Abstract
Background.
The efficacy and toxicity of paclitaxel plus capecitabine (PX) as first-line treatment in advanced gastric cancer (AGC) was evaluated.
Methods.
Patients with previously untreated AGC were included. PX was given every 3 weeks until a maximum of six cycles or progression. Capecitabine monotherapy was continued for patients without disease progression. The primary endpoint was progression-free survival, and secondary endpoints were objective response rate, overall survival (OS), and safety.
Results.
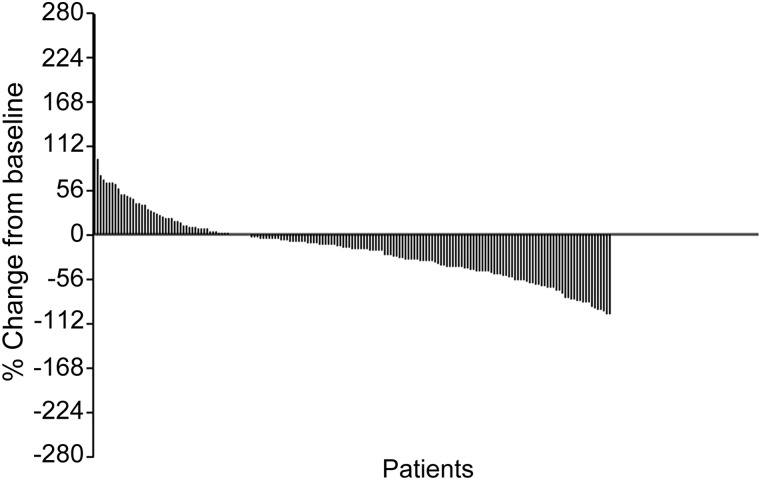
Overall, 194 patients were treated per protocol and one patient was excluded because of allergy to paclitaxel. Response was evaluated in 175 patients, with an objective response rate of 34.8%. After a median follow-up of 33.2 months, disease progression was observed in 141 patients, 137 died, and 16 were lost to follow-up, with progression-free survival of 188 days and OS of 354 days. In multivariate Cox regression analysis, no factor remained an independent predictor of OS. Forty-five patients who received capecitabine monotherapy after PX had longer OS (531 days). Adverse events were mild (Fig. 1), and the most common grade 3–4 toxicities were leucopenia and neutropenia.
Conclusion.
PX as a first-line treatment has promising efficacy in AGC. Based on these data, a phase III study has been launched for further investigation.
Author Summary
Discussion
Chemotherapy can improve survival and quality of life for patients with AGC. The V325 trial established that docetaxel plus 5-fluorouracil and cisplatin would benefit AGC patients; however, a high rate of hematologic toxicity limits the use of the combination regimen. Paclitaxel has good tolerability and efficacy similar to docetaxel. In addition, paclitaxel has synergistic effects with capecitabine. After approval by the ethics committee, we initiated this multicenter, phase II, prospective trial to evaluate the efficacy and toxicity of PX as first-line treatment in AGC.
Overall, 195 patients were enrolled at 18 centers; 175 patients were evaluable for clinical response. The response rate was 34.8%, and the median overall survival was nearly 1 year. These efficacy data were similar to docetaxel plus 5-fluorouracil and cisplatin in the V325 trial. Adverse events were well tolerated. The occurrence of grade 3–4 neutropenia decreased to less than 10%. The most common nonhematologic toxicity was alopecia. Although paclitaxel-related neurotoxicity was observed, most cases were mild and reversible under the weekly schedule. No patients discontinued protocol treatment because of paclitaxel-related neuropathy.
Figure 1.
Waterfall plot of overall response measured by RECIST criteria 1.0.
Abbreviation: RECIST, Response Evaluation Criteria in Solid Tumors.
In vitro models showed that taxanes can upregulate thymidine phosphorylase activity within 4 days with the maximal effect at about 6–8 days after the treatment. Consequently, a weekly schedule of paclitaxel could provide a synergistic effect in combination with capecitabine. Our results demonstrated that the toxicity of this protocol is generally well tolerated. In our study, there was no modification per protocol for elderly patients, and no severe adverse events occurred during the treatment.
The consensus about the number of palliative chemotherapy cycles that should be performed has not yet been reached. Nevertheless, in clinical practice, tolerability often precludes continuing combination chemotherapy until progression. Furthermore, maintenance chemotherapy has shown benefit in colorectal cancer and lung cancer. We explored maintenance therapy in patients with gastric cancer. In this trial, a subset of 45 patients who continued with the capecitabine monotherapy without disease progression after combination therapy seemed to have obtained longer survival benefit (531 days). Hand-foot syndrome was the main toxicity that restricted the use of capecitabine.
In summary, we can conclude that the efficacy and tolerability of PX observed in our study deserves further investigation in a phase III trial (ClinicalTrials.gov identifier NCT01015339).
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81172110), National High Technology Research and Development Program (No. 2006AA 02A 402-B02, 2012AA 02A 504), and Beijing Municipal Science & Technology Commission Program (No. Z11110706730000). Jifang Gong and Bing Hu contributed equally to this manuscript.
Footnotes
Access the full results at: Shen_Gong-13-137.theoncologist.com
Chinese Clinical Trial Registry: ChiCTR-TRC-07000014
Sponsor(s): Lin Shen
Principal Investigator: Lin Shen
IRB Approved: Yes
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.