Abstract
Recent reports have highlighted the role of cellular immunity in anti-tumor defenses. T lymphocytes are known to play important part in anti-cancer immunity. The number and function of T lymphocytes are altered in chronic leukemia patients. CD3+CD56+ T lymphocytes have also been found to be abnormal in cancer patients. We therefore investigated changes in the number and cytotoxicity of CD3+CD56+ T lymphocytes in the peripheral blood of acute leukemia (AL) patients (excluding acute promyelocytic leukemia), to improve our understanding of the role of this T lymphocyte subset. We analyzed CD3+CD56+ T lymphocyte numbers and cytotoxicities in healthy controls, AL patients, and AL patients with complete remission. Lymphocyte counts were performed in peripheral blood and flow cytometry was used to determine cell numbers and cytotoxicities. The absolute number of CD3+CD56+ T lymphocytes was increased in AL patients (including acute myeloid [AML] and acute lymphocytic leukemia [ALL]) compared with healthy controls (P < 0.05), but their functioning was significantly reduced (P < 0.05). The number of CD3+CD56+ T lymphocytes in AML and ALL patients who achieved remission following chemotherapy was close to healthy controls (P > 0.05), but their functioning was still significantly reduced (P < 0.05). In addition, the number of CD3+CD56+ T lymphocytes increased significantly in AML patients with increased peripheral blood white blood cell (WBC) counts, and in ALL patients without increased WBCs. These results suggest that cellular immunity may respond to AML and ALL, but that lymphocyte cytotoxicity remains impaired. Dysfunction of CD3+CD56+ T lymphocytes in AML and ALL patients may contribute to the failure of the host immune response against leukemic blasts.
Keywords: CD3+CD56+ T lymphocyte, cell’s cytotoxicity, cell’s number, primary acute leukemia
Introduction
Immune evasion is an important mechanism in cancer progression. Several studies have identified abnormal cellular immunity in cancer patients, thus indicating potential new strategies for cancer treatment.1 However, successful immunotherapy requires a better understanding of the changes in the immune system in cancer patients.
Acute leukemia (AL) is a malignant tumor of the hematological system, characterized by malignant clones of leukemia cells in the bone marrow. Its clinical manifestations include fever, bleeding, and anemia. Abnormal peripheral blood (PB) cells and malignant leukemic clones can be identified by laboratory tests. AL can be divided into acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL), according to the FAB system. Chemotherapy and stem cell transplantation (SCT) represent the main treatments for AL, and long-term survival may require SCT, which is based on rebuilding the immune system to produce a graft-vs.-leukemia effect.2 However, its high cost, and significant side effects and mortality limit the applicability of SCT in China. Doctors have therefore used novel immunotherapies, and research into abnormalities of the immune system in AL patients has become a hot topic. Several studies have shown changes in the numbers and functions of T lymphocyte subsets in chronic lymphocytic leukemia.3-6
CD3+CD56+ T lymphocytes were first described as a distinct subset of T cells more than a decade ago.7,8 This subset expresses surface receptors that are also found on conventional T cells (CD3), together with receptors characteristic of natural killer (NK) cells (CD56), and they can therefore be referred to as NKT cells. These cells have already been shown to have antitumor cytotoxicity.9,10 Such cells possess a higher level of anti-tumor activity in vivo than interleukin (IL)-2-activated killer (LAK) cells. This property has been demonstrated in various animal tumor models, and survival of severe combined immunodeficient mice (SCID) inoculated with a B-lymphoma cell line was prolonged more by treatment with activated CD3+CD56+ cells than with LAK cells. Furthermore, CD3+CD56+ cells have a higher rate of proliferation than LAK cells.11,12 In another SCID mouse model, CD3+CD56+ cells from patients with chronic myelogenous leukemia were shown to have potent efficacy against autologous tumor cells.13 The anti-tumor efficacy of some immune cells is known to involve perforin, and several studies have shown high levels of perforin expression in CD3+CD56+ cells.14,15 We therefore compared the numbers and cytotoxicities of CD3+CD56+ T lymphocytes in patients with primary AL, healthy controls, and in AL patients who achieved complete remission (CR-AL) following chemotherapy. Only one previous study addressed the issue of CD3+CD56+ T lymphocyte numbers in the PB at the time of diagnosis of AML,16 but no previous studies have investigated these factors at the time of diagnosis of ALL, and at the time of complete remission from AL. Similarly, no studies have examined changes in CD3+CD56+ T lymphocyte numbers and cytotoxicities in AL patients in relation to changes in white blood cell (WBC) counts.
Results
Numbers of CD3+CD56+ T lymphocytes in the PB of patients presenting with AML and ALL
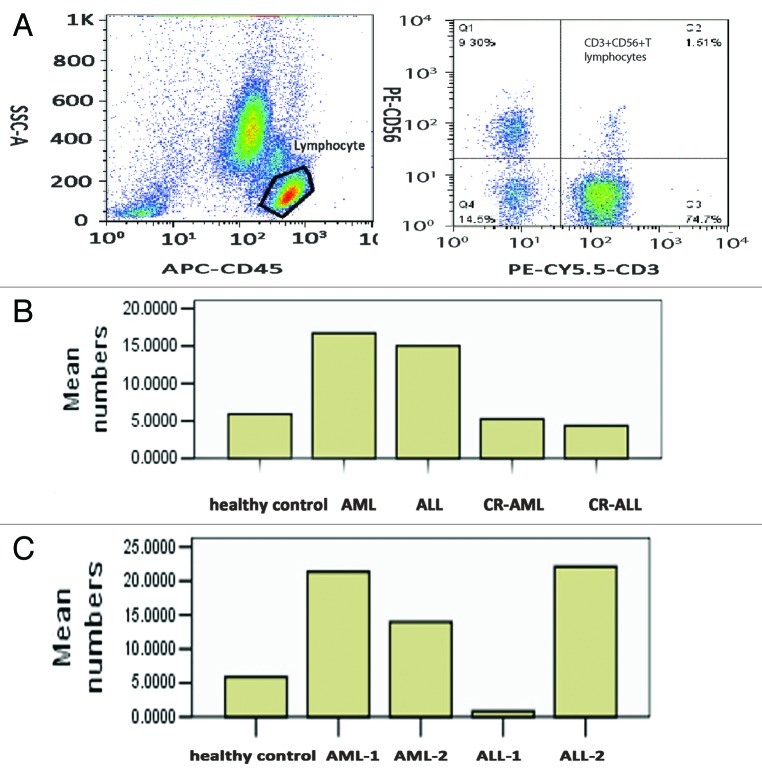
We assessed the proportions and numbers of CD3+CD56+ T lymphocytes in the PB of 24 primary AML patients, 16 primary ALL patients, 14 CR-AML patients, 14 CR-ALL patients, and 20 healthy volunteers. The proportions and numbers of CD3+CD56+ T lymphocytes in the PB of AML and ALL patients were significantly increased compared with healthy controls (both P < 0.05, Table 1; Fig. 1A and B). At the same time, there were significant decreases in the proportions and numbers of CD3+CD56+ T lymphocytes in the PB of CR-AML and CR-ALL patients compared with primary AML and ALL patients (P < 0.05, Table 1; Fig. 1B), but no difference compared with healthy controls (both P > 0.05, Table 1; Fig. 1A and B). There was no significant difference in numbers between AML and ALL (P > 0.05, Table 1). Interestingly, both the proportion and number of CD3+CD56+ T lymphocytes in the PB of AML patients with WBC counts >10 × 109/L at the time of diagnosis (AML-1) were increased more significantly than those in AML patients with WBC counts <10 × 109/L at the time of diagnosis (AML-2, P < 0.05, Table 2; Fig. 1A and C). In contrast, both the proportion and number of CD3+CD56+ T lymphocytes in the PB of ALL patients with WBC counts <10 × 109/L at the time of diagnosis (ALL-2) were increased more significantly than those in ALL patients with WBC counts >10 × 109/L at the time of diagnosis (Table 2; Fig. 1A and C)
Table 1. Numbers of CD3+CD56+ T lymphocytes in the peripheral blood in patients with AML, ALL, CR-AML, CR-ALL, and in healthy controls.
| Healthy controls | AML | ALL | CR-ALL | CR-ALL | |
|---|---|---|---|---|---|
| Proportion | 2.72% ± 1.58% | 6.05% ± 1.83%* | 7.08% ± 3.70%* | 3.58% ± 1.01% | 3.26% ± 1.53% |
| Number (× 106/L) | 58.9 ± 34.7 | 162.4 ± 54.1* | 183.3 ± 91.7 * | 52.4 ± 14.4 | 43.5 ± 3.9 |
AL, acute leukemia; CR, complete remission; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia. *P < 0.05 in one-way ANOVA
Figure 1. Levels and numbers of CD3+CD56+ T lymphocytes in the peripheral blood of patients with AL, CR-AL, and healthy controls. CD3+CD56+ T lymphocytes were significantly increased in AML and ALL patients compared with healthy controls, and levels recovered in AL patients who achieved CR. CD3+CD56+ T lymphocytes were also increased in AML patients with WBC counts >10 × 109/L and ALL patients with WBC counts <10 × 109/L. (A) CD3+CD56+ T lymphocytes. (B) Numbers in healthy controls, AML, ALL, CR-AML, and CR-ALL patients. (C) Numbers in healthy controls, AML-1, AML-2, ALL-1, and ALL-2 patients.
Table 2. Numbers of CD3+CD56+ T lymphocytes in the peripheral blood of patients with AML-1, ALL-1, AML-2, ALL-2, and in healthy controls.
| Healthy controls | AML-1 | ALL-1 | AML- 2 | ALL-2 | |
|---|---|---|---|---|---|
| Proportion | 2.72% ± 1.58% | 7.90% ± 0.72%* | 0.94% ± 0.39% | 3.89% ± 0.54% | 10.15% ± 2.93%* |
| Number (× 106/L) | 58.9 ± 34.7 | 206.5 ± 31.2* | 13.6 ± 1.64 | 116.4 ± 31.8 | 220.9 ± 59.7* |
AML-1, acute myeloid leukemia patients with increased (>10 × 109/L) WBC counts at the time of diagnosis; ALL-1, acute lymphocytic leukemia patients with increased (>10 × 109/L) WBC counts at the time of diagnosis; AML-2, acute myeloid leukemia patients with low WBC counts (<10 × 109/L) at diagnosis; ALL-2, acute lymphocytic leukemia patients with low WBC counts (<10 × 109/L) at diagnosis. *P < 0.05 in one-way ANOVA
Cytotoxicities of CD3+CD56+ T lymphocytes in the PB of patients with AML and ALL
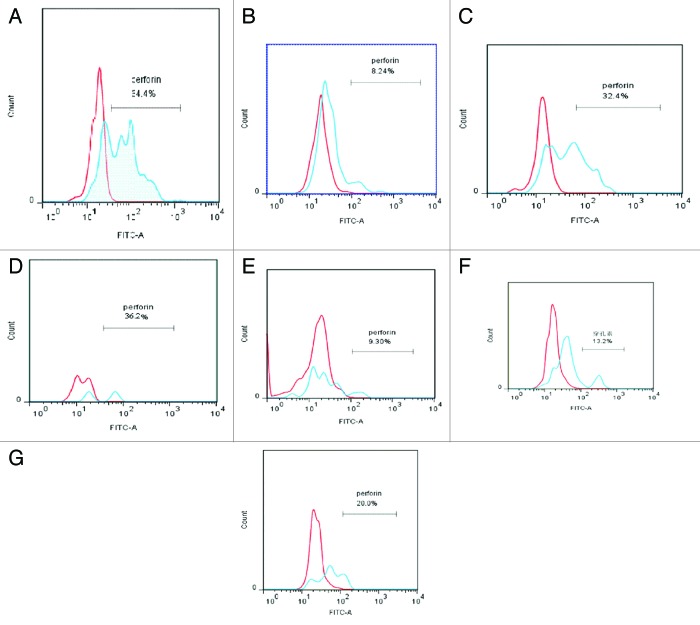
We also examined the cytotoxicities of CD3+CD56+ T lymphocytes in the PB of patients with AML and ALL. Previous studies identified a role for perforin in the anti-tumor effect of CD3+CD56+ T lymphocytes.14,15 We therefore examined the expression of perforin in CD3+CD56+ T lymphocytes and found significant decreases in perforin expression in AML and ALL patients compared with healthy controls (both P < 0.05, Table 3). Although CD3+CD56+ T lymphocyte numbers in the PB recovered to normal levels in AML and ALL patients who achieved CR after chemotherapy, perforin expression levels failed to recover to control levels in these patients (both P < 0.05, Table 3; Fig. 2). There was no difference in perforin levels between AML and ALL patients, but perforin levels were significantly lower in CR-AML patients than in other patients (P < 0.05, Table 3). Further studies with larger sample sizes are needed to confirm this result. In contrast to the numbers of CD3+CD56+ T lymphocytes in the PB, perforin levels were unaffected by changes in WBC numbers in AML and ALL patients, (P > 0.05, Table 4; Fig. 2).
Table 3. Function of CD3+CD56+ T lymphocytes in the peripheral blood of patients with AML, ALL, CR-AML, CR-ALL, and healthy controls.
| Perforin | P* | |
|---|---|---|
| Healthy control | 63.8% ± 7.10% | < 0.05 |
| AML | 26.1% ± 3.34% | < 0.05 |
| ALL | 21.2% ± 4.23% | < 0.05 |
| CR-AML | 13.2% ± 4.81% | < 0.05 |
| CR-ALL | 27.3% ± 2.70% | < 0.05 |
AL, acute leukemia; CR, complete remission; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia. *P < 0.05 in one-way ANOVA
Figure 2. Perforin expression in CD3+CD56+ T lymphocytes in the peripheral blood of patients with AL patient, CR-AL, and healthy controls in relation to WBC count. In each plot, the red peak represents the negative control for perforin, and the blue peak represents perforin expression in each group, respectively.Perforin expression levels in AML and ALL patients were lower than in healthy controls, and remained lower even after CR. Changes in WBC counts in AML and ALL patients had no effect on perforin expression. (A) Healthy controls, (B) AML-1, (C) AML-2; (D) ALL-1, (E) ALL-2, (F) CR-AML, (G) CR-ALL.
Table 4. Function of CD3+CD56+ T lymphocytes in the peripheral blood of patients with AML and ALL in relation to WBC count at diagnosis.
| Perforin | P* | |
|---|---|---|
| AML-1 | 22.5% ± 4.53% | >0.05 |
| AML-2 | 35.5% ± 4.80% | >0.05 |
| ALL-1 | 21.3% ± 8.05% | >0.05 |
| ALL-2 | 21.1% ± 1.82% | >0.05 |
AML-1,indicates acute myeloid leukemia patients with increased (>10 × 109/L) WBC counts at the time of diagnosis;ALL-1, acute lymphocytic leukemia patients with increased (>10 × 109/L) WBC counts at the time of diagnosis;AML-2, acute myeloid leukemia patients whose WBC counts were low (<10 × 109/L) at diagnosis,ALL-2, acute lymphocytic leukemia patients whose WBC counts were low (<10 × 109/L) at diagnosis. *One-way ANOVA.
Discussion
The results of this study demonstrated that CD3+CD56+ T lymphocytes were significantly increased in AML and ALL patients compared with healthy controls, and returned to almost normal levels at the time of CR. These data are in accordance with the results of Le Dieu et al.16 This suggests that the appearance of a malignant clone in AL patients could trigger an immune response, similar to the increases in WBC count and C-reactive protein level that occur in common infections, which return to normal when the infection is controlled. CD3+CD56+ T lymphocyte numbers were also affected by WBC counts. CD3+CD56+ T lymphocytes were significantly increased in AML patients with relatively high WBC counts (>10 × 109/L) at the time of diagnosis, but only slightly increased in patients with lower WBC counts (<10 × 109/L) at the time of diagnosis, compared with healthy controls. This differed from the situation in ALL patients, in whom CD3+CD56+ T lymphocytes were significantly increased in patients with higher WBC counts (<10 × 109/L) at the time of diagnosis, but decreased slightly in patients with lower WBC counts at diagnosis (>10 × 109/L), compared with healthy controls. The clinical significance of this is unclear, but it may reflect the effect of different conditions, such as WBC numbers, on the normal lymphocyte population. However, this would not account for the difference in immune reactions between AML and ALL. Further studies with larger sample sizes are therefore needed to clarify the clinical significance of these findings.
Although the numbers of CD3+CD56+ T lymphocytes, which are known to possess anti-tumor cytotoxicity, were significantly increased in AL compared with healthy controls, they were unable to prevent disease progression. This may be related to dysfunction of these lymphocytes. Immunocytes are known to kill abnormal cells both directly and by other means. Perforin is the main factor involved in direct killing, and the expression levels of perforin in CD3+CD56+ T lymphocytes in vitro has been shown to be very high.14,15 In the current study, however, perforin levels were significantly reduced compared with healthy controls. This suggests that the function of CD3+CD56+ T lymphocytes may be impaired, making them unable to kill the abnormal leukemia cell clone. There was no difference in perforin expression between AML and ALL patients, irrespective of WBC numbers, and perforin levels remained significantly lower than in healthy controls, even in patients who achieved CR. This could help to explain why patients who only receive chemotherapy relapse easily. This is supported by the observation in our hospital that patients who did not receive SCT after consolidation chemotherapy frequently relapsed within one year, and were unable to achieve CR with subsequent chemotherapy, developing refractory leukemia.
Le Dieu et al.16 showed that CD3+CD56+ T lymphocytes are not true NKT cells, though a review of the literature17-21 suggested that CD3+CD56+ T lymphocytes may include several subsets, including NKT and cytokine-induced killer cells (CIK). Future studies are planned to separate the different subpopulations of CD3+CD56+ T lymphocytes, to establish if the increasing cell population represents NKT, CIK, or other CD3+CD56+ T lymphocytes. Changes in the function of the different subsets over time in newly diagnosed patients with AL, and follow-up of patients with CR will also be investigated.
In conclusion, the results of this study suggest that an immune reaction may be triggered in newly diagnosed patients with AML and ALL, but the cytotoxicities of CD3+CD56+ T lymphocytes is impaired, making them unable to kill the leukemia cells. We also showed that the cytotoxicities of CD3+CD56+ T lymphocytes remained impaired in patients who achieved CR. These findings suggest that reinforcing or repairing the function of immunocytes with anti-tumor effects may prolong survival in AL patients unable to undergo SCT, and may thus represent a useful strategy for treating AL.
Materials and Methods
Patients
Fresh PB samples were collected from 40 randomly selected patients with primary AL (24 AML and 16 ALL, excluding acute promyelocytic leukemia) and 28 CR-AL patients (14 AML and 14 ALL, excluding acute promyelocytic leukemia) who visited the Department of Haematology of the Second Affiliated Hospital of Wenzhou Medical College for treatment. There were 14 AML patients and 6 ALL patients with increased (>10 × 109/L) WBC counts at the time of diagnosis (AML-1 and ALL-1, respectively), and 10 AML and 10 ALL patients with low WBC counts (<10 × 109/L) at diagnosis (AML-2 and ALL-2, respectively). The patient characteristics are shown in Table 5. Normal fresh PB samples were obtained from 20 healthy volunteers. No individuals in the control group took any medication or suffered from any known acute or chronic disease. All patients and volunteers had given their signed consent to participate in the study.
Table 5. Characteristics of AML/ALL and CR AML/ALL patients.
| Number | |
|---|---|
| AML | 24 |
| WBC > 10 × 109/L | 14 |
| WBC < 10 × 109/L | 10 |
| ALL | 16 |
| WBC > 10 × 109/L | 6 |
| WBC < 10 × 109/L | 10 |
| CR-AML | 14 |
| CR-ALL | 14 |
AL, acute leukemia; CR, complete remission; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; WBC, white blood cell
Reagents
Allophycocyanin (APC)-anti-human CD45, PerCP-Cy5.5-anti-human CD3, phycoerythrin (PE)-anti-human CD56, fluorescein isothiocyanate (FITC) anti-human perforin were purchased from eBioscience.
Lymphocyte membrane phenotype
Triple-labeling experiments were performed using EDTA-anticoagulated PB samples (AL, CR-AL, and healthy controls). Aliquots of 100 μL were incubated for 30 min at room temperature with pre-titered dilutions of APC-, PE-, and PerCP-Cy5.5-conjugated monoclonal antibodies (mAb) CD45 (HI30), CD3 (OKT3 25 clone, IgG1), and CD56 (IgG1). Isotype-matched control antibodies conjugated with FITC, PE, or PerCP-Cy5.5 were included to establish background fluorescence. Erythrocytes were subsequently lysed by adding 3 mL of NH4Cl for 10 min at room temperature. Cells were then washed in phosphate-buffered saline (PBS) supplemented with 0.1 mM EDTA and 0.02% NaN2 (PBS-EDTA) and kept on ice until flow cytometric examination.
Expression of intracellular perforin
Intracellular staining was performed as described previously.22 Aliquots of surface-stained cells were resuspended in 100 μL of Fixation Medium (Solution A, Fix and Perm™, Caltag Laboratories) and incubated for 15 min at room temperature. After washing in PBS-1% bovine serum albumin, cells were resuspended in 100 μL of permeabilization medium (solution B) and pre-titered saturating amounts of FITC-conjugated anti-Pf mAb (clone dG9, IgG2b), incubated for 30 min at room temperature, washed in PBS-1% BSA and kept on ice until flow cytometric examination.
Flow cytometry
An LSR flow cytometer (FACSCalibur, BD Biosciences) was used for data acquisition and FlowJo (TreeStar Inc.) software was used for analysis.
Statistical analysis
Results were expressed as mean and standard deviation (μ ± SD). The significance of differences was calculated by one-way ANOVA, and the criterion for significance was defined as P < 0.05.
Acknowledgments
We would like to thank Chen Hui and Yang Junjun for their skillful technical assistance. We would also like to thank Zhu Xueqiong for funding assistance. This work was supported by the program of WenZhou Science and Technology Bureau.
Glossary
Abbreviations:
- AL
acute leukemia
- CR
complete remission
- AML
acute myeloid leukemia
- ALL
acute lymphocytic leukemia
- WBC
white blood cell
- PB
peripheral blood
- NK
natural killer
- LAK
interleukin (IL)-2-activated killer
- SCID
severe combined immunodeficient mice
- AML-1
acute myeloid leukemia patients with increased (>10 × 109/L) WBC counts at the time of diagnosis
- ALL-1
acute lymphocytic leukemia patients with increased (>10 × 109/L) WBC counts at the time of diagnosis
- AML-2
acute myeloid leukemia patients with low WBC counts (<10 × 109/L) at diagnosis
- ALL-2
acute lymphocytic leukemia patients with low WBC counts (<10×109/L) at diagnosis
- CIK
cytokine-induced killer cell
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/25938
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. [PubMed] [Google Scholar]
- 3.Görgün G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115:1797–805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorgun G, Ramsay AG, Holderried TA, Zahrieh D, Le Dieu R, Liu F, Quackenbush J, Croce CM, Gribben JG. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106:6250–5. doi: 10.1073/pnas.0901166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–9. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 6.Ramsay AG, Johnson AJ, Lee AM, Gorgün G, Le Dieu R, Blum W, Byrd JC, Gribben JG. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–37. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573–83. doi: 10.1016/S0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 8.Joyce S. CD1d and natural T cells: how their properties jump-start the immune system. Cell Mol Life Sci. 2001;58:442–69. doi: 10.1007/PL00000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148–52. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 10.Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, Hiraide H, Sekine I. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103:281–90. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–96. [PubMed] [Google Scholar]
- 12.Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997;74:51–6. doi: 10.1007/s002770050257. [DOI] [PubMed] [Google Scholar]
- 13.Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92:3318–27. [PubMed] [Google Scholar]
- 14.Gritzapis AD, Dimitroulopoulos D, Paraskevas E, Baxevanis CN, Papamichail M. Large-scale expansion of CD3(+)CD56(+) lymphocytes capable of lysing autologous tumor cells with cytokine-rich supernatants. Cancer Immunol Immunother. 2002;51:440–8. doi: 10.1007/s00262-002-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guven H, Gilljam M, Chambers BJ, Ljunggren HG, Christensson B, Kimby E, Dilber MS. Expansion of natural killer (NK) and natural killer-like T (NKT)-cell populations derived from patients with B-chronic lymphocytic leukemia (B-CLL): a potential source for cellular immunotherapy. Leukemia. 2003;17:1973–80. doi: 10.1038/sj.leu.2403083. [DOI] [PubMed] [Google Scholar]
- 16.Le Dieu R, Taussig DC, Ramsay AG, Mitter R, Miraki-Moud F, Fatah R, Lee AM, Lister TA, Gribben JG. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009;114:3909–16. doi: 10.1182/blood-2009-02-206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada H, Nagamura-Inoue T, Mori Y, Takahashi TA. Expansion of Valpha24(+)Vbeta11(+) NKT cells from cord blood mononuclear cells using IL-15, IL-7 and Flt3-L depends on monocytes. Eur J Immunol. 2006;36:236–44. doi: 10.1002/eji.200526085. [DOI] [PubMed] [Google Scholar]
- 18.Chamoto K, Takeshima T, Kosaka A, Tsuji T, Matsuzaki J, Togashi Y, Ikeda H, Nishimura T. NKT cells act as regulatory cells rather than killer cells during activation of NK cell-mediated cytotoxicity by alpha-galactosylceramide in vivo. Immunol Lett. 2004;95:5–11. doi: 10.1016/j.imlet.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–57. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202:1623–6. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gütgemann S, Frank S, Strehl J, Schmidt-Wolf IG. Cytokine-induced killer cells are type II natural killer T cells. Ger Med Sci. 2007;5:Doc07. [PMC free article] [PubMed] [Google Scholar]
- 22.Rumi C, Rutella S, Teofili L, Etuk B, Ortu La Barbera E, Micciulli G, Voso MT, Leone G. RhG-CSF-mobilized CD34+ peripheral blood progenitors are myeloperoxidase-negative and noncycling irrespective of CD33 or CD13 coexpression. Exp Hematol. 1997;25:246–51. [PubMed] [Google Scholar]