Abstract
Purpose
Retinal pigment epithelium, which forms the outer blood-retinal-barrier, is a critical barrier for transport of drugs to the retina. The purpose of this study was to develop a pigmented MDCK (P-MDCK) cell line as a rapidly established in vitro model for the outer blood-retinal-barrier to assess the influence of melanin pigment on solute permeability.
Methods
A melanin synthesizing P-MDCK cell line was developed by lentiviral transduction of human tyrosinase and p-protein genes in MDCK (NBL-2) cells. Melanin content, tyrosinase activity (conversion of L-dopa to dopachrome), and transepithelial electrical resistance (TEER) were measured. Expression of tyrosinase protein and p-protein in P-MDCK cells was confirmed by confocal microscopy. Effect of L-tyrosine (0 to 2 mM) in culture medium on melanin synthesis in P-MDCK cells was evaluated. Cell uptake and transepithelial transport of pigment-binding chloroquine (Log D = 1.59) and a negative control salicylic acid (Log D = −1.14) were investigated.
Results
P-MDCK cells expressed tyrosinase and p-protein. Tyrosinase activity was 4.5 fold higher in P-MDCK cells as compared to wild-type MDCK cells. The transepithelial electrical resistance stabilized by day 4 in both cell types, with the TEER being 871 ± 30 and 876 ± 53 Ω.cm2 for P-MDCK and wild-type cells, respectively. Melanin content in P-MDCK cells depended on the concentration of L-tyrosine in culture medium, and increased from 3 to 54 µg/mg protein with an increase in L-tyrosine content from 0 to 2 mM. When the cells were grown in 2 mM L-tyrosine, uptake of chloroquine was 2.3 fold higher and the transepithelial transport was 2.2 fold lower in P-MDCK cells when compared to wild-type MDCK cells. No significant difference was observed for both cell uptake and transport of salicylic acid.
Conclusions
We developed a P-MDCK cell line with tunable melanin synthesis as a rapidly developing surrogate for retinal pigment epithelium.
Keywords: P-MDCK cells, melanin, outer blood-retinal-barrier, retinal pigment epithelium
INTRODUCTION
Drug delivery to the back of the eye has received considerable attention in recent years because of the increase in age related and lifestyle related ocular diseases such as age related macular degeneration and diabetic retinopathy1. Delivery of drugs to the posterior tissue is a major challenge because of the stringent, protective barriers of the eye. While various routes of administrations are available for delivery of drugs to the posterior segment of the eye, each one has its own limitations. A noninvasive topical product has yet to be approved for back of the eye diseases because of poor bioavailability in posterior segment tissues. The highly invasive, intravitreal route, which is approved in clinical settings, is associated with various injection related ocular complications such as retinal detachment, endophthalmitis, vitreous hemorrhage, and cataracts2. Drug delivery to the retina via the systemic circulation is limited by nonspecific delivery to the other organs and blood-retinal-barriers in the eye. However, minimally invasive transscleral3 and suprachoroidal4, 5 routes of delivery have shown promising results in preclinical development.
For transscleral drug delivery, the drug needs to cross the sclera, choroid, and retinal pigmented epithelium (RPE) before reaching its target tissue, the neural retina, in treating diseases such as diabetic retinopathy6. More than 80% of resistance to drug transport in transscleral delivery is due to choroid-RPE in bovine and porcine models7. The choroid-RPE is made up of two layers; choroid, a tissue rich in collagen, melanin pigment. and fenestrated blood capillaries and RPE, a single tight layer of pigmented epithelium that forms the outer blood-retinal-barrier (BRB). RPE constitutes a major limiting barrier in transscleral drug delivery because of poor passive permeability and the accumulation of drug due to melanin binding.
Numerous models are described in the literature for the outer BRB, but all have limitations. Freshly isolated sclera-choroid-RPE or choroid-RPE from animal eyes (bovine, porcine and rabbit) are commonly used as in vitro models7–9. Limitations in the availability of fresh tissue on a regular basis makes high throughput difficult. A second available option is the primary culture of RPE cells10; however, isolation and maintenance of these cells is laborious. Another commonly used in vitro model is the ARPE-19 cell line, a human cell line of RPE. Unfortunately these cells take 6 to 8 weeks to form a polarized epithelial monolayer11, and even after 6 to 8 weeks of growth on transwell filters, the transepithelial electrical resistance (TEER) does not exceed 200 Ω.cm2 12, whereas primary human fetal RPE have TEER values of approximately 800 Ω.cm2 by 24 days of growth on transwell filters13. Further, there is no melanin formation in ARPE-19 cells even after 6 weeks of growth14, a key component of RPE that hinders drug delivery. Previous studies from our laboratory as well as other laboratories showed that the transport of lipophilic drug molecules across pigmented choroid-RPE is significantly lower than the transport across non-pigmented choroid-RPE7, 8, 15.
Tyrosinase is a copper containing metalloprotein and the key enzyme involved in synthesis of melanin pigment in pigmented cells16, 17. Tyrosinase catalyzes the conversion of tyrosine to L-dopa and subsequently L-dopa to dopaquinone, which then polymerizes to form melanin pigment. While it has been shown that the transfection of human tyrosinase alone is capable of producing melanin pigment in non-pigmented cells18, 19, tyrosinase is misprocessed sometimes and accumulates in the perinucelar compartment18. Indeed, this misprocessing is similar to that seen in cases of oculocutaneous albinism type 2 (OCA2)18. Molecular studies have mapped this genetic disease to the human homolog of the pink-eye dilution gene, suggesting that the gene product (p-protein) plays some role in the correct processing and localization of tyrosinase18, 20. Expression of human p-protein along with tyrosinase improves the posttranslational processing and transport of tyrosinase to melanosomes18, 21. p-protein forms and stabilizes the melanosomal protein complex, which includes p-protein and tyrosinase. Further, melanin precursors, which are cytotoxic are localized to the melanosome, thereby decreasing their cellular toxicity.
The objective of this study was to develop an in vitro cell culture-based high throughput model for the outer BRB. In the present study we developed melanin pigment expressing MDCK cells as an in vitro cell culture-based high throughput model for the outer-BRB. We stably transfected MDCK cells (NBL-2) with human tyrosinase and p-protein genes using retroviral infection. Melanin synthesis and expression of human tyrosinase and p-protein was confirmed by light microscopy, immunocytochemistry and functional activity assays. Effect of L-tyrosine on melanin synthesis was evaluated by growing the P-MDCK cells in DMEM containing varying concentration of L-tyrosine (0 to 2 mM). In addition, we also evaluated the effect on melanin pigment on uptake and transport chloroquine and salicylic acid.
MATERIALS AND METHODS
Materials
Chloroquine diphosphate salt, salicylic acid, L-tyrosine (~98% purity), and synthetic melanin were purchased from Sigma-Aldrich (St. Louis, MO). HPLC grade acetonitrile and methanol were purchased from Fisher Scientific (Fair Lawn, NJ). Ammonium formate (99.9%) was purchased from Fluka BioChemika (USA). All other chemicals and reagents used in this study were of analytical reagent grade.
Methods
Expression plasmid construction
The tyrosinase gene (NM_000372.4) was constructed (DNA2.0, Inc., Menlo Park, CA, USA) and inserted into pJ201 vector. The plasmid was digested with Xho1/EcoR1 to isolate the full-length human tyrosinase gene. The cDNA insert was then cloned at the XhoI/EcoR1 sites in the mouse stem cell virus IRES CD8 (MiCD8) retroviral expression vector. To generate the expression construct of human p-protein (NM_000275) present in plasmid pOTB7, the plasmid was digested with Mfe1/BamH1 to isolate the full-length human p protein cDNA. The insert cDNA was then cloned at the Bgl2/EcoR1 site in the mouse stem cell virus IRES GFP retroviral vector (a gift from Yosef Refaeli).
Preparation of retrovirus and infection of MDCK cells
High titer retrovirus was generated in 293T1 cells transfected with pCL-Eco packaging plasmid and tyrosinase and p-protein retroviral vector as described22. MDCK cells were infected with retrovirus expressing p-protein by spinfection. Briefly, MDCK cells were grown in 24 well plates for 48 hr. After 48 of incubation (80% confluency), the culture medium was removed, and the cells were centrifuged at 2000 g for 1 hr at 4°C with virus-containing medium isolated from 293F1 cells in the presence of 8 µg/ml polybrene (hexadimethrine bromide; Sigma Chemical Company, St Louis, MO) and Hank’s balanced salt solution (HBSS). The medium was then replaced and cells were allowed to grow up to 4 passages. MDCK cells expressing p-protein also expressed GFP, which was used for cell sorting by flow cytometry. Purified cells were then grown for several passages, reassessed for marker expression, and frozen as stocks. The tyrosinase gene was then introduced into p-protein expressing MDCK cells exactly as described above. Infected MDCK cells stably expressing the tyrosinase gene also expressed CD8 via the viral IRES.CD8 expression was visualized using a Cy5 tagged antibody (Pharmingen, CA) and doubly infected cells were purified by flow cytometry.
Tyrosinase (L-dopaoxidase) activity
Tyrosinase (dopa oxidase) expressing MDCK cells (5 × 105 cells/ per well) were grown for 48 h in a 12 well culture plate. After 48 h, cells were lysed with 300 µl of 1% Triton X-100 in 0.1 M phosphate buffer (pH 6.8). Samples were sonicated and centrifuged at 8000 rpm for 10 min. To 100 µl of the obtained supernatant, 100 µl L-dopa solution (0.15%) was added and incubated at 37 °C for 10 min. Tyrosinase activity was measured by quantifying dopachrome formation using a UV spectrophotometer set at 475 nm.
Measurement of melanin
Melanin content in control and tyrosinase plus p-protein transfected, pigmented MDCK (P-MDCK) cells were measured using a melanin solubilization assay23. To estimate melanin content, both MDCK and P-MDCK cells (4 × 104 cells/ per well) were plated in a 12 well culture plate and grown for 48 hr using DMEM containing varying concentration of L-tyrosine (0 to 2 mM). After 48 hr of incubation, cells were trypsinized and collected in microcentrifuge tubes. The cell pellet was suspended in 100 µl of alkaline solution (1 N NaOH in 10% DMSO) and sonicated on ice bath to lyse the cells. To solubilize the melanin, the above sonicated cell suspension was heated at 70°C for 1 hr. At the end of 1 hr, samples were centrifuged at 8000 rpm for 5 min, and absorbance of supernatant was measured at 475 nm. The calibration curve for melanin estimation was generated using synthetic melanin as a standard. The melanin content was normalized to the total amount of protein.
Immunocytochemistry
For immunocytochemistry experiments, the cells were grown on round cover slip (Fisher Scientific) in 12 well plates (Costar, NY) at 37 °C in humidified CO2 chamber to reach 50–60 % confluency. Cells were fixed with 10 % formalin and treated with 0.1 % Triton–X 100. The cells were labeled overnight with rabbit anti-human tyrosinase antibody (primary antibody, 1:200 dilution) obtained from Abcam (Abcam antibody, MA, USA) at 4 °C. After overnight incubation with primary antibody, cells were washed and incubated with Texas-red labeled goat anti-rabbit IgG antibody (secondary antibody, 1:100 dilutions) obtained from Abcam (Abcam antibody, MA, USA) at room temperature for 2 hr. Nuclei were stained with DAPI and slides were analyzed using a confocal microscope (Nikon C1 si®) set at 60× magnification. Green fluorescence in the images represents p-protein.
Cell uptake study
All cell uptake and transport studies were conducted using sterile isotonic assay buffer (pH 7.4) with the following composition: NaCl (122 mM), NaHCO3 (25 mM), MgSO4 (1.2 mM), K2HPO4 (0.4 mM), CaCl2 (1.4 mM), HEPES (10 mM), and glucose (10 mM). Assay buffer was sterilized by steam sterilization in autoclave at 121 °C for 15 minutes. To study cell uptake of chloroquine and salicylic acid, MDCK and P-MDCK cells (2 × 104 cells/well) were plated in 24 well plates and grown for 48 hr in DMEM medium containing 2 mM L-tyrosine to reach 80–100 % confluency. After 48 hr, the medium was removed and cells were washed with sterile assay buffer solution (pH 7.4). The cells were incubated for 3 hr with assay buffer containing either 25 µg/ml of (chloroquine phosphate (78.2 µM) (a drug that binds to melanin pigment) or salicylic acid (181 µM) (a drug with little or no binding to melanin pigment) at 37 °C in a 5% CO2 incubator. At the end of the 3 hr incubation, the solution was removed, cells were washed three times with assay buffer, and harvested using 250 µl of 1% Triton X-100 in 0.1 M phosphate buffered saline. Cell lysate were stored at −20 °C until LC-MS/MS analysis for drug content. The drug uptake in cells was normalized to the total amount of protein.
Transport study
For transport studies, MDCK and P-MDCK cells were grown on collagen coated transwell filters in the presence of L-tyrosine (Corning, NY). The cells were seeded at a density of 2 × 105 cells/filter in 24 well plates with an insert surface area of 0.33 cm2. One filter was kept as a control without cell seeding for measurement of transepithelial electrical resistance (TEER) of blank filter. The cells were allowed to grow into a confluent monolayer. Medium was replaced every 24 hr, TEER at 37 °C was measured periodically as Ω.cm2 using an epithelial voltmeter (World Precision Instruments). A confluent cell monolayer with stable TEER value was obtained 4–6 days post-seeding. Following subtraction of background TEER from the control filter, both transfected and control MDCK cells showed a TEER of 800–900 Ω.cm2 at 37 °C from day 4 onwards.
Transport experiments were carried out on day 6 post seeding using isotonic assay buffer solution (pH 7.4) at 37 °C in a 5 % CO2 incubator. Prior to introduction of drug, culture medium was removed from the transwell filters and the cell monolayer was washed with sterile assay buffer and allowed to equilibrate with assay buffer for 30 min at 37 °C. After 30 min of equilibration, assay buffer was removed and chloroquine (25 µg/ml; 78.2 µM) or salicylic acid (25 µg/ml; 181 µM) solution (500 µl) in assay buffer was added to the apical side and plain assay buffer (1000 µl) on receiver side of transwells. Cells were incubated at 37°C in 5 % CO2, and 50 µl aliquots were taken from the receiver chamber at 15, 30, 60, 90, 120, 150, and 180 min and the lost volume was compensated with fresh assay buffer pre-equilibrated at 37 °C. Tight junctions of cell monolayer during experiments were monitored by measuring TEER values every hour.
LC-MS/MS
The concentrations of chloroquine and salicylic acid in cell uptake and transport study samples were analyzed using LC-MS/MS. An API-3000 triple quadruple mass spectrometer (Applied Biosystems, Foster City, CA, USA) coupled with a PerkinElmer series-200 liquid chromatography system (Perkin Elmer, Waltham, Massachusetts, USA) was used.
For salicylic acid, samples were analyzed in the negative mode of ionization using diclofenac as an internal standard with the following MRM transitions: 138/94 (salicylic acid); 294/250 (diclofenac). Analytes were separated on a Sunfire C18 column (2.1 × 50 mm, 3 µm) using water containing 0.05 % formic acid (A) and acetonitrile: methanol (1:1 v/v) (B) as mobile phase.
Analysis of chloroquine was carried out in the positive mode of ionization with propranolol as an internal standard with the following MRM transitions: 320/247 (chlororquine); 260/116 (propranolol). Analytes were separated on Zorbax extended C18 column (4.6 × 50 mm, 5 µm) using water containing 5 mM ammonium acetate (A) and acetonitrile: methanol (1:1 v/v) (B) as mobile phase.
Statistical analysis
The data is represented as the mean ± SD. For statistical comparisons between two experimental groups, independent samples Student’s t-test was used (SPSS, version 11.5; SPSS, Chicago, IL). The results were considered statistically significant at P < 0.05.
RESULTS
Characterization of transfected cells for melanin synthesis
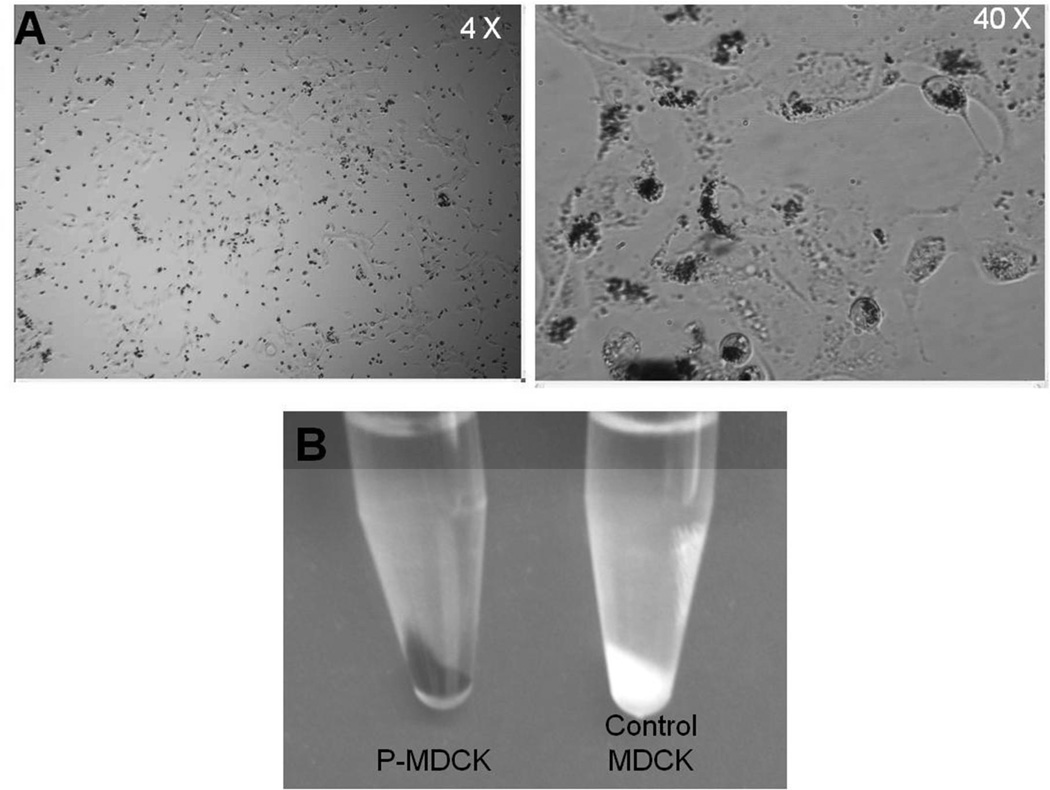
Melanin synthesis in P-MDCK cells was confirmed by light microscopy pictures and photographs of cell pellets (Figure 1). Light microscopy pictures of transfected cells maintained for 48 hrs in media containing 2 mM L-tyrosine showed localization of melanin granules in the perinuclear space. Additionally, cell pellets of P-MDCK cultures appeared totally black, whereas the pellet for wild type cells was white in color (Figure 1B).
Figure 1.
Melanin expression in Tyrosinase and p-protein transfected MDCK (P-MDCK) cells. A) Representative light microscopy pictures of P-MDCK cells showing melanin granules. B) Cell pellets of MDCK and P-MDCK cells.
Fluorescence immunocytochemistry of tyrosinase and p-protein
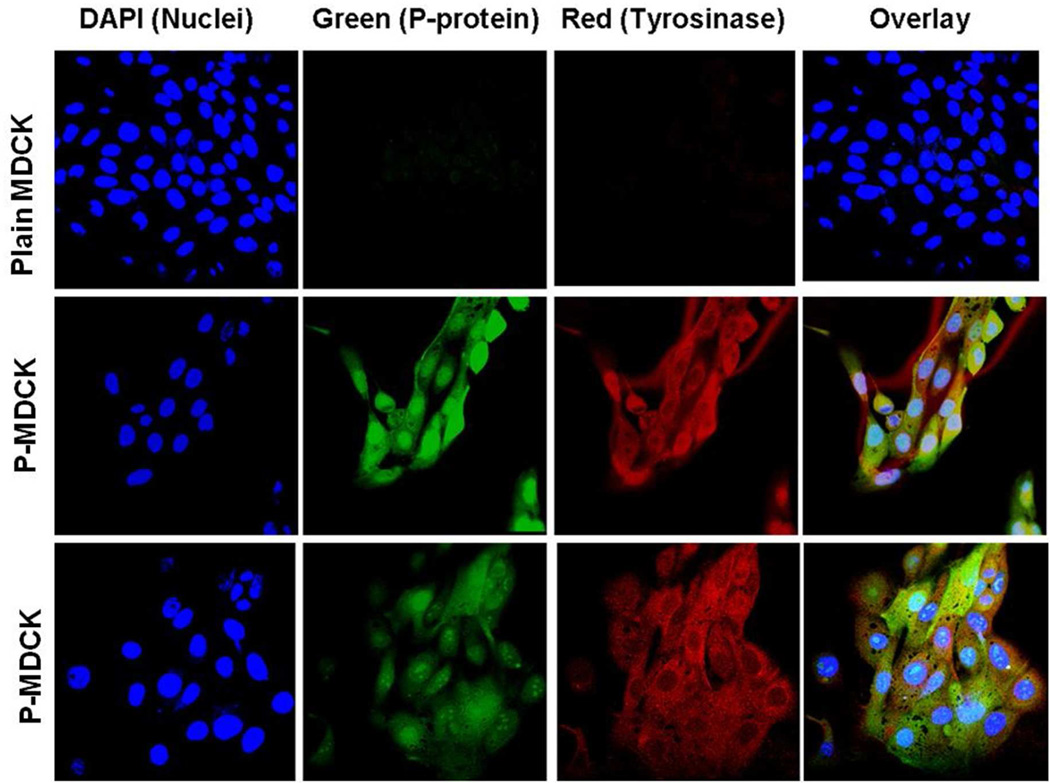
Expression of human tyrosinase and p-protein in P-MDCK and wild type MDCK cells were confirmed by red and green fluorescence signals in confocal images. No tyrosinase or p-protein staining was detected in wild type MDCK cells (Figure 2). P-MDCK cells showed tyrosinase staining throughout the cytoplasm, while p-protein labeling was confined mainly to the nucleus with light staining observed in the cytoplasm.
Figure 2.
Tyrosinase and p-protein are only present in P-MDCK cells and absent in MDCK cells. Tyrosinase showed localization in cytoplasm, whereas p-protein was present in the nucleus. Immunofluorescence confocal microscopy images are shown with immunostaining of tyrosinase (red), nucleus (blue), and p-protein labeled with GFP (green).
Tyrosinase activity in wild type and P-MDCK cells
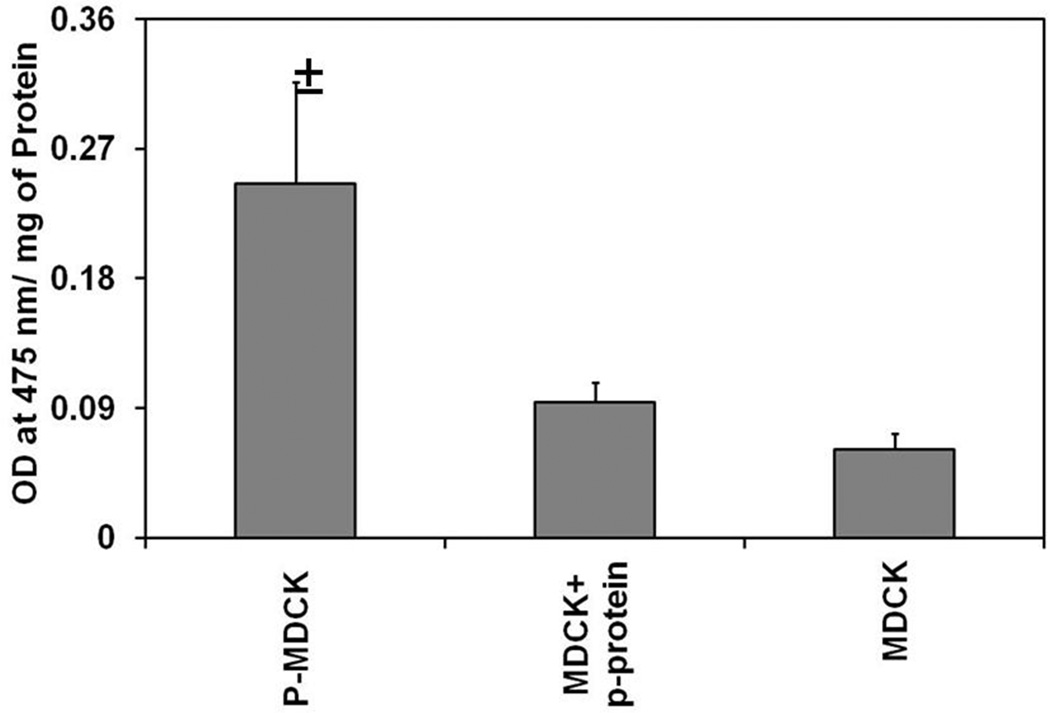
In P-MDCK cells, dopa oxidase activity was significantly higher than the wild type MDCK cells (Figure 3).
Figure 3.
Tyrosinase activity is significantly higher in P- MDCK cells as compared to wild-type and p-protein expressing MDCK cells. Tyrosinase activity was measured by monitoring the conversion of L-tyrosine to L-dopa at 475 nm. Data are expressed as mean ± SD for n = 4. ± P < 0.05 when compared with MDCK cells.
Effect of L-tyrosine concentration on melanin synthesis in transfected cells
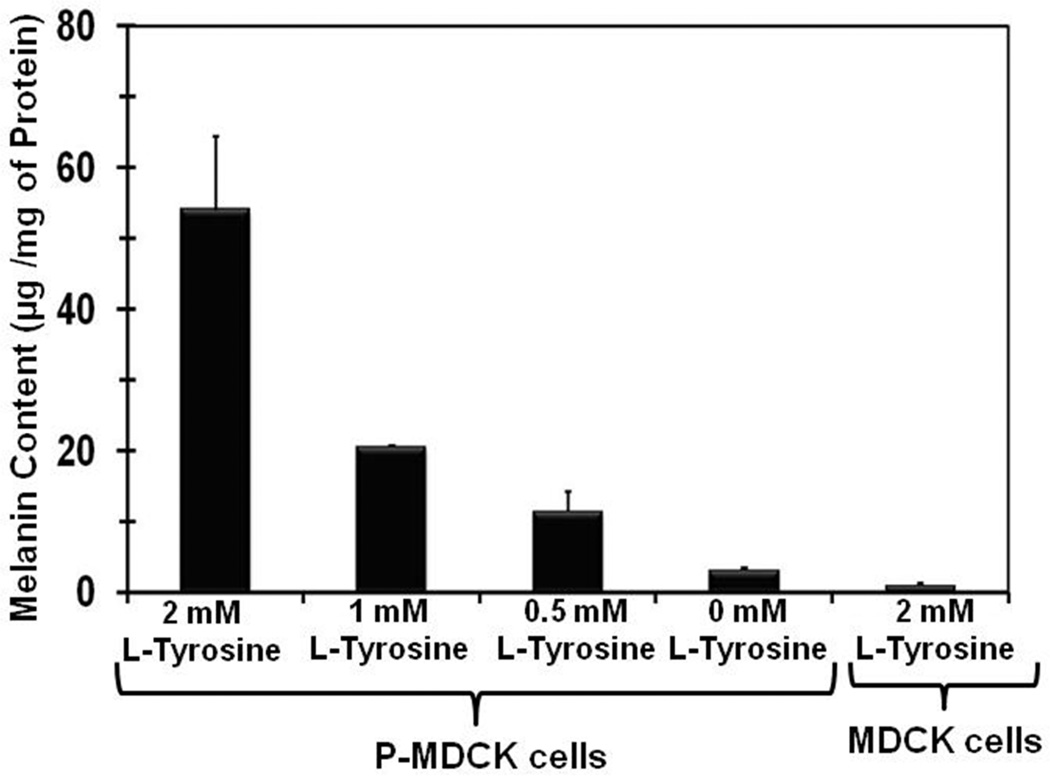
L-tyrosine is the precursor for melanin. L-tyrosine concentration in culture medium had significant influence on melanin synthesis in transfected cells. When transfected cells were grown in medium containing L-tyrosine, the melanin content of cell was significantly increased. There was about 17-fold increase in melanin content with 2 mM L-tyrosine when compared to no tyrosine in culture medium (Figure 4). A decrease in L-tyrosine concentration from 2 mM to 1 mM in culture medium resulted in a 2.6 fold decrease in melanin content in P-MDCK cells.
Figure 4.
L-tyrosine levels correlate with melanin synthesis in P-MDCK cells. P-MDCK cells were grown in DMEM medium containing varying concentrations of L-tyrosine. MDCK cells were grown in DMEM medium containing 2 mM L-tyrosine. Melanin content was measured using a melanin solubilization assay (Materials and Methods) and normalized to protein content. Data are expressed as mean ± SD for n = 4.
Uptake of chloroquine and salicylic acid in wild type and transfected MDCK cells
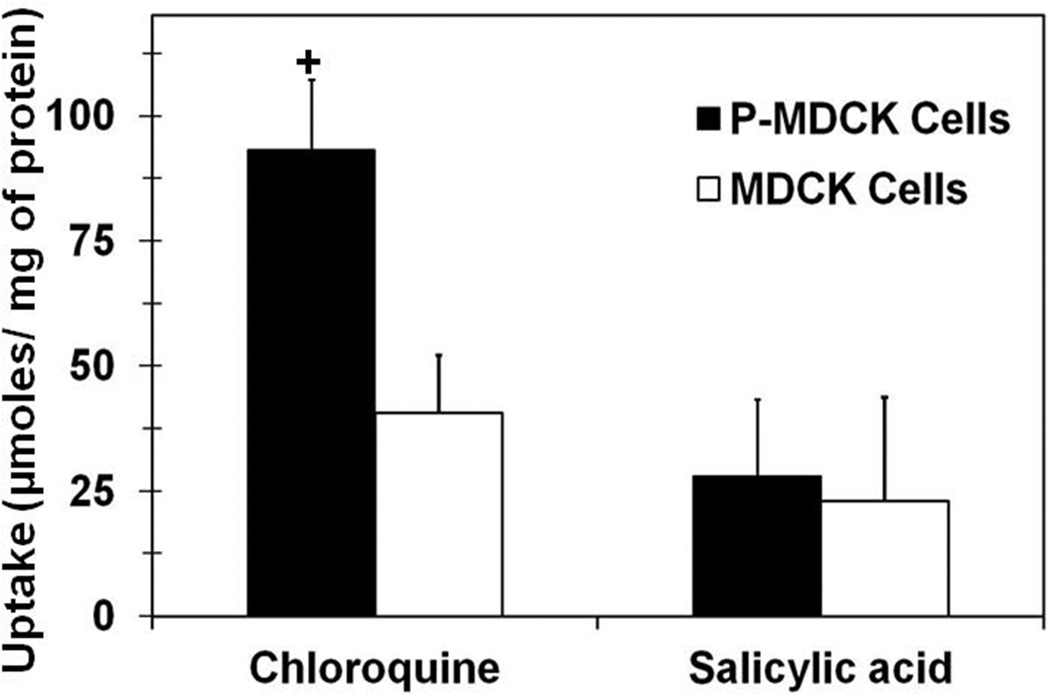
Cell uptake studies of chloroquine and salicylic acid were carried out in wild type and P-MDCK cells to evaluate the effect of melanin content on cellular accumulation of drugs. Chloroquine, a cationic lipophilic drug molecule (Log D (pH 7.4) = 1.59; Log P = 4.5; pKa 10.1)24 has very high affinity for melanin pigment and is commonly used as a positive control in melanin binding studies. Salicylic acid, an anionic drug molecule (Log D (pH 7.4) = − 1.14; Log P = 2.2; pKa 2.97)25 with very low affinity and little or no binding to melanin pigment served as a negative control for melanin binding. Cell uptake of chloroquine was 2.3 fold higher in P-MDCK cells than wild type MDCK cells (p value = 1.598E−05, n = 6); whereas, there was no significant difference in cell uptake of salicylic acid between transfected and wild type cells (Figure 5). The cell uptake of chloroquine was significantly higher than salicylic acid in both wild type as well as transfected MDCK cells, which is due to higher lipophilicity of chloroquine24 as compared to salicylic acid25.
Figure 5.
Uptake of chloroquine is several fold higher in P-MDCK cells than MDCK cells. Cell uptake of chloroquine (positive control for melanin binding) and salicylic acid (negative control for melanin binding) was performed in both cells. P-MDCK and MDCK cells were grown in DMEM medium containing 2 mM L-tyrosine. Data are expressed as mean ± SD for n = 6. † P < 0.05 when compared with MDCK cells.
Transport of chloroquine and salicylic acid across cell monolayer of wild type and transfected MDCK cells
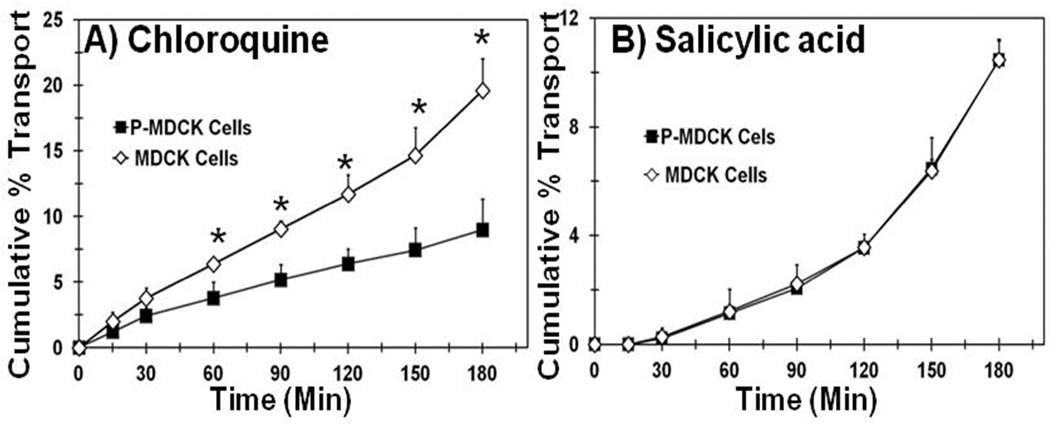
Transport studies of chloroquine and salicylic acid were carried out using wild type and P-MDCK cell monolayers. The transepithelial electrical resistance of P-MDCK and MDCK cell lines after 4 days of growth on transwell filters was 871 ± 30 and 876 ± 53 Ω.cm2, respectively. These values are very close to the ~800 Ω.cm2 TEER for human RPE cells after 24 days of growth13. The cumulative % transport of chloroquine across transfected cell monolayer was 2.2 fold lower than the wild type MDCK cell monolayer (Figure 6A). In the case of salicylic acid cumulative % transport across both cell types were indistinguishable (Figure 6B).
Figure 6.
Transepithelial transport of chloroquine is several folds lower in P-MDCK cells than MDCK cells. Transport of chloroquine and salicylic acid was assessed using P-MDCK and MDCK cell monolayers grown on transwell filter for 6 days in the presence of 2 mM L-tyrosine. Data are expressed as mean ± SD for n = 6. *P < 0.05 when compared with MDCK cells.
DISCUSSION
One important aspect of our study was to develop and validate an in vitro cell culture based model for outer BRB in order to understand the influence of melanin binding on solute transport. To this end, a MDCK cell line that stably expresses human tyrosinase and p-protein was established and characterized.
A very tight polarized barrier (high TEER value) and the presence of melanin pigment are two main prerequisites of the outer BRB formed by RPE. The immortalized human RPE cell line; ARPE-19, is a most commonly used in vitro model for both pathophysiological as well as drug delivery studies involving the outer BRB. Although ARPE-19 is a commonly used in vitro model for RPE cells, this cell line is not able to achieve as high a TEER value (exhibits TEER <200 Ω.cm2)12 as compared to primary cultured human RPE cells (800 Ω.cm2)13 and is devoid of melanin pigment for several months26. The MDCK (NBL-2) cell line, derived from dog kidney, is capable of forming a very tight polarized barrier within 4 to 6 days of growth.27, and also expresses many influx and efflux drug transporters28. One of the major advantages of MDCK (NBL-2) cells over other epithelial cells is short cultivation time that results in significant reduction in labor as well as chances of cell contamination. This ultimately leads to a reduction in cost and an increase in assay throughput29. MDCK cells form tight junction with TEER value of > 500 Ω.cm2 within 4 days of growth on transwell filters, whereas Caco-230 and HCET (human corneal cells)31 take more than 2 weeks to achieve stable TEER. Further, a patent from Rusinko et al.32 showed a good correlation between human corneal permeability and permeability of fluoroquinolones across MDCK cells and suggested that MDCK cells can be used as an in vitro model for predicting the corneal permeability of drug molecules. In general, it is our opinion that passive permeability of various epithelial barriers may be comparable, once the differences in tight junctional properties are accounted for. Thus, passive permeability across MDCK is expected to be comparable to RPE, provided the pigment influence is also accounted for since RPE distinguishes itself from several other epithelial due to its pigmented nature.
Stably transfected MDCK cells (P-MDCK) showed expression of tyrosinase, p-protein, and synthesis of melanin pigment. Expression of tyrosinase was characterized by both immunocytochemistry as well as tyrosine assay. Immunocytochemistry showed the localization of tyrosinase in cytoplasm with diffuse granular pattern (Figure 2). In a previously published report employing human epithelial 293 cells and MDCK cells, localization of tyrosinase in cytoplasm was observed14, 29, similar to what we observed in our experiments18, 33. Using late endosome/lysosome marker Lamp-2 and CD63 and co-localization of signals, these earlier reports proved that tyrosinase was present in lysosomal compartment18, 33. In our study we did not use any lysosomal marker to confirm the localization of tyrosinase in P-MDCK cells but based on previous reports, tyrosinase is possibly localized to the lysosomal compartment. Functional tyrosinase activity was compared in wild type MDCK, p-protein transfected MDCK, and P-MDCK cells using L-dopa to dopachrome conversion assay. P-MDCK showed 4-fold higher tyrosinase activity than plain MDCK cells and 2.6-fold higher activity than MDCK cells transfected with p-protein only (Figure 3).
In Figure 4 we show that melanin content within our P-MDCK cells correlates with the amount of L-tyrosine present in the media. These data demonstrate that our cells are capable of producing a wide range of melanin content (17 fold difference in this study) through changes in substrate availability. This is consistent with an earlier study employing human epithelial 293 cells, where an increase in melanin quantity was observed with an increase in tyrosine concentration in culture media18. Melanin content in choroid-RPE and the iris/ciliary body is significantly higher in people with brown eyes as compared to blue eyes34. Since melanin content varies significantly among humans, the ability of our cells to produce differing amounts of melanin will prove useful in evaluating the effect of pigment variations.
To understand the influence of melanin pigment on epithelial uptake and transport of solutes, we performed and compared uptake and transport of chloroquine and salicylic acid in P-MDCK and wild-type MDCK cell cultures. Lipophilic and basic drug molecules have high affinity to melanin pigment than hydrophilic and negatively charged drug molecules7, 15, 35, 36. Chloroquine, a positively charged. lipophilic drug molecule24 has high affinity for melanin pigment and is commonly used as a positive control for melanin binding37. In contrast, salicylic acid. a negatively charged hydrophilic molecule25 with a very low affinity and little or no binding to melanin pigment serves as a negative control for melanin binding38. Chloroquine showed 2.3-fold higher uptake in P-MDCK cells as compared to plain MDCK cells (Figure 5). This is consistent with the idea that melanin acts as a sink, allowing greater amounts of chloroquine to remain inside the cell. For salicylic acid, there was no significant difference in uptake between P-MDCK and plain MDCK cells. As salicylic acid has a very low affinity for melanin pigment, this is also consistent with the sink hypothesis for pigment binding drugs. Chloroquine cell uptake was significantly higher than salicylic acid in both plain as well as P-MDCK cells. This is due to the fact that chloroquine is highly lipophilic and basic in nature and accumulates more in cells than salicylic acid. Further, basic, lipophilic drugs such as chloroquine accumulate more in lysosomal compartment of cells than acidic drugs, due to ionization of the drug within the acidic lysosomal environment39, 40.
We found that in vitro transport of chloroquine across the P-MDCK and wild-type cell monolayers showed an inverse relationship with cell uptake (Figure 6A). The reason for a lower transport of chloroquine across P-MDCK cells is likely the accumulation of drug within the cell monolayer through interaction with melanin pigment. This is consistent with our previous reports, which demonstrated that lipophilic drugs accumulate to a greater extent in pigmented choroid-RPE than non-lipophilic drugs, thereby reducing transscleral delivery to the retina and vitreous for lipophilic solutes as compared to non-lipophilic drugs35, 41. Further, as predicted, transport of salicylic acid, with low pigment binding affinity, was not influenced by the presence of melanin pigment in P-MDCK cells. Recently Müller et al. showed that chloroquine is a substrate for human multidrug and toxic compound extrusion (MATE1) transporter42 using MDCK cells expressing human MATE1. However, this prior study indicated lack of transporter mediated uptake for chloroquine in control MDCK cells, suggesting lack of transporter activity in native cells. Thus, although MATE1 transporter mediated uptake and transport is a possible contributor for chloroquine delivery, it is likely non-significant in the control MDCK cells. Further, the expression of transporters is expected to be the same in our P-MDCK and MDCK cells. Therefore, the differences in uptake and transport of chloroquine between the two cell lines in this study are expected to be primarily due to differences in pigment content. Additionally, even carrier mediated transport will be influenced by pigment binding since drug bound to pigment within the cell is unavailable for transport across the cell. Thus, P-MDCK cells serve as a surrogate to understand the influence of melanin pigment on solute transport across the retinal pigment epithelium.
CONCLUSIONS
Transfection of human tyrosinase and p-protein plasmid in wild type MDCK cells results in stable expression of tyrosinase and the synthesis of melanin pigment. P-MDCK cells showed an increase in melanin content, in a tyrosine concentration dependent manner, allowing the maintenance of different pigment levels in the cells. The uptake of chloroquine is significantly higher, whereas the transport across cell monolayer was significantly lower in P-MDCK cells when compared to control cells. P-MDCK cells will be useful as a new in-vitro model for the outer blood-retinal-barrier, especially for assessing passive permeability and the influence of RPE pigmentation on solute transport.
ACKNOWLEDGEMENTS
The authors wish to thank Mr. Donald Wilkerson at University of Colorado Anschutz Medical campus for his critical input during the preparation of this manuscript. The authors are thankful to Dr. Yosef Refaeli of University of Colorado Anschutz Medical Campus for providing some of the resources for the construction of P-MDCK cells.
REFERENCES
- 1.Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv. 2010;1(3):435–456. doi: 10.4155/TDE.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah CP, Garg SJ, Vander JF, Brown GC, Kaiser RS, Haller JA. Outcomes and risk factors associated with endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmology. 2010;118(10):2028–2034. doi: 10.1016/j.ophtha.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Investigative ophthalmology & visual science. 2006;47(3):1149–1160. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzo S, Ebert FG, Bartolo ED, Barca F, Cresti F, Augustin C, Augustin A. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina. 2012;32(4):776–784. doi: 10.1097/IAE.0b013e3182278b0e. [DOI] [PubMed] [Google Scholar]
- 5.Tetz M, Rizzo S, Augustin AJ. Safety of submacular suprachoroidal drug administration via a microcatheter: retrospective analysis of European treatment results. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2012;227(4):183–189. doi: 10.1159/000336045. [DOI] [PubMed] [Google Scholar]
- 6.Amrite A, Pugazhenthi V, Cheruvu N, Kompella U. Delivery of celecoxib for treating diseases of the eye: influence of pigment and diabetes. Expert opinion on drug delivery. 2010;7(5):631–645. doi: 10.1517/17425241003663236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadam RS, Cheruvu NP, Edelhauser HF, Kompella UB. Sclera-choroid-RPE transport of eight beta-blockers in human, bovine, porcine, rabbit, and rat models. Investigative ophthalmology & visual science. 2011;52(8):5387–5399. doi: 10.1167/iovs.10-6233. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Cheruvu NP, Kompella UB. Bovine and porcine transscleral solute transport: influence of lipophilicity and the Choroid-Bruch's layer. Investigative ophthalmology & visual science. 2006;47(10):4513–4522. doi: 10.1167/iovs.06-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Investigative ophthalmology & visual science. 2005;46(2):641–646. doi: 10.1167/iovs.04-1051. [DOI] [PubMed] [Google Scholar]
- 10.Steuer H, Jaworski A, Stoll D, Schlosshauer B. In vitro model of the outer blood-retina barrier. Brain Res Brain Res Protoc. 2004;13(1):26–36. doi: 10.1016/j.brainresprot.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Tugizov S, Maidji E, Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol. 1996;77(Pt 1):61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- 12.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62(2):155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 13.Geisen P, McColm JR, King BM, Hartnett ME. Characterization of barrier properties and inducible VEGF expression of several types of retinal pigment epithelium in medium-term culture. Curr Eye Res. 2006;31(9):739–748. doi: 10.1080/02713680600837408. [DOI] [PubMed] [Google Scholar]
- 14.Mannermaa E, Reinisalo M, Ranta VP, Vellonen KS, Kokki H, Saarikko A, Kaarniranta K, A U. Filter-cultured ARPE-19 cells as outer blood-retinal barrier model. Eur J Pharm Sci. 2010;40(4):289–296. doi: 10.1016/j.ejps.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Pescina S, Santi P, Ferrari G, Padula C, Cavallini P, Govoni P, Nicoli S. Ex vivo models to evaluate the role of ocular melanin in trans-scleral drug delivery. Eur J Pharm Sci. 2012;46(5):475–483. doi: 10.1016/j.ejps.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Hirosaki K, Yamashita T, Wada I, Jin HY, Jimbow K. Tyrosinase and tyrosinase-related protein 1 require Rab7 for their intracellular transport. J Invest Dermatol. 2002;119(2):475–480. doi: 10.1046/j.1523-1747.2002.01832.x. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa T, Matsuzaki M, Takeda A, Kikuchi A, Furukawa K, Shibahara Sea. Increased dopamine and its metabolites in SH-SY5Y neuroblastoma cells that express tyrosinase. J Neurochem. 2003;87(2):470–475. doi: 10.1046/j.1471-4159.2003.02008.x. [DOI] [PubMed] [Google Scholar]
- 18.Ni-Komatsu L, Orlow SJ. Heterologous expression of tyrosinase recapitulates the misprocessing and mistrafficking in oculocutaneous albinism type 2: effects of altering intracellular pH and pink-eyed dilution gene expression. Exp Eye Res. 2006;82(3):519–528. doi: 10.1016/j.exer.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard B, Fuller BB, Vijayasaradhi S, Houghton AN. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J Exp Med. 1989;169(6):2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell. 2002;13(6):1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyofuku K, Valencia JC, Kushimoto T, Costin GE, Virador VM, Vieira WD, Ferrans VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA) type II: the pink protein modulates the processing and transport of tyrosinase. Pigment Cell Res. 2002;15(3):217–224. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 22.Swift S, Lorens J, Achacoso P, Nolan GP. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Curr Protoc Immunol. 2001;Chapter 10(Unit 10):17C. doi: 10.1002/0471142735.im1017cs31. [DOI] [PubMed] [Google Scholar]
- 23.Donatien P, Jeffery G. Correlation between rod photoreceptor numbers and levels of ocular pigmentation. Investigative ophthalmology & visual science. 2002;43(4):1198–1203. [PubMed] [Google Scholar]
- 24.McLure JA, Birkett DJ, Elliot DJ, Williams JA, Rowland A, Miners JO. Application of the fluorescent probe 1-anilinonaphthalene-8-sulfonate to the measurement of the nonspecific binding of drugs to human liver microsomes. Drug metabolism and disposition: the biological fate of chemicals. 2011;39(9):1711–1717. doi: 10.1124/dmd.111.039354. [DOI] [PubMed] [Google Scholar]
- 25.Johnson ML, Uhrich KE. Concurrent release of admixed antimicrobials and salicylic acid from salicylate-based poly(anhydride-esters) J Biomed Mater Res A. 2009;91(3):671–678. doi: 10.1002/jbm.a.32288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biesemeier A, Kreppel F, Kochanek S, Schraermeyer U. The classical pathway of melanogenesis is not essential for melanin synthesis in the adult retinal pigment epithelium. Cell Tissue Res. 2010;339(3):551–560. doi: 10.1007/s00441-009-0920-9. [DOI] [PubMed] [Google Scholar]
- 27.Cho MJ, Thompson DP, Cramer CT, Vidmar TJ, Scieszka JF. The Madin Darby canine kidney (MDCK) epithelial cell monolayer as a model cellular transport barrier. Pharmaceutical research. 1989;6(1):71–77. doi: 10.1023/a:1015807904558. [DOI] [PubMed] [Google Scholar]
- 28.Putnam WS, Ramanathan S, Pan L, Takahashi LH, Benet LZ. Functional characterization of monocarboxylic acid, large neutral amino acid, bile acid and peptide transporters, and P-glycoprotein in MDCK and Caco-2 cells. Journal of pharmaceutical sciences. 2002;91(12):2622–2635. doi: 10.1002/jps.10264. [DOI] [PubMed] [Google Scholar]
- 29.Balimane PV, Chong S. Cell culture-based models for intestinal permeability: a critique. Drug Discov Today. 2005;10(5):335–343. doi: 10.1016/S1359-6446(04)03354-9. [DOI] [PubMed] [Google Scholar]
- 30.Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Grove JR. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. Journal of pharmaceutical sciences. 1999;88(1):28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- 31.Xiang CD, Batugo M, Gale DC, Zhang T, Ye J, Li C, Zhou S, Wu EY, Zhang EY. Characterization of human corneal epithelial cell model as a surrogate for corneal permeability assessment: metabolism and transport. Drug metabolism and disposition: the biological fate of chemicals. 2009;37(5):992–998. doi: 10.1124/dmd.108.026286. [DOI] [PubMed] [Google Scholar]
- 32.Rusinko A, Hellberg MR, May JA, Owen GR. Use of MDCK cell line to predict corneal penetration of drugs. 2007 [Google Scholar]
- 33.Simmen T, Schmidt A, Hunziker W, Beermann F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J Cell Sci. 1999;112(Pt 1):45–53. doi: 10.1242/jcs.112.1.45. [DOI] [PubMed] [Google Scholar]
- 34.Menon IA, Wakeham DC, Persad SD, Avaria M, Trope GE, Basu PK. Quantitative determination of the melanin contents in ocular tissues from human blue and brown eyes. J Ocul Pharmacol. 1992;8(1):35–42. doi: 10.1089/jop.1992.8.35. [DOI] [PubMed] [Google Scholar]
- 35.Kadam RS, Kompella UB. Influence of lipophilicity on drug partitioning into sclera, choroid-retinal pigment epithelium, retina, trabecular meshwork, and optic nerve. The Journal of pharmacology and experimental therapeutics. 2010;332(3):1107–1120. doi: 10.1124/jpet.109.161570. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Zane PA, Brindle SD, Gause DO, O'Buck AJ, Raghavan PR, Tripp SL. Physicochemical factors associated with binding and retention of compounds in ocular melanin of rats: correlations using data from whole-body autoradiography and molecular modeling for multiple linear regression analyses. Pharmaceutical research. 1990;7(9):935–941. doi: 10.1023/a:1015997823755. [DOI] [PubMed] [Google Scholar]
- 37.Ono C, Tanaka M. Binding characteristics of fluoroquinolones to synthetic levodopa melanin. The Journal of pharmacy and pharmacology. 2003;55(8):1127–1133. doi: 10.1211/002235703322277168. [DOI] [PubMed] [Google Scholar]
- 38.Sauer MJ, Anderson SP. In vitro and in vivo studies of drug residue accumulation in pigmented tissues. Analyst. 1994;119(12):2553–2556. doi: 10.1039/an9941902553. [DOI] [PubMed] [Google Scholar]
- 39.Kornhuber J, Henkel AW, Groemer TW, Stadtler S, Welzel O, Tripal P, Rotter A, Bleich S, Trapp S. Lipophilic cationic drugs increase the permeability of lysosomal membranes in a cell culture system. J Cell Physiol. 2010;224(1):152–164. doi: 10.1002/jcp.22112. [DOI] [PubMed] [Google Scholar]
- 40.Goldman SD, Funk RS, Rajewski RA, Krise JP. Mechanisms of amine accumulation in, and egress from, lysosomes. Bioanalysis. 2009;1(8):1445–1459. doi: 10.4155/bio.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadam RS, Cheruvu NP, Edelhauser HF, Kompella UB. Sclera-choroid-RPE Transport of Eight Beta-blockers in Human, Bovine, Porcine, Rabbit, and Rat Models. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-6233. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Muller F, Konig J, Glaeser H, Schmidt I, Zolk O, Fromm MF, Maas R. Molecular mechanism of renal tubular secretion of the antimalarial drug chloroquine. Antimicrob Agents Chemother. 2011;55(7):3091–3098. doi: 10.1128/AAC.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]