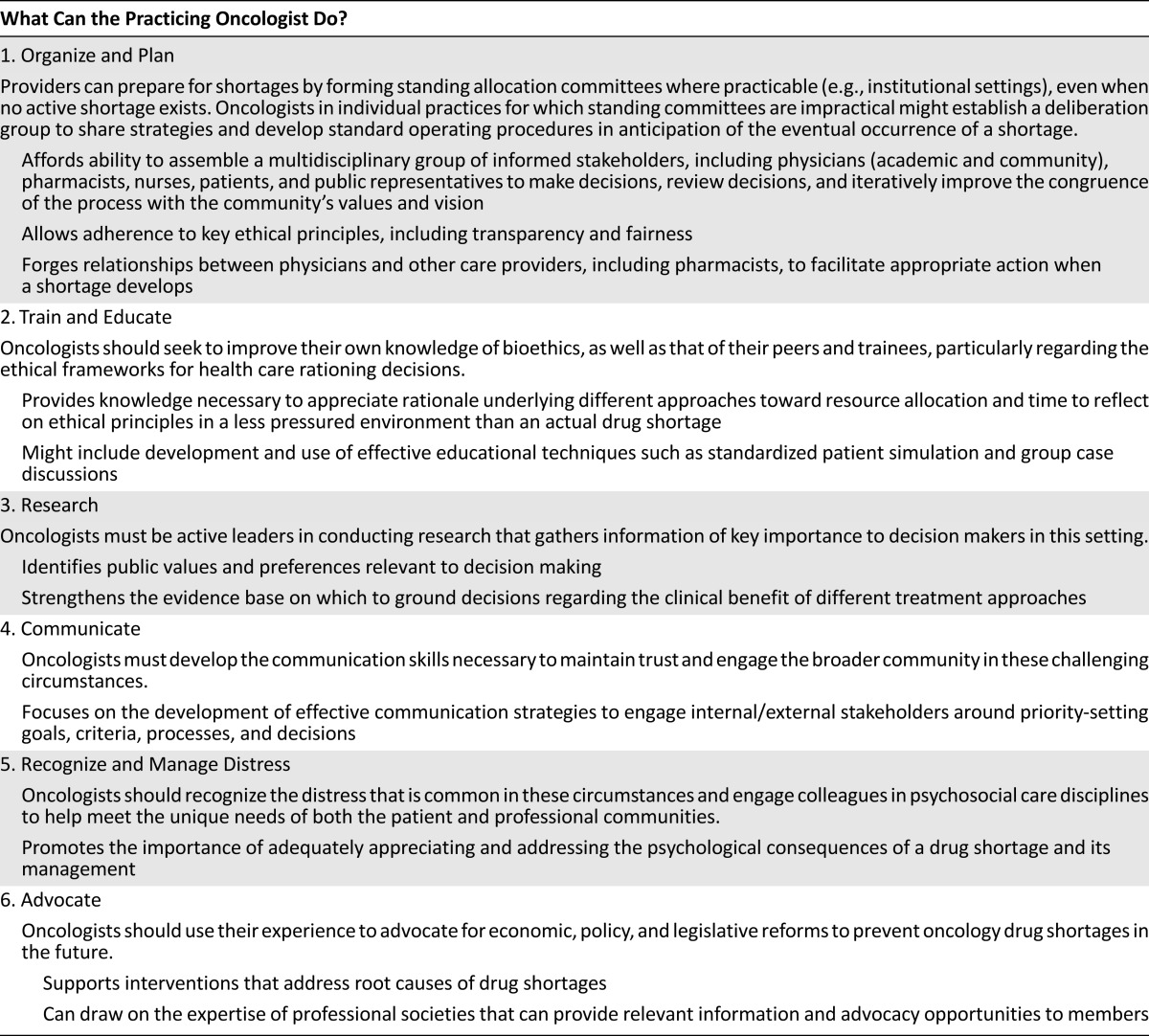
First, because some drug shortages may occur without adequate warning even if new reporting mechanisms are successful, it is important for oncologists in institutional settings to participate in the development of standing institutional allocation committees, even when no active shortage exists. Where an ethics committee or other similar structure already exists within an institution, a subcommittee or working group of members from these existing committees may be arranged to develop procedures and to be called on should the need arise. Such committees are an excellent mechanism by which to ensure legitimacy of the allocation process and adherence to key ethical principles, including transparency and fairness. Ideally, as consistently supported by the scholarly literature reviewed above, such committees would include diverse representatives of the medical and allied professions (including bioethicists where available), administrators, and the patient population and public whom they serve. By creating an infrastructure and process for allocation decisions in advance of any particular shortage situation, oncologists can avoid the ethically challenging situation of bedside rationing that they otherwise may confront during a shortage. Committees might consider criteria such as clinical benefit as described above to avoid first-come/first-served and informal rationing by individual providers.
Of note, differences in practice environments may lead to substantial differences in what resources and needs exist, influencing the approach to decision making in different settings. Physicians practicing outside the context of the large-hospital scenario given detailed attention by bioethicists to date must consider how best to develop a participatory model of allocation within their own environments. Specifically, smaller providers are encouraged to communicate and share strategies for incorporating ethical deliberation in anticipation of the eventual occurrence of a shortage. Instead of a formal committee, providers might establish a deliberation group, which may or may not include all of the participants than an analogous institutional committee would. The prior development of standard operating procedures for these foreseeable circumstances may be beneficial for addressing the potential communication and other challenges to arriving at and implementing ethically sound decisions.
Second, oncologists should educate themselves and their colleagues in the aspects of bioethics likely to be relevant to their practice, including the ethical frameworks that have been developed to guide health care rationing decisions. In particular, consideration should be given to understanding the arguments, both theoretically based and empirically derived from studies of public opinion, that generally weigh against consideration of certain personal characteristics in allocation decisions. In the setting of a shortage, it may become difficult for providers to avoid emotional or inappropriate considerations unless they have previously received training to appreciate the rationale against doing so.
Third, oncologists should contribute to research to better understand public values and preferences regarding allocation deliberation and outcomes, which may help to guide the specific considerations and procedures adopted by allocation committees. Also important is continued pursuit of more traditional forms of clinical research and comparative effectiveness research to better define therapeutic benefits of specific drugs in a variety of clinical circumstances.
Fourth, oncologists should collaborate with experts in communication to identify the best approaches for maintaining trust and engaging internal/external stakeholders around priority-setting goals, criteria, processes, and decisions. Oncologists must develop the skills to communicate effectively in these difficult circumstances. Programming should be tailored to meet the needs of practicing oncologists in a variety of settings, from those in private practice to those in large institutions.
Fifth, allocation decisions are classic “tragic choices” that may result in substantial psychological distress not only for patients but also for providers and those charged with allocation decisions. Oncologists should recognize and seek help in managing this distress, including harnessing supportive care services for both patients and providers. These preparations may help providers manage the complex emotional issues that are likely to arise in the inevitable instance that there is not enough of a drug for all of their patients who might benefit.
Finally, beyond clinicians’ duties to individual patients, the practicing oncologist has a duty to advocate for systemic changes that would effectively address the root causes of drug shortages. Some believe that fundamental changes to the regulatory environment and to the drug reimbursement system are necessary. For example, it may be prudent to establish a pricing floor for generic chemotherapy drugs [40]. Intervention may also be appropriate to decrease the regulatory burden on potential new suppliers, while ensuring that patient safety is not affected. Finally, the FDA must receive adequate support to clear its backlog of unapproved generic applications and decrease the timeline to approval of generic drugs, as it recently did in approving generic doxorubicin, in order to allow suppliers to enter the market when shortage is anticipated [40]. Such policy changes are critically necessary to decrease the number of situations in which the difficult processes of resource allocation discussed above must be applied.
Author Contributions
Conception/Design: Reshma Jagsi, Beverly Moy
Collection and/or assembly of data: Reshma Jagsi
Data analysis and interpretation: Reshma Jagsi, Beverly Moy
Manuscript writing: Reshma Jagsi, Beverly Moy
Final approval of manuscript: Reshma Jagsi, Rebecca Spence, W. Kimryn Rathmell, Angela Bradbury, Jeffrey Peppercorn, Stephen Grubbs, Beverly Moy
Disclosures
Jeffrey Peppercorn: GlaxoSmithKline (E [spouse]); Genentech (C/A), Novartis (RF); Ownership interest: GlaxoSmithKline (OI [spouse]); W. Kimryn Rathmell: GlaxoSmithKline, Siemens, Seattle Genetics, AVEO (RF); Reshma Jagsi: AbbVie (RF [support]); Eviti (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Metzger ML, Billett A, Link MP. The impact of drug shortages on children with cancer—The example of mechlorethamine. N Engl J Med. 2012;367:2461–2463. doi: 10.1056/NEJMp1212468. [DOI] [PubMed] [Google Scholar]
- 2.Valgus J, Singer EA, Berry SR, et al. Ethical challenges: Managing oncology drug shortages. J Oncol Pract. 2013;9:e21–e23. doi: 10.1200/JOP.2012.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blume E. Investigators seek to increase taxol supply. J Natl Cancer Inst. 1989;81:1122–1123. doi: 10.1093/jnci/81.15.1122. [DOI] [PubMed] [Google Scholar]
- 4.Landis N. Provisional observations on drug product shortages: Effects, causes, and potential solutions. Am J Health Syst Pharm. 2002;59:2173–2182. doi: 10.1093/ajhp/59.22.2173. Available at http://www.ashp.org/s_ashp/docs/files/DShort_11b-SF-Witmer.pdf. [DOI] [PubMed] [Google Scholar]
- 5.Park A. Inside America’s drug shortage. Time, March 2012. Available at http://healthland.time.com/2012/03/19/where-have-all-our-drugs-gone/.
- 6.U.S. Food and Drug Administration, Center for Drugs and Evaluation Research Drug Shortage Program. Frequently asked questions about drug shortages. Available at http://www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050796.htm#q1.
- 7.Fox ER, Birt A, James KB, et al. ASHP Expert Panel on Drug Product Shortages ASHP guidelines on managing drug product shortages in hospitals and health systems. Am J Health Syst Pharm. 2009;66:1399–1406. doi: 10.2146/ajhp090026. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman S. The drugs stop here: A public health framework to address the drug shortage crisis. Food Drug Law J. 2012;67:1–22. [PubMed] [Google Scholar]
- 9.Kaakeh R, Sweet BV, Reilly C, et al. Impact of drug shortages on U.S. health systems. Am J Health Syst Pharm. 2011;68:1811–1819. doi: 10.2146/ajhp110210. [DOI] [PubMed] [Google Scholar]
- 10.McBride A, Holle LM, Westendorf C, et al. National survey on the effect of oncology drug shortages on cancer care. Am J Health Syst Pharm. 2013;70:609–617. doi: 10.2146/ajhp120563. [DOI] [PubMed] [Google Scholar]
- 11.Haninger K, Jessup A, Koehler K. Economic analysis of the causes of drug shortages (ASPE issue brief). Washington, DC: United States Department of Health and Human Services, 2011. Available at http://www.aspe.hhs.gov/sp/reports/2011/drugshortages/ib.shtml.
- 12.Chabner BA. Drug shortages—a critical challenge for the generic-drug market. N Engl J Med. 2011;365:2147–2149. doi: 10.1056/NEJMp1112633. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson M, Alpert A, Duarte F. Prescription drug shortages: Reconsidering the role of Medicare payment policies. Available at http://healthaffairs.org/blog/2012/05/29/prescription-drug-shortages-reconsidering-the-role-of-medicare-payment-policies.
- 14.Office of the Press Secretary. We can’t wait: Obama Administration takes action to reduce prescription drug shortages, fight price gouging [press release]. Washington, DC: White House, 2011. Available at http://www.whitehouse.gov/the-press-office/2011/10/31/we-can-t-wait-obama-administration-takes-action-reduce-prescription-drug.
- 15.U.S. Food and Drug Administration. FDA acts to bolster supply of critically needed cancer drugs [press release], 02/2012 update. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm292658.htm
- 16.U.S. Food and Drug Administration. FDA approval of generic version of cancer drug Doxil is expected to help resolve shortage. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm337872.htm.
- 17.U.S. Food and Drug Administration. The Food and Drug Administration Safety and Innovation Act (FDASIA), S. 3187, 2012 . Available at http://www.gpo.gov/fdsys/pkg/BILLS-112s3187pcs/pdf/BILLS-112s3187pcs.pdf.
- 18.Callahan D. Rationing: theory, politics, and passions. Hastings Cent Rep. 2011;41:23–27. doi: 10.1353/hcr.2011.0031. [DOI] [PubMed] [Google Scholar]
- 19.Aaron HJ, Schwartz WB. The Painful Prescription: Rationing Hospital Care. Washington, DC: Brookings Institution; 1984. [Google Scholar]
- 20.Aaron HJ, Schwartz WB. Can We Say NO? The Challenge of Rationing Health Care. Washington, DC: Brookings Institution; 2005. [Google Scholar]
- 21.Ubel PA. Pricing Life: Why It’s Time for Health Care Rationing. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 22.Boulis A, Goold S, Ubel PA. Responding to the immunoglobulin shortage: A case study. J Health Polit Policy Law. 2002;27:977–999. doi: 10.1215/03616878-27-6-977. [DOI] [PubMed] [Google Scholar]
- 23.Health Resources and Services Administration. Organ distribution: Allocation of deceased kidneys, 9/12 update. Available at http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_7.pdf.
- 24.Scribner BH. Lasker Clinical Medicine Research Award. Medical dilemmas: The old is new. Nat Med. 2002;8:1066–1067. doi: 10.1038/nm772. [DOI] [PubMed] [Google Scholar]
- 25. Strosberg M, Wiener J, Baker R et al., eds. Rationing America's Medical Care: The Oregon Plan and Beyond. Washington, DC: Brookings Institution, 1992. [Google Scholar]
- 26.Jagsi R, DeLaney TF, Donelan K, et al. Real-time rationing of scarce resources: The Northeast Proton Therapy Center experience. J Clin Oncol. 2004;22:2246–2250. doi: 10.1200/JCO.2004.10.083. [DOI] [PubMed] [Google Scholar]
- 27.Calabresi G, Bobbitt P. Tragic Choices. New York, NY: W.W. Norton; 1978. [Google Scholar]
- 28.Bloche MG. Clinical loyalties and the social purposes of medicine. JAMA. 1999;281:268–274. doi: 10.1001/jama.281.3.268. [DOI] [PubMed] [Google Scholar]
- 29.Ubel PA, Arnold RM. The unbearable rightness of bedside rationing. Physician duties in a climate of cost containment. Arch Intern Med. 1995;155:1837–1842. [PubMed] [Google Scholar]
- 30.Daniels N, Sabin J. The ethics of accountability in managed care reform. Health Aff (Millwood) 1998;17:50–64. doi: 10.1377/hlthaff.17.5.50. [DOI] [PubMed] [Google Scholar]
- 31.Rosoff PM. Unpredictable drug shortages: An ethical framework for short-term rationing in hospitals. Am J Bioeth. 2012;12:1–9. doi: 10.1080/15265161.2011.634483. [DOI] [PubMed] [Google Scholar]
- 32.Rosoff PM, Patel KR, Scates A, et al. Coping with critical drug shortages: An ethical approach for allocating scarce resources in hospitals. Arch Intern Med. 2012;172:1494–1499. doi: 10.1001/archinternmed.2012.4367. [DOI] [PubMed] [Google Scholar]
- 33.Burda ML. Beyond the framework. Am J Bioeth. 2012;12:11–13. doi: 10.1080/15265161.2011.634957. [DOI] [PubMed] [Google Scholar]
- 34.Goodman A. The tensions and challenges of unpredictable drug shortages. Am J Bioeth. 2012;12:20–22. doi: 10.1080/15265161.2012.634667. [DOI] [PubMed] [Google Scholar]
- 35.Bamford R, Brewer CD, Bucknell B, et al. A paradoxical ethical framework for unpredictable drug shortages. Am J Bioeth. 2012;12:16–18. doi: 10.1080/15265161.2011.634958. [DOI] [PubMed] [Google Scholar]
- 36.Caro JJ, Coleman CN, Knebel A, et al. Unaltered ethical standards for individual physicians in the face of drastically reduced resources resulting from an improvised nuclear device event. J Clin Ethics. 2011;22:33–41. [PubMed] [Google Scholar]
- 37.Thompson AK, Faith K, Gibson JL, et al. Pandemic influenza preparedness: An ethical framework to guide decision-making. BMC Med Ethics. 2006;7:E12. doi: 10.1186/1472-6939-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson JL, Bean S, Chidwick P, et al. Ethical framework for resource allocation during a drug supply shortage. Healthc Q. 2012;15:26–35. doi: 10.12927/hcq.2013.23040. [DOI] [PubMed] [Google Scholar]
- 39.Peppercorn J, Armstrong A, Zaas DW, et al. Rationing in urologic oncology: Lessons from sipuleucel-T for advanced prostate cancer. Urol Oncol. 2013; 31:1079–1084. doi: 10.1016/j.urolonc.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Link MP, Hagerty K, Kantarjian HM. Chemotherapy drug shortages in the United States: Genesis and potential solutions. J Clin Oncol. 2012;30:692–694. doi: 10.1200/JCO.2011.41.0936. [DOI] [PubMed] [Google Scholar]


