Abstract
We observed a cell concentration-dependent differentiation switch among cultured dendritic cells (DCs) triggered by lactic acid, a product of glycolytic metabolism. In particular, while interleukin (IL)-12, IL-23, and tumor necrosis factor α (TNFα)-producing, migratory DCs developed in sparse cultures, IL-10-producing, non-migratory DCs differentiated in dense cultures. This points to a novel opportunity for tailoring DC-based anticancer therapies through metabolism modulation in developing DCs.
Keywords: dendritic cell, glycolysis, lactic acid, dendritic cell vaccine, dendritic cell differentiation
Dendritic cell (DC)-based anticancer vaccines have been shown to induce tumor-specific immune responses in a wide range of experimental settings as well as in multiple clinical trials.1,2 The administration of DCs to tumor-bearing hosts can potentially deliver tumor-associated antigens (TAAs) to lymphoid tissues and activate antigen-specific CD4+ and CD8+ T cells that are capable of homing to neoplastic lesions and exert potent effector functions. However, the results of early clinical trials testing DC-based anticancer vaccines have not entirely fulfilled general expectations. Thus, various strategies have been devised to improve the efficiency of DC-based vaccination, including approaches to optimize the capacity of injected DCs to present TAAs and deliver T-cell co-stimulatory signals, resulting in the robust priming of TAA-specific CD8+ T cells.1 Of note, the molecular mechanisms that program DCs to induce diverse types of immune responses, ranging from antigen-specific tolerance to the development of different TH lineages, are not properly understood, potentially hindering the development of efficient DC-based anticancer vaccines. In addition, the differentiation of DCs from monocytic precursors is relatively little studied and therefore it remains questionable whether the culture systems historically established and widely used for in vitro DC differentiation are truly adequate to set the stage for potent antitumor response.
We and others have previously shown that at least two co-existing DC subsets, namely CD1a+CD14− and CD1a−CD14+ DCs can develop within the same culture.3,4 Although different DC precursors and culture conditions were used in these studies, unique functional abilities have been associated with CD1a+ and CD1a− DCs. In particular, the CD1a+CD14− DC subset appeared to be superior to its CD1a−CD14+ counterpart in inducing TH1 polarization3 and cytotoxic T lymphocyte (CTL) activity.4 Importantly, the CD1a+/CD1a− DC ratio varied greatly among blood donors,3 raising the possibility that either endogenous features of monocytic precursors or subtle differences in cell culture conditions would strongly influence the development of CD1a+ vs. CD1a− DCs. Such variability in the composition of DC preparations may impact on the efficacy of DC-based vaccines. In particular, limited TH1 responses would be expected in patients who are immunized with preparation containing mainly CD1a− DCs.
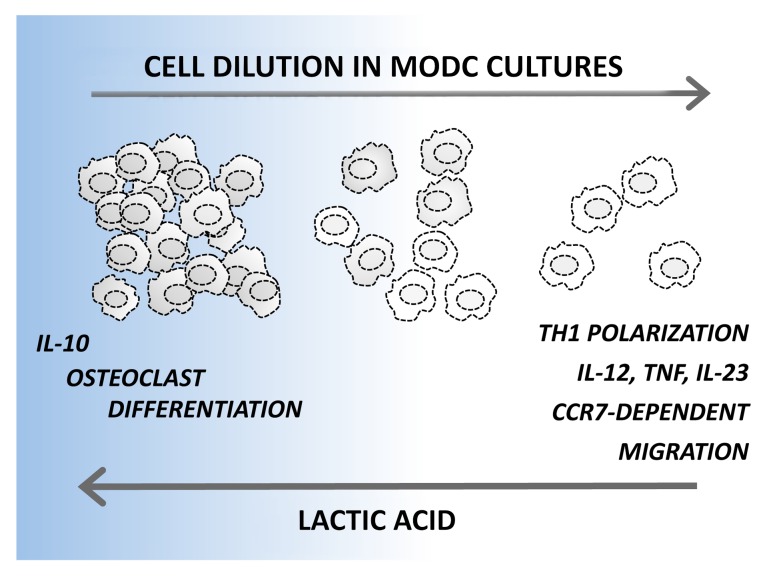
We have recently identified a potent autocrine pathway that operate on developing DCs and provide a surprising contribution to the phenotypic and functional heterogeneity of monocyte-derived DCs.5 We demonstrated that lactic acid, an abundant product of aerobic glycolysis, accumulates in dense DC cultures and induces a powerful anti-inflammatory differentiation program. Indeed, high cell culture densities (2 x 106 monocytes/mL) resulted in the development of a mixture of CD1a+CD14−CD209+ and CD1a−CD14+CD209+ DCs that produced high levels of interleukin (IL)-10 but no pro-inflammatory cytokines upon Toll-like receptor (TLR) stimulation. In addition, the DCs developing in dense cultures exhibited defective migratory responses to the lymphoid tissue-derived chemotactic agent chemokine (C-C motif) ligand 19 (CCL19) and remained uncommitted to DC functions, as demonstrated by their trans-differentiation to osteoclasts. By decreasing culture density to 0.5–1 x 106 monocytes/mL, we mainly obtained CD1a+CD14− DCs, which produced modest levels of both IL-10 and pro-inflammatory cytokines upon activation. Conversely, the further dilution of cell cultures (0.125–0.25 x 106 monocytes/mL) primed DCs to produce very high levels of IL-12, IL-23 and tumor necrosis factor α (TNFα) upon activation (but no IL-10), and these cells were easily mobilized by CCL19 (Fig. 1). These DCs obtained their functional polarization already in the first 1–2 d of culture, and changing the culture density later did not influence their cytokine secretion profile.
Figure 1. Rewiring dendritic cell differentiation upon the accumulation of lactic acid. Monocyte-derived dendritic cells (DCs) developing in sparse monocytic cultures show a superior ability to produce pro-inflammatory cytokines, to elicit TH1 responses and to migrate toward the lymphoid tissue-derived chemotactic agent chemokine (C-C motif) ligand 19 (CCL19). On the contrary, DCs differentiating in dense cultures produce interleukin (IL)-10 but no pro-inflammatory cytokines upon activation. In addition, DCs originating in dense cultures maintain a relatively high plasticity and can trans-differentiate to osteoclasts. A key role for lactic acid in rewiring DC functions was demonstrated by interfering with lactic acid production in dense cultures, which increased IL-12 and decreased IL-10 production, and by adding lactic acid to sparse cultures, which resulted in opposite effects. CCR7, chemokine (C-C motif) receptor 7; TNFα, tumor necrosis factor α.
Interfering with glycolytic energy production during the early stages of differentiation increased IL-12 and decreased IL-10 production by DCs developing in dense cultures, whereas lactic acid added to sparse cultures resulted in the opposite effect, indicating a key role of this glycolytic product in the control of DC differentiation.5 High levels of lactic acid in dense monocyte cultures induced an anti-inflammatory program in developing DCs resembling the one previously attributed to lactic acid released by malignant cells within the tumor microenvironment, which contributes to the non-inflammatory profile of tumor-infiltrating DCs.6
The capacity of cell culture density and lactic acid to influence the functional polarization of DCs may have significant implication for DC research and DC-based therapies. First, the culture density chosen by investigators will largely determine how DCs behave in different experimental settings, rendering the results of studies based on DCs generated in vitro highly context-dependent. DCs obtained from dense monocytic cultures, for example, may not respond to anti-inflammatory interventions with a reduced production of pro-inflammatory cytokines, as these cells already secrete very little of such cytokines. Conversely, DCs from sparse cultures may be particularly sensitive to anti-inflammatory interventions, as these cells are primed to produce high levels of IL-12, IL-23, and TNFα in baseline conditions.5
To understand the relevance of our findings for DC-based therapies it will be essential to clarify whether DCs generated in dense or sparse cultures maintain their functional polarization in vivo. If so, DCs not exposed to lactic acid during development might be superior to induce inflammation, TH1 polarization and CTL responses as well as to deliver TAAs to lymphoid tissues. On the other hand, DCs developing in dense cultures may exert robust immunosuppressive effects, due to their capacity to produce high levels of IL-10. If DCs developing in dense or sparse cultures can indeed induce different types of immune responses in vivo, it will be important to clarify whether the efficacy of current DC-based vaccines is influenced by such a density-dependent mechanism. As sparse cultures are costly, most laboratories might be prone to choose cell culture densities that are suboptimal to generate DCs that migrate to lymph nodes, secrete pro-inflammatory cytokines and elicit TH1 responses. However, avoiding the anti-inflammatory effects of lactic acid by diluting monocyte cultures might represent a remarkably simple and efficient means of boosting the ability of DCs to induce antitumor immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Nasi A, Rethi B. Disarmed by density: A glycolytic break for immunostimulatory dendritic cells?. OncoImmunology 2013; 2:e26744; 10.4161/onci.26744
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26744
References
- 1.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuler G. Dendritic cells in cancer immunotherapy. Eur J Immunol. 2010;40:2123–30. doi: 10.1002/eji.201040630. [DOI] [PubMed] [Google Scholar]
- 3.Gogolak P, Rethi B, Szatmari I, Lanyi A, Dezso B, Nagy L, Rajnavolgyi E. Differentiation of CD1a- and CD1a+ monocyte-derived dendritic cells is biased by lipid environment and PPARgamma. Blood. 2007;109:643–52. doi: 10.1182/blood-2006-04-016840. [DOI] [PubMed] [Google Scholar]
- 4.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavölgyi E, Catrina AI, Wheelock CE, Vivar N, Rethi B. Dendritic cell reprogramming by endogenously produced lactic Acid. J Immunol. 2013;191:3090–9. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 6.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–21. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]