Abstract
The ability of regulatory T cells (Tregs) to promote immunological tolerance represents an important obstacle in cancer immunotherapy. We have recently discovered that the clinically established immunotherapeutic agent interferon α (IFNα) inactivates the suppressive functions of human Tregs. Here, we outline the mechanisms whereby IFNα mediates this important function and discuss its therapeutic implications for cancer immunotherapy.
Keywords: regulatory T cells, IFN-alpha, cAMP, cancer, tolerance, NK cells, PDE
Activated CD4+CD25+FOXP3+ regulatory T cells (Tregs) prevent the development of efficient antitumor immune responses, hence dampening the efficacy of multiple immunotherapeutic regimens. Whereas in animal models the depletion of Tregs effectively unleashes antitumor immune responses, the attempts to remove Tregs in cancer patients are generally hampered by the relative inefficiency and poor selectivity of current Treg-targeting agents. Moreover, efficient strategies for the inactivation of Tregs remain to be identified.
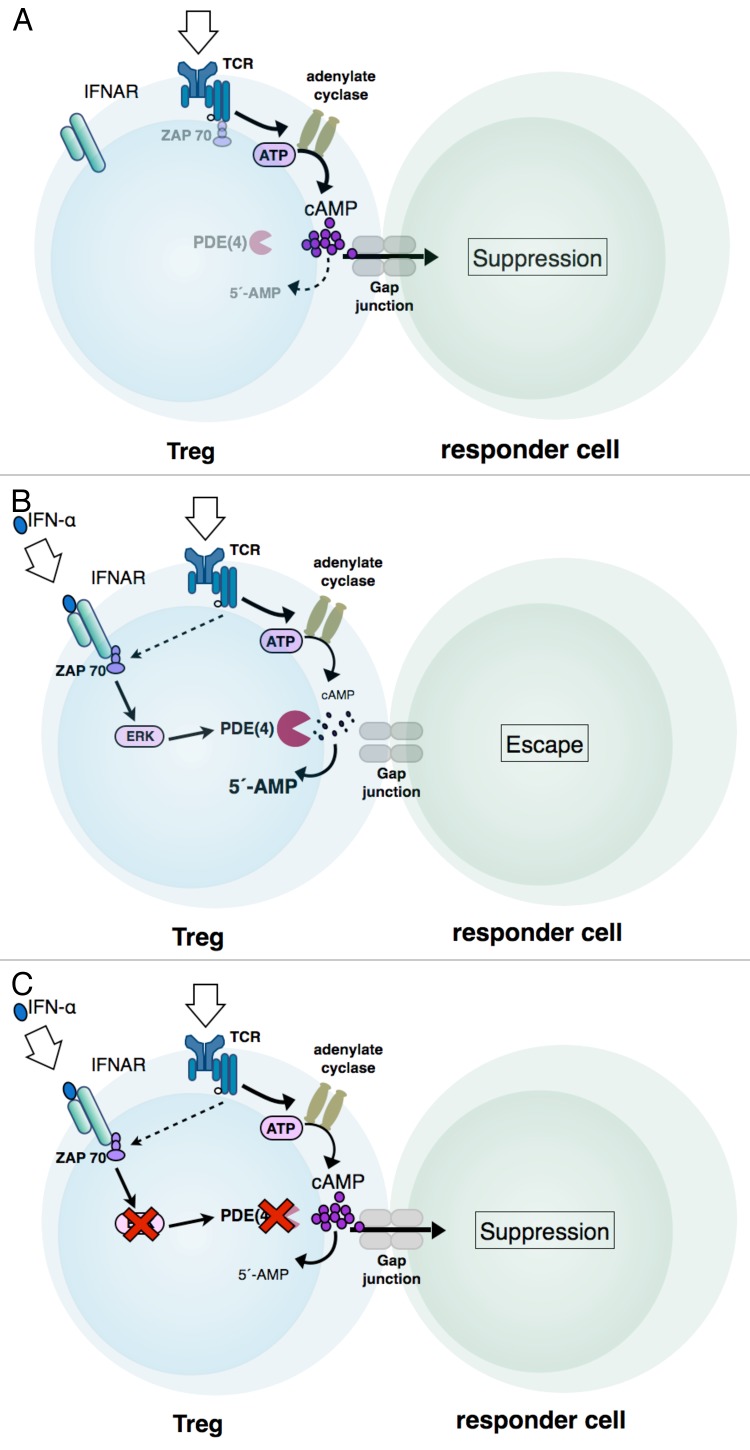
Interferon α (IFNα) exerts a robust immunostimulatory activity and is therapeutically effective against a wide range of solid and hematologic tumors. By investigating the effects of IFNα on human Tregs, we have recently demonstrated that IFNα specifically inactivates their immunosuppressive functions by repressing the production of the second messenger cyclic adenosine monophosphate (cAMP) (Fig. 1B).1 The ability of Tregs to mediate immunosuppressive effects has previously been shown to depend on increases in the intracellular concentration of cAMP2-4 (Fig. 1A). Intracellular cAMP levels reflect the rate of cAMP synthesis by adenylate cyclases (ACs), degradation by phosphodiesterases (PDEs), or excretion (Fig. 1A). In Tregs, the activity of AC9 and the expression of PDE3B are regulated by the lineage-defining transcription factor forkehead box P3 (FOXP3).4,5 However, the IFNα-mediated inactivation of Tregs did not affect FOXP3 expression levels. In line with this notion, IFNα also failed to alter the methylation state of the FOXP3 locus, which is required for stable FOXP3 expression and suppressive functions.1 Thus, IFNα disturbs the immunosuppressive activity of human Tregs without affecting their imprinted lineage phenotype.
Figure 1. Interferon α signaling interferes with the TCR-dependent production of cAMP in regulatory T cells. (A) T-cell receptor (TCR) signaling causes an increase in the intracellular pool of cyclic adenosine monophosphate (cAMP) of regulatory T cells, as well as the transmission of cAMP via gap junctions to responder cells. In natural killer (NK) cells or effector T lymphocytes targeted by Tregs, cAMP limits effector functions by a hitherto undefined mechanism. (B) The binding of interferon α (IFNα) to its receptor activates the MEK-ERK signaling pathway, probably via the JAK-STAT axis. Activated ERK in turn stimulates the enzymatic activity of short phosphodiesterase 4 isoforms predominantly expressed in T cells, namely, PDE4B and PDE4D, resulting in a decrease in intracellular (and hence transmittable) cAMP. (C) Blocking the MEK-ERK signaling pathway or the enzymatic activity of PDE4 with specific inhibitors counteracts the ability of IFNα to repress cAMP production and hence restores the immunosuppressive functions of Tregs.
A prominent and well-known activity of IFNα consists in the activation of the MEK/ERK signaling pathway,5 and active mitogen-activated protein kinase 1 (MAPK1, also known as ERK2) is able to stimulate the enzymatic activity of 2 short PDE4 isoforms that are predominantly expressed in T cells, namely PDE4B and PDE4D (Fig. 1B).6 Consistent with the ability of IFNα to activate PDE4 via ERKs, the global inhibition of ERKs or PDEs, as well as the specific inhibition of PDE4, restored the immunosuppressive activity of Tregs exposed to IFNα (Fig. 1C). In addition to regulating the intracellular pool of cAMP, IFNα decreased the T-cell receptor (TCR)-induced activation of ζ chain (TCR) associated protein kinase 70kDa (ZAP70) in Tregs, supposedly by recruiting ZAP70 molecules to the interferon-α receptor (IFNAR), hence limiting their availability in TCR-mediated signal transduction.7
In co-cultures of human Tregs and CD4+ T lymphocytes or NK cells, IFNα restored the ability of CD4+ T cells to proliferate and secrete cytokines or the cytotoxic functions of NK cells, respectively (Fig. 1C).1 Interestingly, using a xenogeneic graft vs. host disease (GvHD) model applicable for the functional analysis of human Tregs, we found that the pre-incubation of Tregs with IFNα abrogated their ability to protect from disease onset.1 The loss of immunosuppressive activity upon IFNα treatment, however, did not affect the survival of Tregs, and Tregs did not lose their anergic phenotype, in vitro and in vivo.
While short-term IFNα signaling may transiently inhibit the immunosuppressive activity of Tregs, continued IFNα treatment may inactivate Tregs in a permanent manner. Temporary increases in systemic IFNα levels may thus allow for the generation of effector T cells in the absence of any interference from Tregs, whereas sustained Treg inactivation by persistently high levels of IFNα may lead to excessive immune activation. Consistent with this notion, the activity of Tregs negatively correlates with IFNα levels in patients with acute viral infections or autoimmune diseases such as Sjögren’s syndrome, systemic lupus erythematosus (SLE), inflammatory myositis and Type I diabetes.8
The administration of IFNα has been previously observed to reduce the number of Tregs in melanoma and renal cell carcinoma patients.9 Conversely, declining IFNα levels and impaired IFNα signaling have been correlated with an elevation in the number of Tregs as well as with an increased risk of tumor progression.10 The repression of human Treg functions by IFNα may significantly contribute to its antineoplastic effects and provides a rationale for considering IFNα as a potent Treg-inactivating agent in the context of anticancer vaccination. In line with this assumption, improved immune responses have been reported in renal, pancreatic, and colorectal cancer patients upon the co-administration of IFNα with peptide vaccines. However, whether the prolonged administration of IFNα to cancer patients can recapitulate and/or stimulate all or only specific subsets of tumor-specific T cells remains to be investigated. Moreover, in accordance with its role in autoimmune diseases, the continuous administration of therapeutic doses of IFNα also increases the risk of developing autoimmune symptoms, as frequently observed in cancer patients receiving IFNα-based immunotherapy.
Altogether, our findings reveal an hitherto unrecognized function of IFNα that provides a logical explanation for its robust immunostimulatory activity in cancer patients, including frequently observed autoimmune symptoms upon continuous therapeutic application.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
This work was supported by the German Research Foundation (German research Foundation (DFG): Transregio 52/A7, STE791/6-1, initiative CRC 1066/B6) to KS, by the initiative CRC 1066/B8 to CB and TB, by the German Cancer Aid (KS) and by intramural grants to KS, C.B. and NB (MAIFOR, NMFZ).
Submitted: 09/10/2013; Accepted: 12/14/2013
Citation: Becker C, Bopp T, Steinbrink K. Interferon α interferes with immunological tolerance. OncoImmunology 2013; 2:e27528; 10.4161/onci.27528
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27528
References
- 1.Bacher N, Raker V, Hofmann C, Graulich E, Schwenk M, Baumgrass R, Bopp T, Zechner U, Merten L, Becker C, et al. Interferon-α suppresses cAMP to disarm human regulatory T cells. Cancer Res. 2013;73:5647–56. doi: 10.1158/0008-5472.CAN-12-3788. [DOI] [PubMed] [Google Scholar]
- 2.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–10. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein M, Vaeth M, Scheel T, Grabbe S, Baumgrass R, Berberich-Siebelt F, Bopp T, Schmitt E, Becker C. Repression of cyclic adenosine monophosphate upregulation disarms and expands human regulatory T cells. J Immunol. 2012;188:1091–7. doi: 10.4049/jimmunol.1102045. [DOI] [PubMed] [Google Scholar]
- 4.Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX, Zhang GM, Feng ZH. miR-142-3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009;10:180–5. doi: 10.1038/embor.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–5. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 6.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 7.Peter D, Jin SL, Conti M, Hatzelmann A, Zitt C. Differential expression and function of phosphodiesterase 4 (PDE4) subtypes in human primary CD4+ T cells: predominant role of PDE4D. J Immunol. 2007;178:4820–31. doi: 10.4049/jimmunol.178.8.4820. [DOI] [PubMed] [Google Scholar]
- 8.Petricoin EF, 3rd, Ito S, Williams BL, Audet S, Stancato LF, Gamero A, Clouse K, Grimley P, Weiss A, Beeler J, et al. Antiproliferative action of interferon-alpha requires components of T-cell-receptor signalling. Nature. 1997;390:629–32. doi: 10.1038/37648. [DOI] [PubMed] [Google Scholar]
- 9.Borg FA, Isenberg DA. Syndromes and complications of interferon therapy. Curr Opin Rheumatol. 2007;19:61–6. doi: 10.1097/BOR.0b013e328010c547. [DOI] [PubMed] [Google Scholar]
- 10.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S, et al. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:5188–97. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]