Abstract
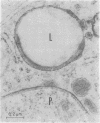
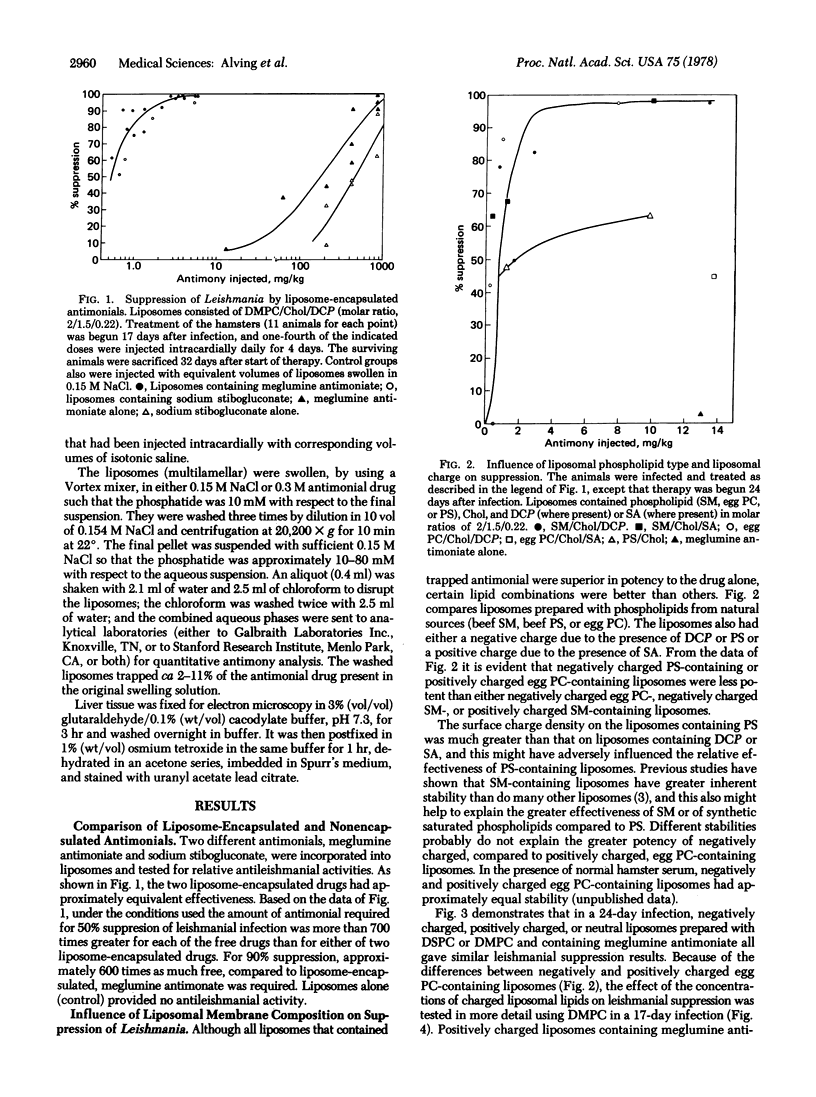
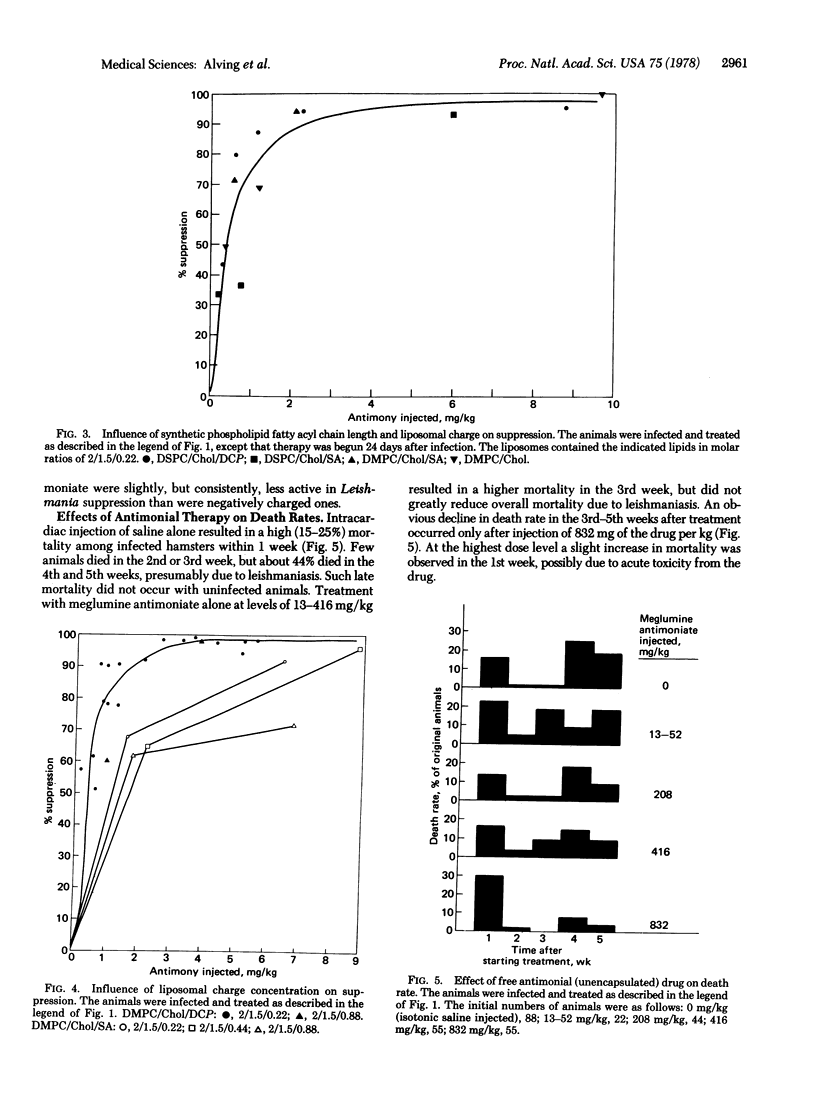
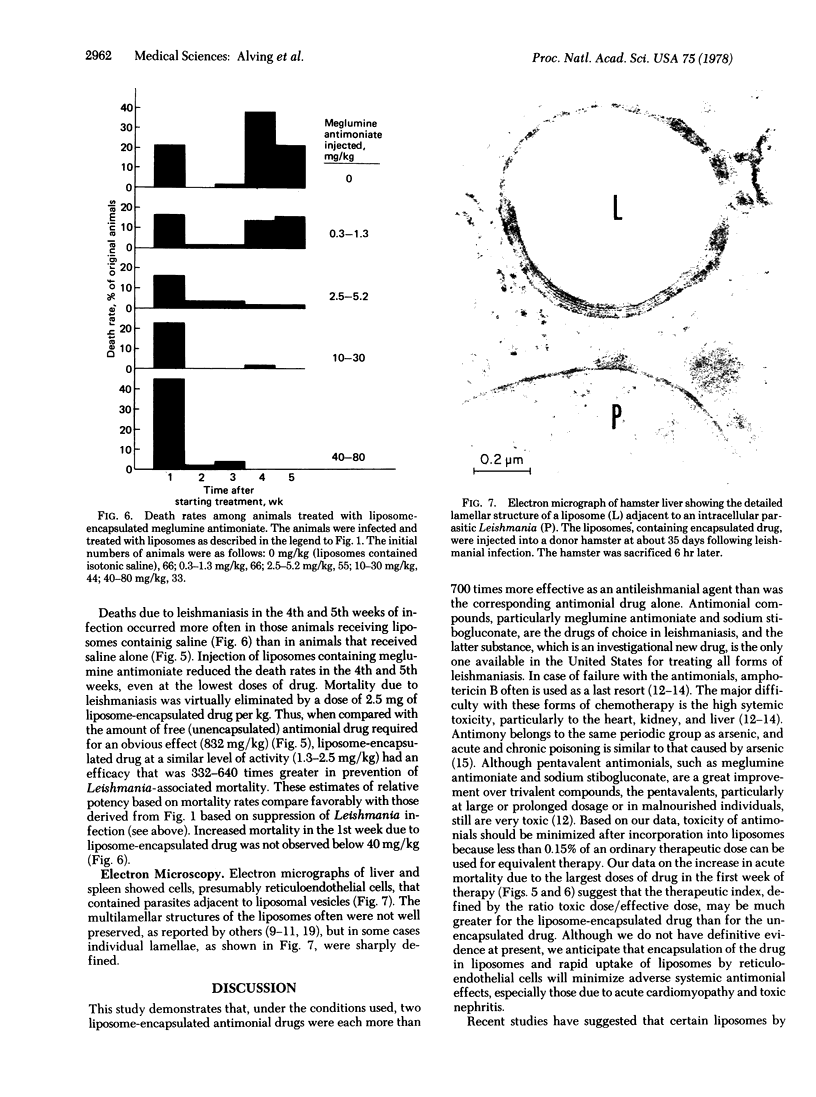
Liposomes containing antimonial compounds trapped in the aqueous phase were tested in the treatment of experimental leishmaniasis. The rationale of this approach was based on the hypothesis that the liposomes and the parasite are taken up by the same cell, the reticuloendothelial cell, and we present electron microscopic evidence that supports this hypothesis. Suppression of leishmaniasis was quantified by determining the total number of parasites per liver from impression smears. When two antimonials, meglumine antimoniate and sodium stibogluconate, were encapsulated within liposomes, each was more than 700 times more active compared to either of the free (unencapsulated) drugs. After infection, if untreated, all of the hamsters eventually would die from the disease. Liposome-encapsulated meglumine antimoniate was about 330-640 times more effective in causing a drop in the death rate than was the free antimonial. The efficacy of treatment was influenced by the lipid composition and charge of the liposomes. For example, positively charged liposomes containing egg phosphatidylcholine were much less effective than negatively charged ones. In contrast, positively and negatively charged sphingomyelin liposomes were equally effective. Liposomes containing phosphatidylserine (which were negatively charged, but also had a much higher charge density) were among the less-effective preparations. Among those tested, the most consistently efficacious liposomes contained highly saturated long-chain phospholipids (eg., dipalmitoyl phosphatidylcholine), cholesterol, and a negative charge.
We conclude that liposomes may be useful as carriers of drugs to treat infectious diseases involving the reticuloendothelial system. The toxicities of antimony are very similar to those of arsenic. Encapsulation of antimonial drugs and reduction of the dose required for effective therapy should minimize such systemic toxicities as acute cardiomyopathy and toxic nephritis.
Keywords: antimonial compounds, phospholipids, model membranes, parasites
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Joyce G., Richardson V. J., Ryman B. E., Wiśniewski H. M. Liposome toxicity in the mouse central nervous system. J Neurol Sci. 1977 Mar;31(2):173–179. doi: 10.1016/0022-510x(77)90104-6. [DOI] [PubMed] [Google Scholar]
- Alving C. R., Steck E. A., Hanson W. L., Loizeaux P. S., Chapman W. L., Jr, Waits V. B. Improved therapy of experimental leishmaniasis by use of a liposome-encapsulated antimonial drug. Life Sci. 1978 Mar;22(12):1021–1026. doi: 10.1016/0024-3205(78)90270-9. [DOI] [PubMed] [Google Scholar]
- Bangham A. D. Membrane models with phospholipids. Prog Biophys Mol Biol. 1968;18:29–95. doi: 10.1016/0079-6107(68)90019-9. [DOI] [PubMed] [Google Scholar]
- Black C. D., Watson G. J., Ward R. J. The use of Pentostam liposomes in the chemotherapy of experimental leishmaniasis. Trans R Soc Trop Med Hyg. 1977;71(6):550–552. doi: 10.1016/0035-9203(77)90155-9. [DOI] [PubMed] [Google Scholar]
- Bruni A., Toffano G., Leon A., Boarato E. Pharmacological effects of phosphatidylserine liposomes. Nature. 1976 Mar 25;260(5549):331–333. doi: 10.1038/260331a0. [DOI] [PubMed] [Google Scholar]
- Fendler J. H., Romero A. Liposomes as drug carriers. Life Sci. 1977 Apr 1;20(7):1109–1120. doi: 10.1016/0024-3205(77)90481-7. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Lysosomal localization of -fructofuranosidase-containing liposomes injected into rats. Biochem J. 1972 Aug;129(1):123–133. doi: 10.1042/bj1290123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G. The carrier potential of liposomes in biology and medicine (second of two parts). N Engl J Med. 1976 Sep 30;295(14):765–770. doi: 10.1056/NEJM197609302951406. [DOI] [PubMed] [Google Scholar]
- Hanson W. L., Chapman W. L., Jr, Kinnamon K. E. Testing of drugs for antileishmanial activity in golden hamsters infected with Leishmania donovani. Int J Parasitol. 1977 Dec;7(6):443–447. doi: 10.1016/0020-7519(77)90004-2. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S. Algorithms in the diagnosis and management of exotic diseases. XXIV. Leishmaniases. J Infect Dis. 1977 Jul;136(1):160–163. doi: 10.1093/infdis/136.1.160. [DOI] [PubMed] [Google Scholar]
- Masturzo P., Gallamini A., Murialdo G., Nizzo M. C., Toffano G. Dopaminergic pathway and prolactin: effect of a liposomal preparation. N Engl J Med. 1977 Aug 11;297(6):338–339. doi: 10.1056/NEJM197708112970618. [DOI] [PubMed] [Google Scholar]
- Mattenberger-Kreber L., Auderset G., Schneider M., Louis-Broillet A., Benedetti M. S., Malnoë A. Phagocytose des liposomes par les macrophages péitonéaux de souris. Experientia. 1976 Dec 15;32(12):1522–1524. doi: 10.1007/BF01924429. [DOI] [PubMed] [Google Scholar]
- New R. R., Chance M. L., Thomas S. C., Peters W. Antileishmanial activity of antimonials entrapped in liposomes. Nature. 1978 Mar 2;272(5648):55–56. doi: 10.1038/272055a0. [DOI] [PubMed] [Google Scholar]
- Rahman Y. E., Wright B. J. Liposomes containing chelating agents. Cellular penetration and a possible mechanism of metal removal. J Cell Biol. 1975 Apr;65(1):112–122. doi: 10.1083/jcb.65.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Wills E. J., Richmond J. E., Slavin G., Black C. D., Gregoriadis G. Morphological observations on the cellular and subcellular destination of intravenously administered liposomes. Br J Exp Pathol. 1974 Aug;55(4):320–327. [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Heath T. D., Colley C. M., Ryman B. E. New aspects of liposomes. Biochim Biophys Acta. 1976 Dec 14;457(3-4):259–302. doi: 10.1016/0304-4157(76)90002-2. [DOI] [PubMed] [Google Scholar]
- de Barsy T., Devos P., Van Hoof F. A morphologic and biochemical study of the fate of antibody-bearing liposomes. Lab Invest. 1976 Mar;34(3):273–282. [PubMed] [Google Scholar]