The objective of this study was to evaluate preferences associated with grade I/II and grade III/IV chemotherapy side effects among breast cancer patients receiving chemotherapy. This study also assessed trade-offs that patients are willing to make between treatment side effects and the route and schedule of treatment administration. Results identified relative preferences among both mild/moderate to severe side effects from the patient perspective; patients appear to be willing to make trade-offs between side effects and different regimens.
Keywords: Conjoint, Preferences, Breast cancer, Side effects, Tradeoffs
Abstract
Objective.
Our objective was to evaluate preferences associated with grade I/II and grade III/IV chemotherapy side effects among breast cancer patients receiving chemotherapy. We also assessed trade-offs that patients are willing to make between treatment side effects and the route and schedule of treatment administration.
Methods.
In this cross-sectional study, patients receiving chemotherapy for breast cancer completed a one-time Web survey. Conjoint analysis was used to elicit preferences for 17 grade I/II and III/IV side effects associated with available chemotherapies and regimens. In the analysis, the risk of each side effect was increased by 5%, holding all others constant, and the respective impact on patient preferences was identified.
Results.
A total of 102 women participated (mean age 54 ± 11). Among the grade I/II side effects, a 5% reduction in the risk of sensory neuropathy, nausea, and motor neuropathy had the highest impact on preferences. Among grade III/IV side effects, motor neuropathy, nausea/vomiting, and myalgia made the most difference. An oral twice-daily regimen was most preferred; however, patients were willing to receive an intravenous regimen relative to oral to avoid an increased risk of 5% in the majority of side effects. Avoiding an increased chance of grade III/IV motor neuropathy was associated with willingness to tolerate one of the least preferred administration schedules.
Conclusion.
This study identified relative preferences among both mild/moderate to severe side effects from the patient perspective. Patients appear to be willing to make trade-offs between side effects and different regimens. These findings may help to inform medical decision-making processes.
Implications for Practice:
Patient care has shown a gradual shift from a paternalistic model, in which the patient serves a passive role, to a model of shared decision making, in which patiets become active participants in their health care plans. The current literature has suggested that this approach can result in increased satisfaction and adherence. In the current study, patients with breast cancer were able to express clear preferences for and make trade-offs among various grade I/II and grade III/IV side effects. Understanding these preferences and incorporating them into their treatment plans for breast cancer patients could lead to substantially improved clinical and quality of life outcomes.
Introduction
Chemotherapy regimens for breast cancer vary greatly with respect to their constituent agents, frequency, and route of administration, effectiveness, and side effects. Given that available chemotherapies have advantages and disadvantages relative to each other, it would be useful for health care providers to understand how these differences may influence individual patient preferences. As suggested by Chung and Carlson [1], the goals of treatment for patients with breast cancer are influenced by estimates of prognoses as well as a balance between physician and patient preferences regarding efficacy and toxicity.
Cancer patients will make trade-offs between chemotherapy regimens and specific side effects [2]. Previous studies regarding patient perceptions of chemotherapy side effects in advanced breast cancer patients showed that differences in the risks of hospitalization for neutropenia, diarrhea, nausea, and fatigue had the most influence of patient preferences, whereas the risk of myalgia, stomatitis, and hand-foot syndrome had the least influence of patient preferences [3]. Similar studies in patients with ovarian cancer [4] and advanced non-small cell lung cancer [5] found that nausea and vomiting were the most important side effects that would influence choice of chemotherapy.
In addition to studies assessing the impact of regimen toxicity on treatment preferences, how the route and schedule of treatment administration (e.g., oral versus intravenous 3-weekly treatment versus intravenous weekly treatments) may impact preferences is also of interest. One study suggested that patients with incurable cancers have a clear preference for oral versus infusion-based treatment, although they do not retain this preference if, in doing so, they must accept a lower response rate [6].
We are unaware of any patient preference studies in breast cancer that have included the full range of severity levels of side effects, both mild/moderate and severe, as well as trade-offs that patients are willing to make between drug side effects and the convenience of treatment administration. Conjoint analysis is a useful methodology for evaluating the impact of different attributes on patient preferences as it explores the trade-offs that patients are willing to make to avoid or accept specific treatment features or attributes. For example, are patients willing to accept a 5% increase in the risk of a side effect in exchange for a more convenient regimen [7, 8]? The goal of the present study was to use conjoint analysis to capture patient preferences for both the side effects of varying severity levels associated with chemotherapies for breast cancer as well as their preferences for regimens that vary in frequency and mode of administration.
Materials and Methods
This cross-sectional Web survey was implemented at the Ottawa Hospital Cancer Centre and Irving Greenberg Family Cancer Centre (Ottawa, Canada). Eligibility included female breast cancer patients, with disease of any stage, who were currently receiving neo/adjuvant or palliative chemotherapy. Participants were required to have adequate written and oral fluency in English. After providing written informed consent, patients had a choice to complete the survey either on a laptop in the chemotherapy unit or at their home. The participants completed surveys at their own pace, and research team members were available either in person or via telephone and/or E-Mail if the patient required additional assistance. The study received local Research Ethics Board approval.
Survey Design
This research was implemented following published methodological guidelines for conjoint studies [9]. Attributes of commonly used breast cancer chemotherapy treatments were identified from a literature review, a detailed assessment of breast cancer forum discussions, and consultation with clinical experts. These attributes included seven side effects, presented in both grade I/II and grade III/IV severity levels, as well as neutropenia requiring hospitalization, alopecia, and administration regimen. Attributes were described in lay terminology and informed by the Common Toxicity Criteria for Adverse Events grading descriptions [10]. Each side effect attribute had three levels, reflecting different risks of occurring. The highest and lowest reflected the highest and lowest estimates observed in breast cancer studies; the middle estimate generally was halfway between the highest and lowest. The regimen attribute had seven levels, each describing a different chemotherapy administration protocol (e.g., “21-day cycle; oral tablets taken twice daily for first 2 weeks,” and “21-day cycle; 30-minute infusion on days 1 and 8”).
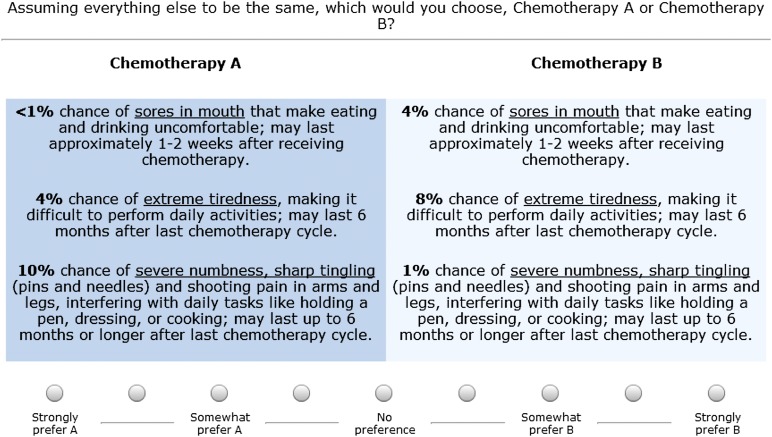
An adaptive conjoint analysis (ACA) approach was used to design the survey [11]. The conjoint exercise involved a series of paired comparison questions, which elicited treatment preferences by asking respondents to make trade-offs among attributes and choose from a pair of hypothetical treatments (Fig. 1 shows an example item). In each of these questions, the profiles of two hypothetical treatments—labeled simply chemotherapy A and chemotherapy B—were presented with different levels of the same three attributes. No chemotherapies were named in the survey. Respondents used a seven-point scale to indicate not only their preference but also the strength of their preference; response options ranged from “strongly prefer A” to “strongly prefer B.” The questions that appeared were based on the respondent's previous choices. Thus, the survey could focus on those attributes that the respondent considered as most important and those attribute levels regarded as most relevant.
Figure 1.
Example of paired comparison question.
Analysis
The conjoint data were analyzed using Sawtooth Software (Sequim, WA, http://www.sawtoothsoftware.com) v7.0.26, the ACA/Hierarchical Bayes (HB) module, and the Sawtooth Market Simulator. The preference weights (part-worth utilities) were calculated for each attribute level using ordinary least squares regression. The absolute values of the final utilities are arbitrary; what is important is the magnitude of the difference between them. These weights are “zero-centered,” whereby the utility weight of the attribute level falling in the middle has a value that approximates zero and the more favorable level is higher, or positive, and the less favorable level is lower, or negative. Finally, HB was applied to the data to further refine the precision of the utility estimates [12]. HB produces more robust parameter estimates than regression alone because it looks for patterns at both the individual and group levels (rather than just at the group level, as in traditional conjoint). Based on the Sawtooth Software market simulator, which is a software program that estimates the percentages of respondents who would choose one product profile over another (i.e., market share for a product profile), two sets of simulations with the preference data were conducted that accounted for potential imperfections, or error, in market share estimation. In the first set of simulations, two product profiles were compared that differed based on a 5% difference in the risk of each side effect, holding all other attributes constant, and differences in preferences for each product profile were identified.
In the second set of simulations, we evaluated patients’ willingness to receive treatment by different administration protocols, for example, oral versus intravenous, as well as frequency and duration of visits for intravenous therapy. Again we assessed patient preference for each protocol (based on mean utility weights for each regimen) in exchange for avoiding a 5% increase in each side effect. In other words, we evaluated how willing patients would be to accept a chemotherapy administration protocol that was most likely a less convenient regimen to avoid a 5% increased risk of each side effect.
Results
From June to December 2012, a total of 102 breast cancer patients completed the Web survey. Table 1 reports their demographic and clinical characteristics. The mean age was 54 ± 11 years; 10% had stage 1 disease, 34% had stage 2, 28% had stage 3, and 28% had stage 4. The majority of patients (79%) were married or had a partner, and 80% reported that their health was the same or worse relative to 1 year ago. At the time of completing the survey, patients were receiving: docetaxel (45), paclitaxel (27), anthracycline (14), capecitabine (5), nabpaclitaxel (4), cyclophosphamide, methotrexate, and fluorouracil (CMF) (3), vinorelbine (1), eribulin (1), and trastuzumab-DM1 (1). Less than one fifth of patients reported having ever previously missed a chemotherapy session, discontinuing a chemotherapy, or switching a chemotherapy medication because of side effects (Table 1).
Table 1.
Demographic and clinical characteristics
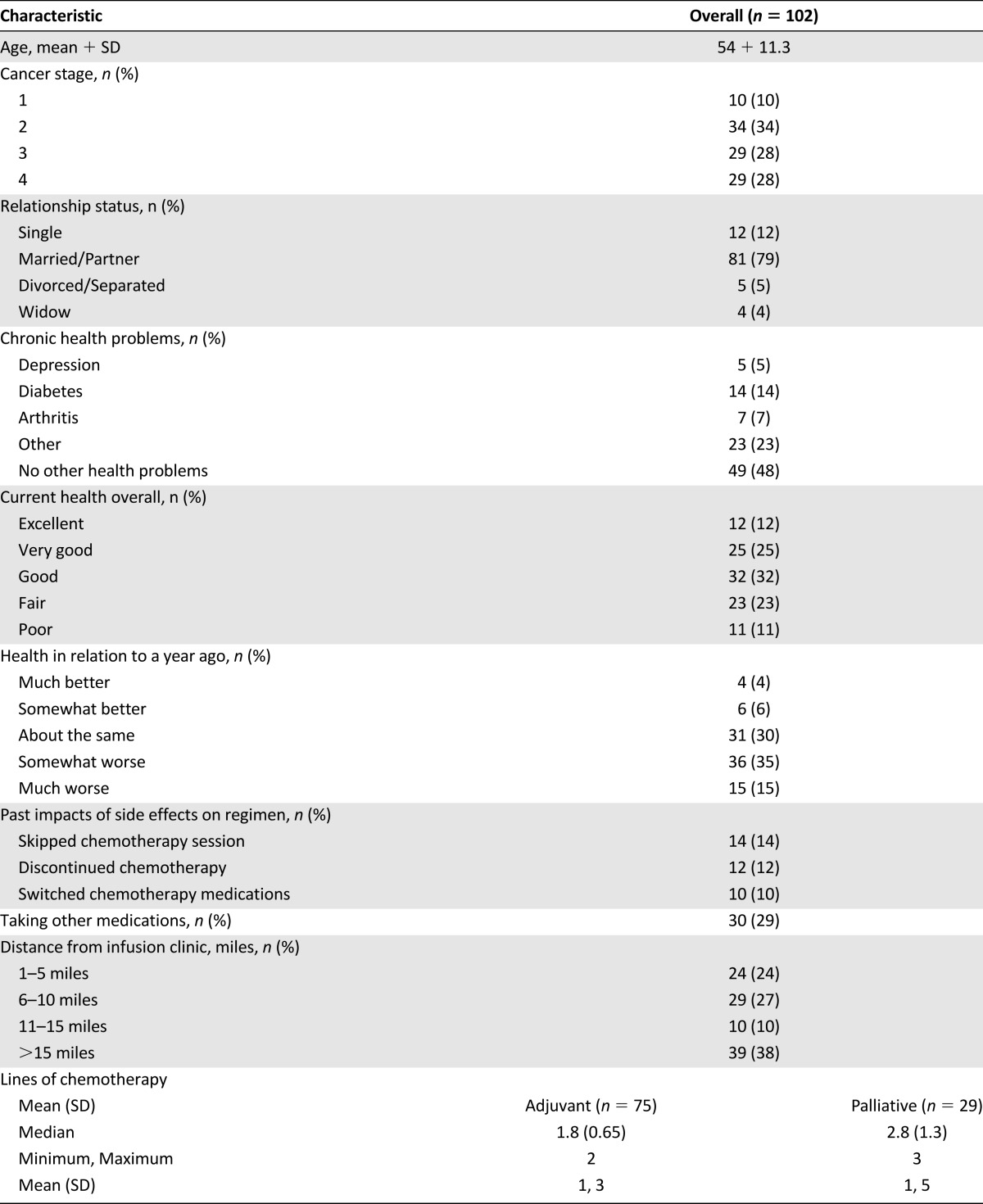
Table 2 shows the median utilities for each attribute level included in the survey. Given the magnitude of the differences within each attribute, the findings show that, in general, a small change in the risk of a grade III/IV side effect is relatively comparable to a larger difference in the risk of a grade I/II side effect. For example, the importance of reducing the risk of grade I/II sensory neuropathy from 71% (utility = −54.8) to 6% (utility = 52.2) is approximately comparable to reducing the risk of grade III/IV sensory neuropathy from 21% (utility = −56.7) to 1% (utility = 55.2). With respect to administration regimen, a “21-day cycle; oral tablets taken twice daily for 2 weeks” was most preferred (utility = 64.0), and a “21-day cycle; 3-hour infusion on days 1, 8, and 15” was least preferred (utility = 63.0).
Table 2.
Median utilities of attribute levels
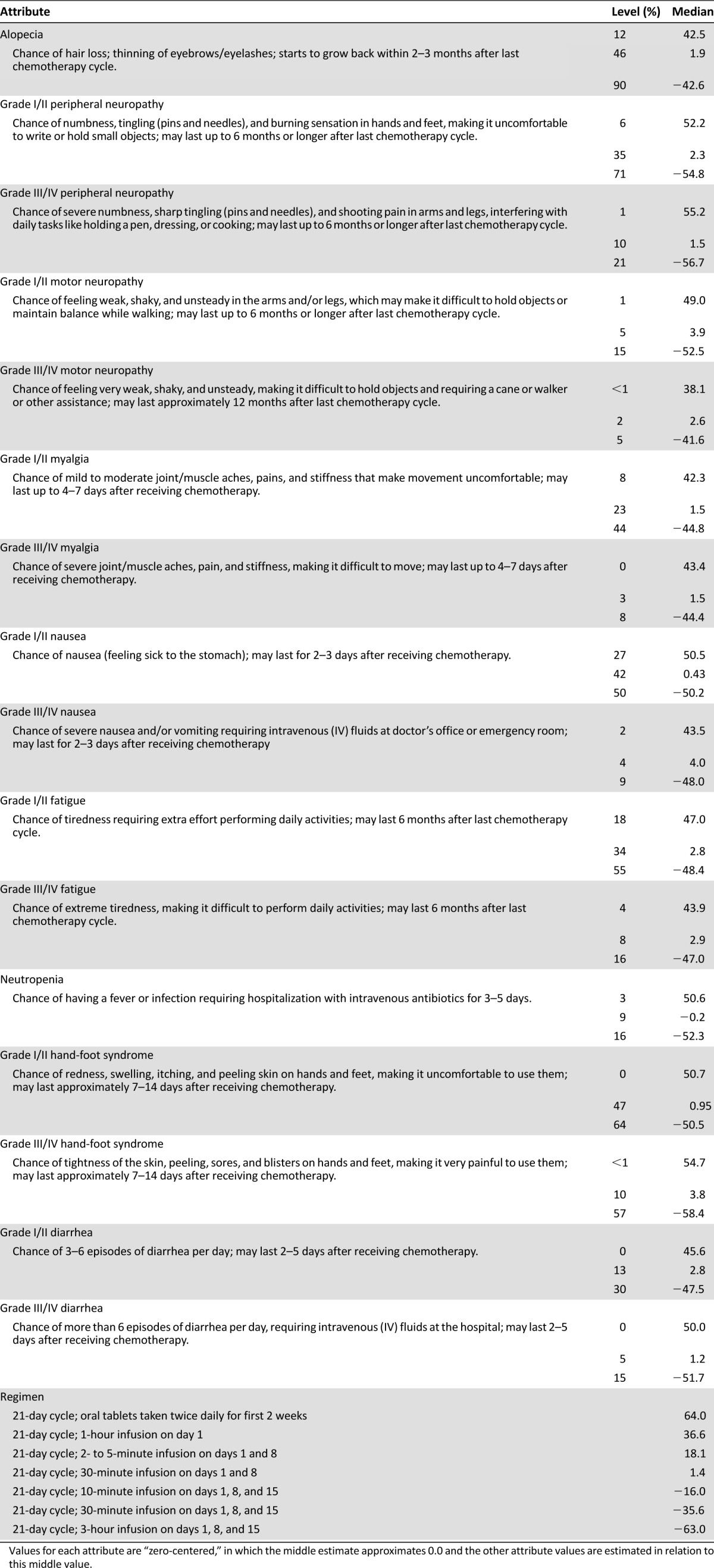
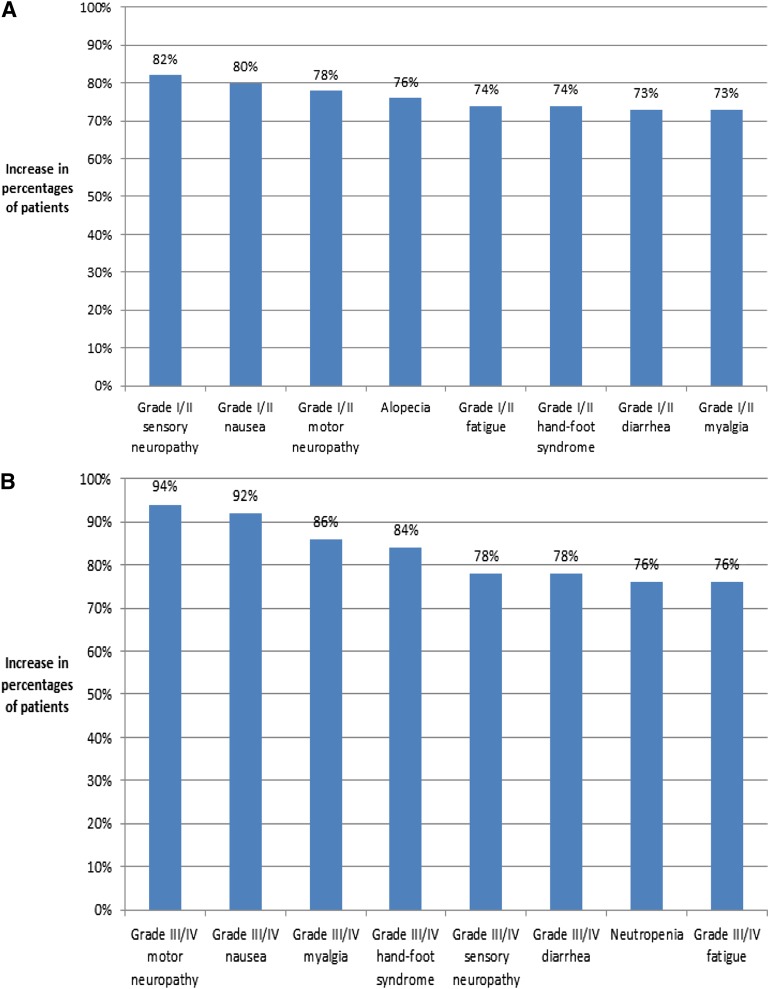
Figure 2A shows the increase in patient preferences associated with a decrease by 5% in the risk of each grade I/II side effect, and Figure 2B reports the increase in patient preferences associated with a decrease by 5% in the risk of each grade III/IV side effect. In general, the increase in preferences for a 5% reduction in the risk of grade III/IV side effects was higher than the increase in preferences for a 5% reduction in the grade I/II side effects. However, the increase in preferences for a 5% reduction in grade I/II sensory neuropathy, grade I/II nausea, and grade I/II motor neuropathy (82%, 80%, and 78%, respectively) was higher or equivalent to the increase in preferences for a 5% reduction in grade III/IV diarrhea, sensory neuropathy, fatigue, and neutropenia (preference increases ranging from 73% to 78%).
Figure 2.
Increase in patient preferences. (A): Increase in percentage of patients preferring treatment with a 5% reduction in each grade I/II side effect. (B): Increase in percentage of patients preferring treatment with a 5% reduction in each grade III/IV side effect.
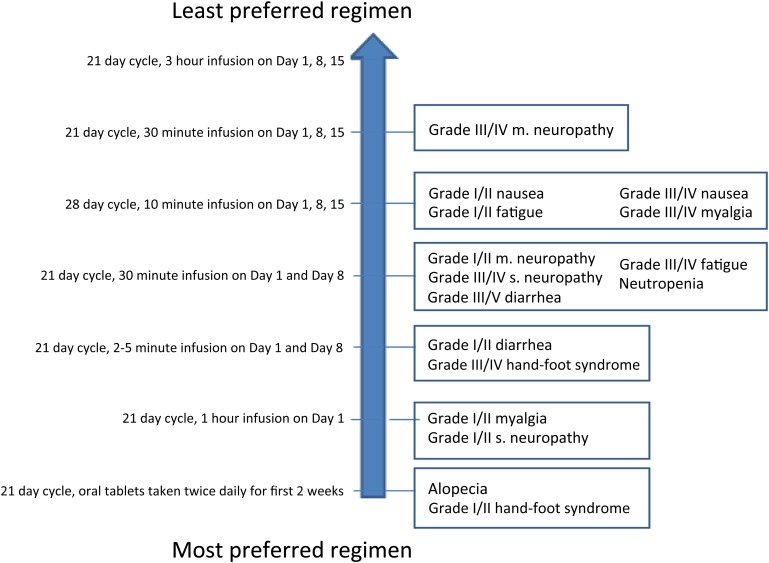
Figure 3 shows, for each side effect, the least preferred administration regimen that the majority (>50%) of patients would tolerate before switching their preference for a treatment with a 5% increased risk of the respective side effect coupled with the most preferred regimen (oral). Patients were not willing to tolerate a less preferred regimen relative to an oral regimen (which was most preferred) in exchange for a 5% reduced risk of alopecia or grade I/II hand-foot syndrome. The least preferred administration regimen that patients were willing to tolerate was a 21-day cycle, 30-minute infusion on days 1, 8, and 15 in exchange for a 5% increased risk of grade III/IV motor neuropathy (with oral regimen).
Figure 3.
Administration regimen that the majority of patients are willing to tolerate in exchange for a 5% reduction in each side effect.
Discussion
Chemotherapy remains an essential component of breast cancer therapy for patients with both early- and late-stage disease. However, given the increasing importance placed on patient quality of life, it is critical that patients and physicians fully understand the potential impact of available treatment options. To this end, this study provides information about commonly occurring side effects associated with chemotherapy from the patient perspective. It builds on the current literature on patient preferences in breast cancer by not only focusing on one level of toxicity but also including mild/moderate as well as severe side effects. We have also evaluated the importance of these side effects in comparison with the convenience of administration of different chemotherapy regimens. The findings suggest that patients may not only be willing to make trade-offs between different regimens based on their toxicities but will also make trade-offs between different toxicities and the schedule of administration of different regimens.
The findings of this study suggest, as expected, that the risks of grade III/IV side effects are more influential drivers of preferences for chemotherapy than the risks of the corresponding grade I/II side effects. Reducing the risk of sensory neuropathy had the largest impact on preferences among grade I/II side effects, and reducing the risk of motor neuropathy had the largest impact on preferences among grade III/IV side effects. These findings are consistent with those of previous research in breast cancer, in which both sensory and motor neuropathies were deemed highly important in terms of predicting preferences [13]. This most likely is attributable to the frequency and prolonged duration of these side effects [14]. This may also explain why the increases in preferences for a reduced risk of grade I/II sensory neuropathy and motor neuropathy were higher than those associated with a reduced risk of grade III/IV diarrhea and fatigue. The finding that a reduced risk of grade I/II sensory neuropathy was associated with a greater increase in preference relative to a reduced risk of grade III/IV sensory neuropathy was unexpected; it may be that the descriptions of these two severity levels were not sufficiently distinctive for patients when completing the survey.
Consistent with previous research, patients were able to make trade-offs between risks of individual side effects and the convenience of how the regimen is administered [2]. Specifically, patients were willing to tolerate an increased risk of certain side effects, such as alopecia, in exchange for having an oral regimen. When it came to other more severe side effects, such as grade III/IV motor neuropathy, patients were willing to accept a less convenient regimen to avoid an increased risk of these side effects. These results are again consistent with findings in the previous study of patient preferences for chemotherapies used to treat breast cancer [13] as well as a study in which patients with non-small cell lung cancer were more likely to trade less convenience to avoid severe side effects but would choose more convenience if the side effect was mild [15].
This study has a number of limitations. While the common toxicity criteria (CTC) verbiage from patient web forums and patient feedback in the pilot test informed the health state descriptions, the descriptions still may not represent the average experience for patients experiencing grade I/II or grade III/IV side effects. Nevertheless, given that the descriptions were standardized as much as possible in relation to the CTC and pilot tested among patients, we believe that they resonate with both patients and clinicians. There is clearly patient selection bias as a result of this methodology, as patients had to be comfortable using a computer. In addition, the survey was lengthy, taking an average of 63 minutes to complete. As such, patients may have grown fatigued and less careful about their responses.
In conclusion, this survey evaluated a comprehensive set of mild and severe side effects and regimen attributes observed with chemotherapies for breast cancer. Our study showed that patients have clear preferences for these attributes and are willing to make trade-offs among side effects differing in severity as well as between the toxicities of individual regimens and the convenience of how the regimen is administered. The findings from this research may be useful in incorporating patients’ views into medical decision-making processes, patient education, cost resource allocations, and drug development. A better understanding of patients’ preferences may help to improve patient satisfaction and compliance with treatment regimens.
Acknowledgments
We thank all the patients and their families for their help with this project. This work was supported by an unrestricted educational grant from Eisai Pharmaceuticals. Eisai Pharmaceuticals had no access to the study data or role in the study analysis.
Data have been previously presented as a poster presentation (abstract 6595) at the American Society of Clinical Oncology meeting, June 2013, Chicago, IL.
Author Contributions
Conception and design: Jessica Grinspan, Kathleen Beusterien, Nathaniel Bouganim, Lisa Vandermeer, Susan Dent, Sasha Mazzarello, Mark Clemons
Provision of study material or patients: Nathaniel Bouganim, Iryna Kuchuk, Lisa Vandermeer, Susan Dent, Sasha Mazzarello, Mark Clemons
Collection and/or assembly of data: Iryna Kuchuk, Mark Clemons
Data analysis and interpretation: Jessica Grinspan, Kathleen Beusterien, Iryna Kuchuk, Mark Clemons
Manuscript writing: Jessica Grinspan, Kathleen Beusterien, Mark Clemons
Final approval of manuscript: Jessica Grinspan, Kathleen Beusterien, Nathaniel Bouganim, Lisa Vandermeer, Susan Dent, Sasha Mazzarello, Mark Clemons
Disclosures
Jessica Grinspan: Oxford Outcomes (E); Mark Clemons: Esai Pharmaceuticals (RF); Kathy Beusterien: Oxford Outcomes (E [previous employment]). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. The Oncologist. 2003;8:514–520. doi: 10.1634/theoncologist.8-6-514. [DOI] [PubMed] [Google Scholar]
- 2.Young A, Topham C, Moore J, et al. A patient preference study comparing raltitrexed (‘Tomudex’) and bolus or infusional 5-fluorouracil regimens in advanced colorectal cancer: Influence of side-effects and administration attributes. Eur J Cancer Care. 1999;8:154–161. doi: 10.1046/j.1365-2354.1999.00152.x. [DOI] [PubMed] [Google Scholar]
- 3.Beusterien KM, Davies J, Leach M, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: A cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50. doi: 10.1186/1477-7525-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: Insights from experienced patients with ovarian cancer. Support Care Cancer. 2005;13:219–227. doi: 10.1007/s00520-004-0710-6. [DOI] [PubMed] [Google Scholar]
- 5.Dubey S, Brown RL, Esmond SL, et al. Patient preferences in choosing chemotherapy regimens for advanced non-small cell lung cancer. J Support Oncol. 2005;3:149–154. [PubMed] [Google Scholar]
- 6.Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–115. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 7.Green PE, Srinivasan V. Conjoint analysis in marketing: New developments with implications for research and practice. J Mark. 1990;54:3–19. [Google Scholar]
- 8.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–1533. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: A report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 11.Sawtooth Software. ACA/Web Technical Paper. Available at http://sawtoothsoftware.com. Accessed August 15, 2009
- 12.Ryan M, Scott DA, Reeves C, et al. Eliciting public preferences for healthcare: A systematic review of techniques. Health Technol Assess. 2001;5:1–186. doi: 10.3310/hta5050. [DOI] [PubMed] [Google Scholar]
- 13.Beusterien K, Grinspan J, Tencer T, et al. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279–287. doi: 10.2147/IJWH.S31331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: A retrospective, outcomes-based survey. Curr Oncol. 2010;17:42–47. doi: 10.3747/co.v17i4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridges JF, Mohamed AF, Finnern HW, et al. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: A conjoint analysis. Lung Cancer. 2012;77:224–231. doi: 10.1016/j.lungcan.2012.01.016. [DOI] [PubMed] [Google Scholar]