Abstract
Like higher eukaryotic cells in tissues, microbial cells in a community act in concert in response to environmental stimuli. They coordinate gene expression and their physiological and morphological states through intercellular communication mediated by matricellular signals. The adhesion protein Cfl1 was recently shown to be a matricellular signal in regulating morphogenesis and biofilm formation in the eukaryotic microbe Cryptococcus neoformans. Cfl1 is naturally highly expressed in the hyphal subpopulation during the mating colony development. Some Cfl1 proteins are cleaved and released to the ECM (extracellular matrix). The released exogenous Cfl1 activates Cryptococcus cells to express their endogenous Cfl1, to undergo filamentation, and to form structured biofilm colonies. In this study, we demonstrate that the N-terminal signal peptide and the novel conserved cysteine-rich SIGC domain at the C-terminus are critical for the adherence property and the signaling activity of this multifunctional protein. The investigation of this fungal matricellular signaling network involving Cfl1 and the master regulator of morphogenesis Znf2 provides a foundation to further elucidate intercellular communication in microbial development.
Keywords: matricellular signal, autoinducer, filamentation, biofilm, paracrine
It is becoming increasingly evident that microbes, like multicellular higher eukaryotes, have evolved complex social behaviors in preparing their adaptation to environmental fluctuations.1 In a community, cells coordinate their physiological and morphological states through intercellular communication using long range signals made of diffusible low-molecular-weight molecules and short range signals made of less diffusible macro-molecules. Gradients generated from these signals help establish position-specific cues in the community, which in turn create different dynamic microenvironments to regulate the differentiation of subpopulations.2-4 Extracellular matrix (ECM) is considered a scaffold for signal molecules, which ultimately provides the position cues.5 In higher eukaryotes, cellular interactions with ECM shape embryogenesis, cell locomotion/migration, tissue/organ development, and even organismal survival.6-9
In the eukaryotic microbe Cryptococcus neoformans, it is shown recently that released adhesion protein Cfl1 in the ECM acts as a matricellular signal to regulate cellular morphological differentiation and the formation of structured biofilm colonies.10 In this study, we further demonstrated that secretion and the conserved C-terminal SIGC domain of Cfl1 are critical for its multi-functions. (1) The absence of the N-terminal signal peptide resulted in the intracellular accumulation of Cfl1 and abolished its ability to promote the formation of structured biofilm colonies; (2) The deletion of the SIGC domain in Cfl1 reduced the ability of this protein to promote the formation of biofilms; and (3) Donor strains expressing this mutant allele failed to evoke any response from the nearby wildtype recipient. By contrast, donor strains expressing the wildtype CFL1 allele elicit the recipient strain to produce filaments and form biofilm colonies.10
Several lines of evidence presented in this study and the previous one10 point to the signaling function of this novel and yet conserved adhesion protein. First, the expression of CFL1 can be induced to a high level comparable to other genes producing autoinducers that are well-known regulators of community behaviors in bacteria.11 During mating colony development, CFL1 is expressed at an equivalent level as the pheromone gene MF1α, although MF1α is induced earlier to initiate early mating events while the induction of Cfl1 is concomitant with the later filamentation events. Accordingly, the Cfl1 protein is specifically expressed in the hyphal but not the yeast subpopulation.10 Second, the deletion of CFL1 (cfl1∆) attenuates filamentation and colony adherence to agar. Conversely, the overexpression of CFL1 (CFL1oe) enhances filamentation and stimulates the formation of a structured biofilm. Interestingly, neighboring wildtype colonies in close proximity to the CFL1oe strain also filament and develop biofilm that phenotypically mimicking the CFL1oe donor strain. Consistently, the endogenous Cfl1-mCherry in the wild type recipient strain is induced by released signals from the donor strain in a distance-dependent manner, implicating extracellular Cfl1 as a paracrine signal.10 Third, the released Cfl1 (rCfl1), with two-thirds of the full length protein from the C-terminus, is enriched in the ECM. When purified rCfl1 is added to the wildtype recipient strain, the recipient undergoes morphogenesis and forms biofilm, similar to the changes observed when the recipient is placed adjacent to the CFL1oe donor strain.10 Fourth, as expected for a signal molecule, the secretion of Cfl1 is critical for its function. The intracellularly expressed Cfl1 mutant protein lacking the N-terminal signal peptide [CFL1(sigPΔ)] does not accumulate in the ECM.10 Donor strains expressing such a mutated allele of CFL1 do not form biofilm colonies (Fig. 1A) and fail to elicit changes in morphology and colony architecture in the nearby wildtype recipient strain.10 Fifth, although the truncation of Cfl1’s C-terminus (aa 230–309Δ) reduces the ability of Cryptococcus cells to adhere and to undergo filamentation, such mutation does not abolish these properties (Fig. 1A). However, donor strains expressing this truncated CFL1 allele are incapable of eliciting responses from the recipient (Fig. 1B). Thus, the C-terminus is important for Cfl1’s function as an adhesion protein, but it is absolutely critical for the function of Cfl1 as a matricellular signal. Finally, although no annotated domain is indicative of rCfl1’s signaling activity, the cysteine-rich C-terminus (80 amino acid residues) termed SIGC is highly conserved among fungal species in the phylum of Basidiomycota. Intriguingly, about 90% of Cfl1 homologs with SIGC are predicted to be secreted and 50% domains associated with SIGC are involved in extracellular ligand-receptor interaction.10 Collectively, these findings suggest that the SIGC domain is involved in the matricellular signal transduction.
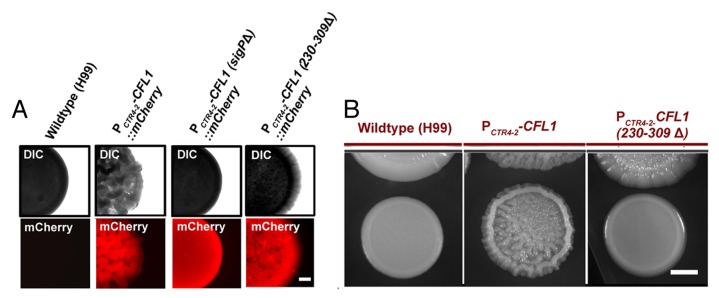
Figure 1. Secretion and the C-terminal SIGC domain are important for Cfl1’s multi-functions. (A) The expression of the full length and the truncated Cfl1-mCherry proteins [CFL1(sigPΔ) and CFL1(230–309Δ)] was driven by the promoter of the copper transporter CTR4 (PCTR4–2). Indicated strains were grown on YPD agar medium supplemented with the inducer BCS (copper chelator) at 200 μM. Cells were cultured at 22°C for 3 d before being examined under a fluorescence stereoscope. The degree in colony wrinkledness reflects the strength in cell-cell adhesion and the complexity in colony morphology (or biofilm). The deletion of the N-terminal signal peptide (sigPΔ) or the SIGC domain (230–309Δ) did not significantly affect the protein expression level based on their fluorescence intensity. The C. neoformans strain expressing CFL1(sigPΔ) did not form biofilm colonies while the strain expressing the SIGC truncated allele CFL1(230–309Δ) showed decreased wrinkledness in colony morphology. Scale bar: 1 mm. (B) The donor strain expressing the SIGC truncated CFL1 allele failed to evoke the nearby wildtype recipient to form a structured biofilm colony. The confrontation assay was performed on YPD agar medium with 200 μM BCS. The donor strains were precultured for 3 d and then the XL280 recipient strain was placed near the donor colonies. The cells were then photographed after being cultured for additional 5 d. Scale bar: 2 mm.
Signaling mediated by Cfl1 requires the network controlled by the transcription factor Znf2. Znf2 is the irreplaceable decision maker of morphological transition between the yeast form and the filamentous form in Cryptococcus.12-14 Disruption of ZNF2 in the recipient strain completely prevents the recipient from responding to the Cfl1 matricellular signal released from the donor.13 By comparison, disruption of Cfl1 in the recipient reduces but does not abolish its response to the exogenous Cfl1 signal. These observations indicate that Cfl1 is the critical signal in this paracrine regulation, but it is only one of the targets.
Taken together, we propose the following model to illustrate the intercellular communication mediated by the rCfl1 matricellular signal in this eukaryotic microbe (Fig. 2). The released exogenous Cfl1 in the ECM binds to its putative receptor on the cell surface, which then activates Znf2 directly or indirectly. Activated Znf2 induces the expression of its downstream targets, including other cell surface proteins and Cfl1. The nascent full length Cfl1 is then synthesized and transported in vesicles. The signal peptide is then removed and the resulting mature full length protein is attached to the cell wall and functions as an adhesion protein. Some of the Cfl1 proteins are cleaved and released from the cell wall to the ECM. The released Cfl1 in turn activates the Cryptococcus cells that it comes into contact to express the endogenous Cfl1, forming a positive feedback loop (Fig. 2). As the first example of matricellular signaling in fungi, this Cfl1 signaling network provides a paradigm to advance our understanding of intercellular communication in microbes. Furthermore, given the link between morphogenesis and virulence in fungi and other eukaryotic pathogens,15-19 such investigation could also help our understanding of microbial pathogenesis.
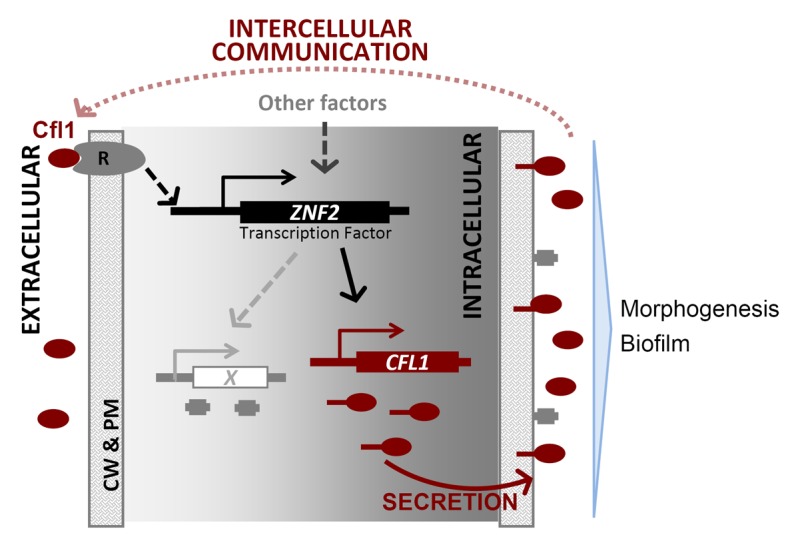
Figure 2. Working model of the paracrine signaling network mediated by Cfl1. (X: cell surface proteins; R: Cfl1 receptor; CW: cell wall; PM: plasma membrane)
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIAID/NIH (grants R01AI097599 and R21AI107138 to XL). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dylan Foyle for critical reading and Dr. L. Rene Garcia for his assistance in microscopy.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26444
References
- 1.Crespi BJ. The evolution of social behavior in microorganisms. Trends Ecol Evol. 2001;16:178–83. doi: 10.1016/S0169-5347(01)02115-2. [DOI] [PubMed] [Google Scholar]
- 2.Mehta P, Goyal S, Long T, Bassler BL, Wingreen NS. Information processing and signal integration in bacterial quorum sensing. Mol Syst Biol. 2009;5:325. doi: 10.1038/msb.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ham JH. Intercellular and intracellular signalling systems that globally control the expression of virulence genes in plant pathogenic bacteria. Mol Plant Pathol. 2013;14:308–22. doi: 10.1111/mpp.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JY. Regulation of short-distance transport of RNA and protein. Curr Opin Plant Biol. 2005;8:45–52. doi: 10.1016/j.pbi.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zusman S, Patel-King RS, Ffrench-Constant C, Hynes RO. Requirements for integrins during Drosophila development. Development. 1990;108:391–402. doi: 10.1242/dev.108.3.391. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy J, Turley EA. Effects of extracellular matrix components on cell locomotion. Crit Rev Oral Biol Med. 1993;4:619–37. doi: 10.1177/10454411930040050101. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee S, Isaacman-Beck J, Schneider VA, Granato M. A novel role for Lh3 dependent ECM modifications during neural crest cell migration in zebrafish. PLoS One. 2013;8:e54609. doi: 10.1371/journal.pone.0054609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song J, Lokmic Z, Lämmermann T, Rolf J, Wu C, Zhang X, Hallmann R, Hannocks MJ, Horn N, Ruegg MA, et al. Extracellular matrix of secondary lymphoid organs impacts on B-cell fate and survival. Proc Natl Acad Sci U S A. 2013;110:E2915–24. doi: 10.1073/pnas.1218131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Tian X, Gyawali R, Lin X. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc Natl Acad Sci U S A. 2013;110:11571–6. doi: 10.1073/pnas.1308173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schauder S, Bassler BL. The languages of bacteria. Genes Dev. 2001;15:1468–80. doi: 10.1101/gad.899601. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Jackson JC, Feretzaki M, Xue C, Heitman J. Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same-sex mating in Cryptococcus neoformans. PLoS Genet. 2010;6:e1000953. doi: 10.1371/journal.pgen.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Zhai B, Lin X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 2012;8:e1002765. doi: 10.1371/journal.ppat.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai B, Zhu P, Foyle D, Upadhyay S, Idnurm A, Lin X. Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infect Immun. 2013;81:2626–37. doi: 10.1128/IAI.00259-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–49. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 16.Nemecek JC, Wüthrich M, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–8. doi: 10.1126/science.1124105. [DOI] [PubMed] [Google Scholar]
- 17.Webster RH, Sil A. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proc Natl Acad Sci U S A. 2008;105:14573–8. doi: 10.1073/pnas.0806221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bibel DJ, Crumrine DA, Yee K, King RD. Development of arthrospores of Trichophyton mentagrophytes. Infect Immun. 1977;15:958–71. doi: 10.1128/iai.15.3.958-971.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews KR. The developmental cell biology of Trypanosoma brucei J Cell Sci 2005; 118:283-90; 10.1242/jcs.01649. [DOI] [PMC free article] [PubMed]


