Abstract
Background
Although caries is prevalent in adults, few preventive therapies have been tested in adult populations. This randomized clinical trial evaluated the effectiveness of xylitol lozenges in preventing caries in elevated caries-risk adults.
Methods
X-ACT was a three-site placebo-controlled randomized trial. Participants (n=691) ages 21–80 consumed five 1.0 g xylitol or placebo lozenges daily for 33 months. Clinical examinations occurred at baseline, 12, 24 and 33 months.
Results
Xylitol lozenges reduced the caries increment 11%. This reduction, which represented less than one-third of a surface per year, was not statistically significant. There was no indication of a dose-response effect.
Conclusions
Daily use of xylitol lozenges did not result in a statistically or clinically significant reduction in 33-month caries increment among elevated caries-risk adults.
Clinical Implications
These results suggest that xylitol used as a supplement in adults does not significantly reduce their caries experience.
Keywords: Xylitol, Dental Caries Prevention, Randomized Controlled Trial, Adults
Many adults continue to develop dental caries throughout their lifespan, and caries activity in this population is at least as extensive as it is in children and adolescents.1 However, dental caries prevention efforts have historically focused on children rather than adults. Public oral health programs targeting caries prevention for adults are uncommon, and provision of caries preventive treatment to adult dental patients at elevated caries-risk is relatively infrequent.2,3 A possible contributing reason for this lack of attention may be that knowledge of effectiveness of caries prevention methods for adults is incomplete. The 2001 NIH Consensus Development Conference Statement on the Diagnosis and Management of Dental Caries Throughout Life expressed concern over the paucity of studies in adults, noting that “almost all of the relevant studies included populations of children between 6 and 15 years of age.”4 Both this report and a Centers for Disease Control and Prevention report that same year recommended evaluation of several preventive interventions in adult populations, particularly adults at elevated risk of caries.4,5
One potentially effective preventive intervention identified in the systematic review prepared for the NIH Consensus Development Conference was xylitol.6 Xylitol acts to reduce levels of mutans streptococci in the plaque and saliva. The efficacy of xylitol-based interventions has been controversial primarily due to differences in opinion regarding the quality of the published trials.7–9 Several reviews of portions of the evidence for the effectiveness of xylitol have appeared over the past decade.10–17 The conclusions of these reviews differ, principally due to their assessments of the quality of the studies. Some conclude that there is evidence for a caries preventive effect of xylitol, and others indicate that the evidence is inconclusive. However, all of the reviews indicate that the existing evidence should be supplemented by well-designed randomized controlled trials. This is the motivation for the Xylitol for Adult Caries Trial (X-ACT). X-ACT tested the hypothesis that daily use of xylitol lozenges reduces dental caries incidence in adults at elevated risk of caries.
Methods
Because a detailed description of study methods appeared previously,18 they are only summarized here, together with one important change in the analysis plan.
Study Design
X-ACT was a 33 month, multi-center, placebo-controlled, double-blind, Phase III randomized clinical trial that tested the effectiveness of daily xylitol lozenge use (up to 5 grams /day) versus placebo lozenge use to prevent caries in adults at elevated risk of caries. The primary outcome was the increment of cavitated lesions. The trial randomized 691 participants, 92% of the goal of 750, with assignments stratified by site and age, and randomly allocated in blocks of varying size within strata.
The trial was conducted at three clinical centers located in the dental schools of the University of North Carolina-Chapel Hill (UNC), the University of Alabama-Birmingham (UAB), and the University of Texas Health Sciences Center-San Antonio (UTHSCSA). The Kaiser Permanente Center for Health Research-Portland OR served as the Data Coordinating Center (DCC). The study was approved by the Institutional Review Board at each site and all participants provided written informed consent.
Study Procedures
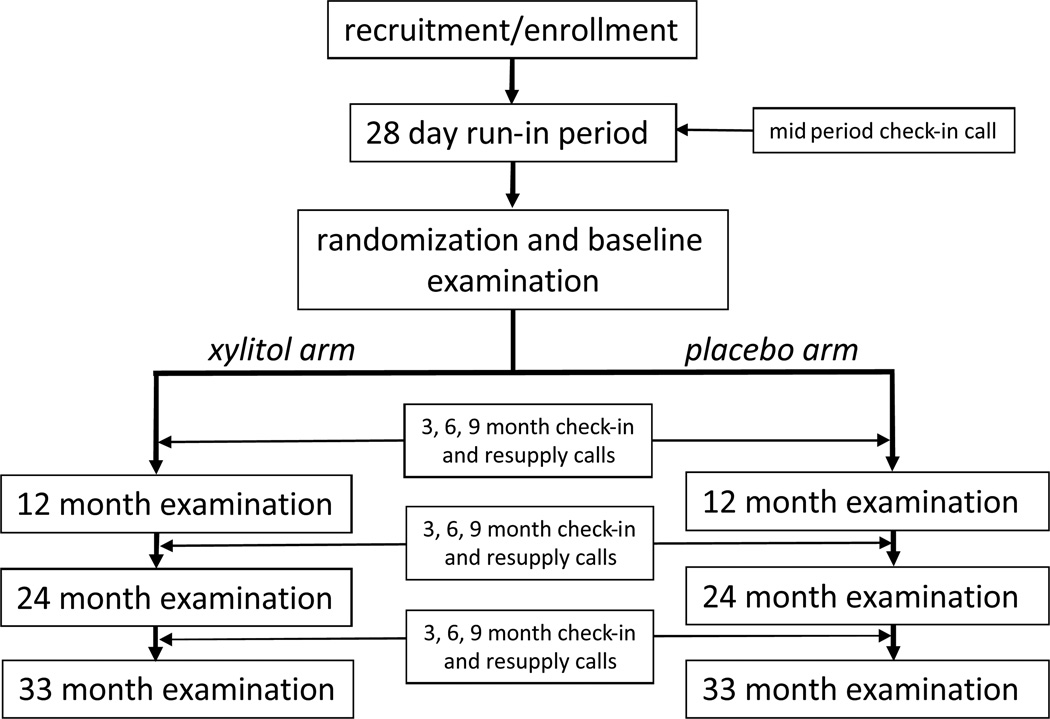
Figure 1 summarizes the trial events from a participant’s perspective. The study required a participant to visit the dental school a minimum of 5 times. Initial telephone or in-person screening preceded an enrollment visit to confirm eligibility and obtain consent, which was followed by a 4-week run-in period (using placebo lozenges) to allow both potential participants and study coordinators to evaluate willingness to adhere to the study regimen.19 Adherent, eligible participants were randomized at a baseline visit and scheduled to return at 12 months, 24 months and 36 months for caries examinations. Before the 24-month visits began, the final examination schedule was shifted to 33 months, to adjust for slower than expected completion of enrollment. Quarterly telephone contacts occurred between the annual follow-up visits to arrange for re-supply of lozenges, inquire about adherence, assess side effects, and screen for possible serious adverse events (SAE).
Figure 1.
Sequence of Trial Events
Study Population
Inclusion criteria were age 21–80 years; the presence of at least one coronal or root surface cavitated caries lesion (present at screening, or documented in the patient record or by self-report as having been restored in the previous year); presence of at least 12 teeth; ability to read and understand English; and ability to give informed consent. Exclusion criteria were the presence of extensive caries (more than 10 teeth with lesions); periodontal disease requiring aggressive treatment; residing in same household with another participant; or anticipated move within three years.
Study Treatments
The intervention consisted of five lozenges spaced across the day and dissolved in the mouth. Each active lozenge contained 1.0 g of xylitol as a sweetening agent. The placebo lozenge was identical in size and color to the active lozenge but was sweetened with sucralose, which lacks any plausible biologic cariostatic or cariogenic properties other than sugar substitution. Both the active and placebo lozenges were peppermint-flavored. A second placebo with different flavoring was used for the run-in period. At baseline, sufficient lozenges for three months were provided to participants (seven containers of 75 lozenges), with the quarterly telephone contacts used to determine the number of additional containers needed for the next three months.
Caries Examinations
Trained and calibrated examiners visually identified caries lesions by using a CPITN-E probe, a non-magnifying plane mirror, and standard dental operating light and chair. Loupes were used at the discretion of the examiner, but consistently within each examiner. Tooth surfaces were dried for five seconds with an air/water syringe. Examiners used a modification of the International Caries Detection and Assessment System II (ICDAS II) criteria,20 with four disease levels possible for each coronal surface; sound (S), non-cavitated enamel lesion (D1)(ICDAS codes 1&2), cavitated lesion penetrating the enamel, or shadowing (D2)(ICDAS codes 3&4), and cavitated lesion penetrating into the dentin (D3)(ICDAS codes 5&6). Root surfaces were scored as sound (S), lesion with estimated depth <0.5mm (D1), and lesion with estimated depth ≥0.5mm (D2). Other surface conditions noted were pits and fissure sealants (P), restorations (F), crowns (C), missing teeth (M), and surfaces unable to score (Y). Examiners made one classification per tooth surface, and each tooth was deemed to have five coronal (including the incisal) surfaces and four root surfaces.
A primary examiner at each clinical center completed almost all examinations, 100% at UAB, 98% at UNC, and 96% at UTHSC-SA, although back-up examiners were available as needed. To the extent possible, all follow-up examinations were performed by the same examiner who conducted the baseline examination. A recorder was present for all caries examinations. Primary and back-up examiners and recorders from all three clinical centers participated in a four-day training and calibration session with a gold standard examiner,21 as well as refresher sessions prior to the 12-, 24-, and 33-month examinations. All primary examiners completed second examinations of approximately 5% of participants annually to determine intra-examiner reliability. Secondary examiners also examined these participants.
Study Outcomes and Statistical Power
The primary study outcome was the cumulative Decayed or Filled Surface (D2 FS) increment (root and coronal surfaces combined, D2 and D3 lesions combined for coronal surfaces) from baseline through the three follow-up examinations, which we expressed in terms of an annualized increment. In the event of missed visits, we based the increment on the observable transitions, so that only participants with no follow-up visits (5% of all randomized participants) had missing increment scores. The D2 FS increment was computed as the weighted sum of changes in surface status associated with 64 pre-defined transitions in tooth-surface status.18 Reversals were considered invalid transitions and scored 0 (effectively excluding them from the analyses). Null transitions (e.g., F to F), transitions from D2 to treated status (F or C), or to or from an unscorable status (i.e., Y or M) were also scored 0. Transitions from sound (S) to crowned (C) were scored 0 to minimize the influence of non-caries–related treatment on increment counts. The study was designed to provide 80% power to detect a 20% reduction in the D2FS increment assuming a two-tailed test with type 1 error rate of 5%. The target sample size of 750 allowed a 10% attrition rate per year.
Covariates
We created several measures to characterize participants’ baseline oral health and oral healthcare practices. These included a binary indicator of a routine dental visit (exam or cleaning) in the past year; a three-level indicator of over-the-counter (OTC) fluoride use (toothpaste, mouthrinse, or both), a five-level indicator of oral hygiene practices (from infrequent to frequent brushing and flossing) and the baseline D2FS count. We also assessed participants’ adherence by comparing the number of containers of resupply lozenges to the number that would be used under ideal adherence. Forms used for all data collection are available on the study’s public website, http://www.xactstudy.org.
Safety Monitoring
We probed for hospital admissions, gastrointestinal (GI) symptoms, and mouth symptoms at three-month intervals throughout the study. We also asked participants to characterize symptoms as severe or not severe.
Statistical Methods
We multiply imputed missing data using data augmentation with Markov Chain Monte Carlo sampling (SAS® 9.1 PROC MI). We used an inclusive approach22 to imputation that included a wide range of baseline and interim variables to minimize bias. All analyses were carried out identically on all 8 copies of the imputed data and then combined according to Rubin's rules23 to adjust statistical test results for the uncertainty inherent in the imputation process.
We conducted the primary outcome analysis on the intent-to-treat (ITT) sample using negative binomial regression to model the ln(annual incidence) as a function of xylitol arm while adjusting for clinical center, age, age-squared, dental cleaning history, OTC fluoride use, oral hygiene practices, and severity of baseline caries (D2FS). The regression coefficients from this model have the interpretation of ln(rate ratios), but have been re-expressed as rate ratios (RR) for the tables, which can be interpreted as relative risks. We included the natural log of person-years at risk as an offset to adjust for varying length of time from randomization to last oral examination obtained across participants.
During the final study year, the DCC uncovered irregularities in participant data at the UTHSCSA site. After subsequent investigation, the study’s Data Safety and Monitoring Board (DSMB) deemed that, although the caries examination data appeared sound, the adherence data were unreliable. In addition, 10 individuals who were household members of existing participants had been randomized in violation of the protocol. The DSMB also could not discount the possibility that some participants received incorrect lozenges. The DSMB therefore recommended that the primary analysis be based on data from only the unaffected sites, UNC and UAB, while secondary analyses could use the caries examination data from all three sites. They also agreed that, per protocol, the 10 participants who had been randomized in error should be excluded from all analyses. Because its adherence data were unusable, missing data from the UTHSCSA site were not imputed. Thus, secondary analyses that include the UTHSCSA data do not include 12 UTHSCSA participants with no follow-up data, 10 participants who had been randomized in error, and an additional 5 participants with missing covariate data.
Additional secondary analyses reported here assess the effect of xylitol in subgroups defined by baseline D2FS score, baseline D2S score, adherence to the study protocol (i.e., lozenge usage), sex, and race/ethnicity. For the D2FS score we used a binary split (0–20 vs. 21+, roughly a median split), while for D2S we defined groups as none vs. any, and for adherence we defined three levels (0–40%, 41–80%, and 81–100%, corresponding to roughly 20%, 40%, and 40% of the sample) based on the quantity of lozenges supplied as a percentage of the quantity that would have been used with perfect adherence. For the primary examiners, we report intra-examiner reliability scores based on a roughly 5% convenience sample of participants who returned for repeat examinations during the study. We report simple pairwise kappa statistics for each year for the combined examiners’ reliability in distinguishing D2D3 lesions from all other surface conditions.
Results
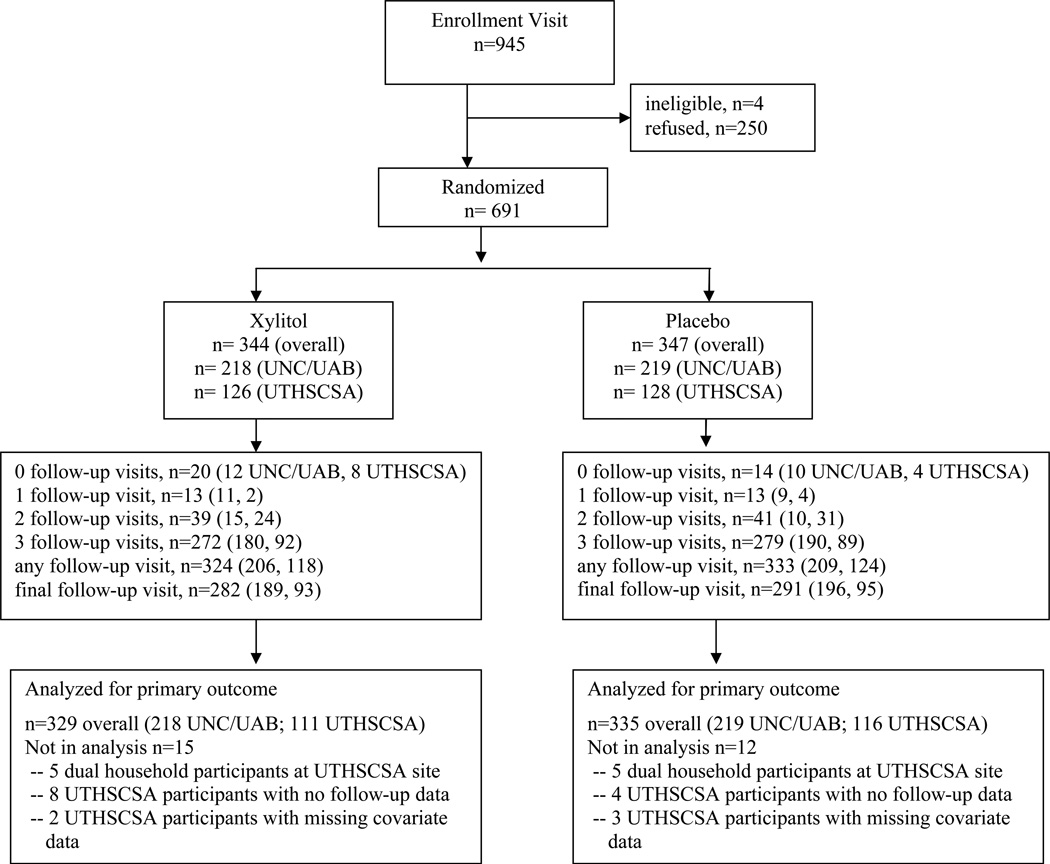
Figure 2 indicates the flow of participants through the study. We screened informally in clinics and did not record these contacts. Of 945 participants who attended the enrollment visit, 691, 73%, were ultimately randomized (344 in the xylitol arm and 347 in the placebo arm). Of the 254 who were not randomized, 81 withdrew during the run-in period, and 173 elected not to continue after completing the run-in period. Mean duration of follow-up was 2.56 years for UNC and UAB participants, and loss to follow-up was balanced between study arms.
Figure 2.
Participant Flow
Participant characteristics are shown in Table 1. Mean age was 47 years (range 21–80), and 65% were female. The majority brushed at least twice/day, and were exposed to fluoride through office visits or toothpaste, or both (all three sites were fluoridated). A small minority reported having dry mouth. No marked differences were noted between the xylitol and placebo groups in these observations. We observed similar participant characteristics among the primary analysis sample (the 437 randomized participants from UNC and UAB—tables for each site are available at http://www.xactstudy.org).
Table 1.
Baseline Participant Characteristics
| Xylitol | Placebo | Total | |
|---|---|---|---|
| All sites | n=339 | n=342 | n=681 |
| Race/Ethnicity | |||
| Non-Hispanic white | 46.6% | 50.3% | 48.5% |
| Non-Hispanic black | 27.1% | 25.4% | 26.3% |
| Hispanic | 23.0% | 21.4% | 22.2% |
| Other | 3.0% | 2.9% | 2.9% |
| Age | 46.3 (13.5)1 | 47.7 (13.7)1 | 47.0 (13.6)1 |
| Female | 62.2% | 67.0% | 64.6% |
| Brushes 2+ times/day | 63.1% | 69.3% | 66.2% |
| Flosses 1+ times/day | 47.5% | 46.8% | 47.1% |
| Routine (exam/clean) dental visit in past year | 32.2% | 30.1% | 31.6% |
| Self-report dry mouth | 4.7% | 9.1% | 6.9% |
| Extent of fluoride exposure | |||
| Toothpaste or prof. topical fluoride | 52.8% | 60.3% | 56.5% |
| Both toothpaste and prof. topical fluoride | 37.5% | 31.0% | 34.2% |
Standard deviation
Examiner Reliability
The target intra-examiner reliability value (unweighted kappa; D2D3 versus all else) for primary examiners during examiner training was 0.70. The mean value achieved for the three primary examiners was 0.72.18 The mean values achieved during the participant examinations were 0.58, 0.88, 0.67, and 0.71 for the baseline and 12-, 24-, and 33-month examinations, respectively. The baseline value was lower primarily due to extremely small numbers of observed lesions at one site.
Primary Outcome Analysis
Among participants from the UNC and UAB sites, the crude annualized D2FS increment in the xylitol group was 2.69 compared to 2.98 in the placebo group, a 10% lower increment. This difference did not achieve statistical significance (95% confidence interval (CI) for RR = 0.78, 1.03). The full regression model (not shown) indicated that annualized caries increments increased significantly with increasing baseline D2FS score, but did not differ by site, age, routine dental visit history, OTC fluoride use, or frequency of oral hygiene (full regression model tables for all analyses are available at http://www.xactstudy.org). Similar results were seen when the UTHSCSA data were included (Table 2).
Table 2.
Summary of Principal Outcome Analysis for D2FS increment
| Xylitol | Placebo | Rate Ratio1 | 95% CI1 | p-value1 | |
|---|---|---|---|---|---|
| Excluding UTHSCSA | |||||
| n=218 | n=219 | ||||
| follow-up (years)2,3 | 2.54 (.55) | 2.58 (.50) | |||
| baseline D2FS score2 | 21.3(13.6) | 21.4 (13.3) | |||
| annualized D2FS increment4 | 2.69 | 2.98 | 0. 90 | (0.78, 1.03) | 0.14 |
| Including UTHSCSA | |||||
| n=331 | n=338 | ||||
| follow-up (years)2,3 | 2.55 (.49) | 2.57 (.47) | |||
| baseline D2FS score2 | 18.8 (12.8) | 18.5 (12.5) | |||
| annualized D2FS increment4,5 | 2.64 | 2.96 | 0.89 | (0.80, 1.01) | 0.063 |
based on negative binomial model adjusting for age at randomization, age-squared, dental cleaning or exam in the last 12 months, OTC fluoride use, usually brush/floss, baseline severity, site
data expressed as mean (SD)
time from baseline to last observed follow-up visit
includes imputed data (from baseline to first annual visit) for 22 individuals from UAB or UNC with no follow-up data
based on analysis excluding an additional 5 individuals with missing covariate data
Subgroup Analyses
Because baseline D2FS score was such a highly statistically significant covariate in the primary analysis, we conducted a secondary analysis that assessed treatment effects separately for those with baseline D2FS scores of 20 or less and those with scores of 21 or greater. We observed no evidence of a treatment effect in the former group (RR=1.00), but a significant effect in the higher D2FS group (RR=0.80, p=0.027) (Table 3). However, when the UTHSCSA data were included, the treatment effect was reduced in the high D2FS group and it was no longer statistically significant (RR=0.83, p=0.053) (Table 3). In both analyses, the interaction p-value exceeded 0.12.
Table 3.
Summary of Analyses for Selected Subgroups
| Xylitol | Placebo | Rate Ratio1 | 95% CI1 | p-value1 | |
|---|---|---|---|---|---|
| Excluding UTHSCSA | |||||
| Baseline D2FS | |||||
| 0–20 D2FS | n=109 | n=119 | |||
| annualized D2FS increment | 2.23 | 2.24 | 1.00 | (0.81, 1.22) | 0.96 |
| 21+ D2FS | n=109 | n=100 | |||
| annualized D2FS increment | 3.37 | 4.21 | 0.80 | (0.61, 0.99) | 0.027 |
| Baseline D2S | |||||
| 0 D2S | n=94 | n=94 | |||
| annualized D2FS increment | 2.34 | 2.75 | 0.85 | (0.68, 1.07) | 0.16 |
| 1+ D2S | n=124 | n=125 | |||
| annualized D2FS increment | 3.07 | 3.23 | 0.95 | (0.75, 1.15) | 0.62 |
| Lozenge Adherence | |||||
| 0–40% | n=42 | n=46 | |||
| annualized D2FS increment | 2.57 | 2.96 | 0.87 | (0.58, 1.30) | 0.48 |
| 41–80% | n=72 | n=96 | |||
| annualized D2FS increment | 2.50 | 2.90 | 0.86 | (0.66, 1.07) | 0.16 |
| 81–100% | n=104 | n=77 | |||
| annualized D2FS increment | 2.85 | 3.09 | 0.92 | (0.73, 1.12) | 0.43 |
| Including UTHSCSA | |||||
| Baseline D2FS | |||||
| 0–20 D2FS | n=194 | n=211 | |||
| annualized D2FS increment | 2.22 | 2.36 | 0.94 | (0.80, 1.10) | 0.44 |
| 21+ D2FS | n=135 | n=124 | |||
| annualized D2FS increment | 3.57 | 4.28 | 0.83 | (0.65, 1.02) | 0.053 |
| Baseline D2S | |||||
| 0 D2S | n=114 | n=117 | |||
| annualized D2FS increment | 2.30 | 2.72 | 0.85 | (0.68, 1.05) | 0.13 |
| 1+ D2S | n=215 | n=218 | |||
| annualized D2FS increment | 3.02 | 3.18 | 0.95 | (0.79, 1.11) | 0.51 |
based on negative binomial model adjusting for age at randomization, age-squared, dental cleaning or exam in the last 12 months, OTC fluoride use, usually brush/floss, baseline severity, site
To investigate whether this apparent subgroup effect might be more closely related to lesions at baseline, we conducted a similar analysis for the D2S score (Table 3). Here neither the group with no D2 surfaces at baseline nor the group with one or more D2 surfaces at baseline displayed a significant treatment effect. Similar results were found when the analyses were re-run including the UTHSCSA data.
We evaluated treatment effects in subgroups defined by adherence to the 5x daily lozenge protocol (Table 3). We found a non-significant RR associated with lozenge use in all three adherence groups, and no evidence of any treatment-by-dose interaction. We also observed no effect of adherence on D2FS increment in analyses restricted to placebo group participants. Similar results were observed when UTHSCSA data were included. Finally, when we examined treatment effects by sex and race/ethnicity, we found no difference between males and females in either the two or three site samples, but we did find that the treatment effect was significant for non-Hispanic whites, but not for Hispanics or Others (primarily African Americans) in the two site sample but not in the three site sample. These analyses are shown in the public website.
Safety Outcomes
Including the UTHSCSA data, randomized participants reported a total of 46 SAEs (22 in the xylitol arm and 24 in the placebo arm). Of these, one (a diagnosis of longstanding gastroparesis) was considered possibly study-related while an additional three were classified as unknown study-relatedness because participants declined to provide information. In addition, severe oral side-effects were reported at rates of less than 0.5% for all reporting periods, while severe GI side-effects were reported at a rate of 1% or less during the study. Reports of any oral or GI side-effects ranged from 3.2% to 8.4% for the mouth and from 3.3% to 11.4% for the GI tract over the duration of the study. The majority of oral side-effects were sores, while GI side-effects were distributed across reports of cramps, bloating, constipation, flatulence, and loose stool/diarrhea. For all effects, patterns of were similar for the two study groups.
Discussion
This clinical trial did not demonstrate a statistically significant reduction in 33 month caries incidence in either the primary analysis or in the secondary analysis that included all three sites. This finding is at odds with some reviews of xylitol studies that conclude definitively that xylitol is effective in reducing caries increments,10,12,14,,15,17 but supports other reviews concluding that the evidence is inconclusive, or that xylitol has little or no effect on caries increments.11,13,16 It is important to realize that this is the first large-scale, placebo-controlled, multi-site, randomized, double-blind study of xylitol as a caries preventive agent. All previous studies lacked one or more of these essential features intended to minimize the likelihood of bias.24 The study also differs from most previous reports in that it evaluated lozenges (mints), rather than chewing gum, thereby rendering inoperative one possible method of action, mechanical plaque removal.25 Mints may be less effective than gum for this reason. Also, and perhaps most importantly, this study evaluated xylitol in adults, rather than children and adolescents, the subjects of virtually every preceding xylitol study. Finally, the majority of the participants lived in fluoridated areas, used fluoridated toothpaste, and, due to our recruitment strategies, could be characterized as recent dental attenders.18 The results of this study help explain the existing controversy regarding xylitol’s effectiveness. At best, xylitol’s effect appears to be modest in this study group. We observed a reduction in the risk of dental caries on the order of 10%, which was not statistically significant. Such a small effect would be easy to miss in some trials, and, perhaps in other trials, open to exaggeration through the unintentional incorporation of bias. We saw essentially the same magnitude of risk reduction at each of the three sites, which gives us confidence that our methods, especially our caries measurements, were well-standardized.
Among this group of adults at elevated risk of caries, this reduction amounts to 0.29 “surfaces saved” per year. However, our observed crude increments, on the order of 3 decayed and/or filled surfaces per year, are higher than what would be expected from the literature we reviewed to estimate the power of our study18 because we chose not to adjust the increments for “reversals,” i.e., improbable calls.18 Thus, the “surfaces saved” estimate would be lower if we had adjusted for reversals. While not negligible, the magnitude of surfaces saved is not encouraging for the widespread adoption of xylitol as either a public health measure or a dentist-recommended supplemental preventive intervention for adults at elevated risk of caries who already use fluoride toothpaste.
We did find a significant reduction in the incidence of caries when we analyzed the treatment effect in subgroups stratified by baseline D2FS. Those with D2FS scores higher than 20 experienced a reduction of 20%, while those with D2FS scores of 20 or below showed essentially no reduction. However, this reduction was not statistically significant when the UTHSCSA participants were included in the analysis. When we explored this relationship further by stratifying participants by their baseline D2 counts, we observed no similar trend toward increased effectiveness in those with any, versus no cavitated lesions at baseline. Thus, at two sites, those with more filled surfaces seemingly experience a greater reduction in caries incidence with the use of xylitol, but those with active disease do not enjoy any increased effect. We speculate that we have observed this contradictory effect because, in this group of individuals who have received recent dental care, D2FS may be a better reflection of recent caries activity than the presence of cavitated lesions.
We also observed no apparent xylitol dose-response effect, nor did we observe an effect associated with adherence in the placebo group. In the placebo group, the extra salivary stimulation from consuming more lozenges apparently was insufficient to affect caries incidence. Similarly, exposure to increasing amounts of xylitol also had no additive effect on caries incidence. It is possible that the trial protocol specified a daily dose that is below an as-yet-undetermined threshold of daily consumption for effectiveness. At the time the study was designed, the typical daily dose in reported studies was between 3 and 5 gr/day, and the range was 0.7 to 7.0 gr/day.13 We chose the “high side” of the range to ensure that the trial would provide an effective dose. Further we recommended 5 one-lozenge doses to minimize under-dosing if one dose was missed. Recently, a threshold dose of 5–6 grams daily has been suggested.26 However, because the most compliant participants were consuming xylitol near or at this threshold, some effect might be expected. Another explanation might be that frequency of exposure is more important than total dose. Because even moderately compliant subjects would likely experience the recently suggested three daily exposures,25 exposure-response would be difficult to detect. In any event, dosage protocols requiring more than 5 grams daily and/or near-perfect adherence may well mean that benefits experienced in practice will not approach the optimum benefit possible.
Future trials of caries prevention interventions in adults can benefit from the experiences in this trial. We included only adults at elevated risk of caries, defined as having or having recently had a cavitated lesion. However, the presence of one lesion may not necessarily indicate a true “high-risk” individual. Thus, trials seeking to assess effectiveness in high-risk populations may need to establish a longitudinal assessment of caries activity. In addition, recruitment of participants from dental clinic populations may mask subtle treatment effects due to extensive restorative activity that may not be caries-related. Our trial experienced excellent participant retention, possibly due both to our use of a run-in period, and to frequent contacts from study coordinators, who deliberately sought to establish continuing relationships with participants. We observed adherence that was higher than what we expected given the length of the trial and the need for daily action. Again we attribute this to pre-selecting adherent individuals and establishing close personal relationships with them.
The results of this trial may not apply to younger populations, in which almost all of the previous research on xylitol as a caries preventive agent has been conducted. Nevertheless, the lack of a statistically significant effect in this elevated caries-risk adult population should serve to temper over-optimistic expectations that xylitol lozenges used as a supplement in patients or the public at large with access to fluoride will substantially reduce their caries experience.
Supplementary Material
Acknowledgement
This work was supported by the following National Institute of Dental and Craniofacial Research grants: U01DE018038,U01DE018047, U01DE018048, U01DE018049, and U01DE018050. These are, respectively, the “Xylitol Adult Caries Trial (X-ACT) Study Chair,”“Xylitol Adult Caries Trial(X-ACT) Data Coordinating Center,” “Xylitol Adult Caries Trial (X-ACT) UNC Clinical Center,” “Xylitol Adult Caries Trial (X-ACT) UAB Clinical Center,” and the “Xylitol Adult Caries Trial (X-ACT) UTHSC San Antonio Clinical Center. We thank the X-ACT Data Safety and Monitoring Board and special thanks go to the X-ACT participants and to Dr. Christina Gullion. In addition to the authors of this paper, the X-ACT collaborative research group is comprised of the following people: From the University of North Carolina – Chapel Hill, NC: Jan Carlton Holland, RDH, MS, Debbie S Robinson, CDA, MS, From the University of Alabama – Birmingham, AL: Mona Z. Anabtawi, DDS, MS. From the University of Texas Health Sciences Center –San Antonio, TX: Anna Theresa Vega, RDA, Bithiah Radcliffe, Belinda Vitolas, RDA From the Coordinating Center at the Kaiser Permanente Center for Health Research, OR: Donna J. Eubanks, BSCS, Kelly Kirk, C. Chen, PhD, Deborah Reck, Jeanette Bardsley, BA, Arthur R. Dixon, MSTM, Elizabeth J. Esterberg, MS. From the NIDCR Project Office: Jane C. Atkinson, DDS.
References
- 1.Thomson WM. Dental caries experience in older people over time: what can the large cohort studies tell us? Brit Dent J. 2004;196:89–92. doi: 10.1038/sj.bdj.4810900. [DOI] [PubMed] [Google Scholar]
- 2.Bader J, Shugars D, White B, Rindal D. Development of effectiveness of care and use of services measures for dental care plans. J Public Health Dent. 1999;59:142–149. doi: 10.1111/j.1752-7325.1999.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 3.Bader J, Shugars D, Kennedy J, Hayden W, Baker S. A pilot study of risk-based prevention in private practice. J Am Dent Assoc. 2003;134:1195–1202. doi: 10.14219/jada.archive.2003.0354. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health; Office of Medical Application of Research. Consensus Development Conference Statement: Diagnosis and Management of Dental Caries Throughout Life. 2001 Mar 26–28; accessed at: http://consensus.nih.gov/2001/2001DentalCaries115html.htm.
- 5.Centers for Disease Control and Prevention. Recommendations for using fluoride to prevent and control dental caries in the United states. MMWR Morb Mortal Wkly Rep. 2001;50(RR-14):1–42. Accessed at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5014a1.htm. [PubMed] [Google Scholar]
- 6.Bader J, Shugars D, Bonito A. A systematic review of selected caries prevention and management methods. Community Dent Oral Epidemiol. 2001;29:399–411. doi: 10.1034/j.1600-0528.2001.290601.x. [DOI] [PubMed] [Google Scholar]
- 7.Scheie A, Fejerskov O. Xylitol in caries prevention: what is the evidence for clinical efficacy. Oral Diseases. 1998;4:268–278. doi: 10.1111/j.1601-0825.1998.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 8.Twetman S. Current controversies--is there merit? Adv Dent Res. 2009;21:48–52. doi: 10.1177/0895937409335624. [DOI] [PubMed] [Google Scholar]
- 9.Makinen K. Xylitol-based caries prevention: is there enough evidence for the existence of a specific xylitol effect? Oral Diseases. 1998;4:226–230. doi: 10.1111/j.1601-0825.1998.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayes C. The effect of non-cariogenic sweeteners on the prevention of dental caries: a review of the evidence. J Dent Educ. 2001;65:1106–1109. [PubMed] [Google Scholar]
- 11.Lingström P, Holm A, Ingegerd M, et al. Dietary factors in the prevention of dental caries: a systematic review. Acta Odont Scand. 2003;61:331–340. doi: 10.1080/00016350310007798. [DOI] [PubMed] [Google Scholar]
- 12.Maguire A, Rugg-Gunn A. Xylitol and caries prevention--is it a magic bullet? Brit Dent J. 2003;194:429–436. doi: 10.1038/sj.bdj.4810022. [DOI] [PubMed] [Google Scholar]
- 13.van Loveren C. Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res. 2004;38:286–293. doi: 10.1159/000077768. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande A, Jadad A. The impact of polyol-containing chewing gums on dental caries. J Am Dent Assoc. 2008;139:1602–1614. doi: 10.14219/jada.archive.2008.0102. [DOI] [PubMed] [Google Scholar]
- 15.Mäkinen KK. Sugar alcohols, caries incidence, and remineralization of caries lesions: a literature review. Int J Dent. 2010;2010:981072. doi: 10.1155/2010/981072. Epub 2010 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonio AG, Pierro VS, Maia LC. Caries preventive effects of xylitol-based candies and lozenges: a systematic review. J Pub Health Dent. 2011;71:117–124. doi: 10.1111/j.1752-7325.2010.00208.x. [DOI] [PubMed] [Google Scholar]
- 17.Rethman MP, Beltrán-Aguilar ED, Billings RJ, Hujoel PP, Katz BP, Milgrom P, Sohn W, Stamm JW, Watson G, Wolff M, Wright JT, Zero D, Aravamudhan K, Frantsve-Hawley J, Meyer DM. American Dental Association Council on Scientific Affairs Expert Panel on Nonfluoride Caries-Preventive Agents. Nonfluoride caries-preventive agents: executive summary of evidence-based clinical recommendations. J Am Dent Assoc. 2011;142:1065–1071. doi: 10.14219/jada.archive.2011.0329. [DOI] [PubMed] [Google Scholar]
- 18.Bader JD, Shugars DA, Vollmer WM, Gullion CM, Gilbert GH, Amaechi BT, Brown JP. Design of the xylitol for adult caries trial (X-ACT) BMC Oral Health. 2010 Sep 29;10:22. doi: 10.1186/1472-6831-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bader JD, Robinson DS, Gilbert GH, Ritter AV, Makhija SK, Funkhouser KA, Amaechi BT, Shugars DA, Laws R. Four "lessons learned" while implementing a multi-site caries prevention trial. J Public Health Dent. 2010;70:171–175. doi: 10.1111/j.1752-7325.2010.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Caries Detection and Assessment System Coordinating Committee: Criteria Manual: International Caries Detection and Assessment System (ICDAS II) Baltimore, MD: Report of a Workshop; 2005. Mar 12–14, accessed at http://www.dundee.ac.uk/dhsru/docs/ICDAS II criteriadocument September 10.doc. [Google Scholar]
- 21.Banting DW, Amaechi BT, Bader JD, Blanchard P, Gilbert GH, Gullion CM, Holland JC, Makhija SK, Papas A, Ritter AV, Singh M, Vollmer WM. Examiner training and reliability in two randomized clinical trials of adult dental caries. J Pub Health Dent. 2011;71:335–344. doi: 10.1111/j.1752-7325.2011.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins LM, Schafer JL, Kam C. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Meth. 2001;6:330–351. [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple Imputations for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 24.Twetman S. Consistent evidence to support the use of xylitol- and sorbitol-containing chewing gum to prevent dental caries. Evid Based Dent. 2009;10:10–11. doi: 10.1038/sj.ebd.6400626. [DOI] [PubMed] [Google Scholar]
- 25.Addy M, Perriam E, Sterry A. Effects of sugared and sugar-free chewing gum on the accumulation of plaque and debris on the teeth. J Clin Periodontol. 1982 Jul;9(4):346–354. doi: 10.1111/j.1600-051x.1982.tb02101.x. [DOI] [PubMed] [Google Scholar]
- 26.Milgrom P, Ly KA, Rothen M. Xylitol and its vehicles for public health needs. Adv Dent Res. 2009;21:44–47. doi: 10.1177/0895937409335623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.