Abstract
Introduction
Human saphenous vein (HSV) is the most widely used bypass conduit for peripheral and coronary vascular reconstructions. However, outcomes are limited by a high rate of intimal hyperplasia (IH). HSV undergoes a series of ex vivo surgical manipulations prior to implantation, including hydrostatic distension, marking, and warm ischemia in solution. We investigated the impact of surgical preparation on HSV cellular function and development of IH in organ culture. We hypothesized that oxidative stress is a mediator of HSV dysfunction.
Methods
HSV was collected from patients undergoing vascular bypass before and after surgical preparation. Smooth muscle and endothelial function were measured using a muscle bath. Endothelial preservation was assessed with immunohistochemical staining. An organ culture model was used to investigate the influence of surgical preparation injury on the development of IH. Superoxide levels were measured using a high-performance liquid chromatography-based assay. The influence of oxidative stress on HSV physiologic responses was investigated by exposing HSV to hydrogen peroxide (H2O2).
Results
Surgical vein graft preparation resulted in smooth muscle and endothelial dysfunction, endothelial denudation, diminished endothelial nitric oxide synthase staining, development of increased IH, and increased levels of reactive oxygen species. Experimental induction of oxidative stress in unmanipulated HSV by treatment with H2O2 promoted endothelial dysfunction. Duration of storage time in solution did not contribute to smooth muscle or endothelial dysfunction.
Conclusions
Surgical vein graft preparation causes dysfunction of the smooth muscle and endothelium, endothelial denudation, reduced endothelial nitric oxide synthase expression, and promotes IH in organ culture. Moreover, increased levels of reactive oxygen species are produced and may promote further vein graft dysfunction. These results argue for less injurious means of preparing HSV prior to autologous transplantation into the arterial circulation.
Approximately 1,000,000 aortocoronary and peripheral vascular reconstructions are performed annually using human saphenous vein (HSV). The leading cause of vein graft failure is intimal hyperplasia (IH).1 This process leads to pathologic narrowing of the vessel lumen, graft stenosis, and ultimately graft failure.2 IH remains the primary factor limiting the durability of vein bypass grafts and contributes to significant morbidity, reintervention, limb loss, myocardial infarction, and death. While technical errors, poor outflow, thrombosis, and vasospasm are the principle etiologies of vein graft failure in the immediate postoperative period (<30 days), IH and atherosclerosis are the leading causes of vein graft failure in the short-term (30 days to 2 years) and long-term (>2 years) time frames, respectively.3 Two recent large phase III multicenter, randomized, double-blinded, placebo-controlled clinical trials have examined outcomes in coronary artery bypass grafting and peripheral vascular bypass grafting. The Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT) III trial demonstrated a 61% primary patency rate at 1 year following peripheral vascular bypass grafting.4 The per patient vein graft failure rate after coronary artery bypass grafting was 45% in the PREVENT IV trial at 12 to 18 months.5 These trials set a modern standard for benchmark outcomes from these procedures.
Successive and additive levels of vein graft injury occur during vein conduit harvest and preparation. These include mechanical stretch,6 conduit distension using a hand-held syringe for identification of leaks and side branches,7 marking of the conduit using surgical skin markers for purposes of orientation,8 and vein conduit storage in acidic solutions.9 The surgical literature has focused primarily on the histologic and morphologic changes occurring to the vein graft following surgical harvest and preparation, including disruption of the vasa vasorum,10 surgical trauma to the endothelium,11 and tunica media.12 However, the degree to which these morphologic changes impact the cellular viability and physiology of the HSV graft has not been well investigated. Moreover, the standard process of vein graft preparation including distension, marking, and warm ischemia in solution has never been validated or demonstrated to adequately preserve tissue viability.
Oxidative stress is a well-known mechanism mediating vascular injury in multiple cardiovascular diseases.13 The production of reactive oxygen species becomes magnified and dysregulated in pathophysiologic states and serves as a secondary mediator of injury. Oxidative stress has been postulated to contribute to vein graft dysfunction,14 but evidence of this is lacking in human tissue. Therefore, we investigated the influence of surgical vein graft preparation on cellular viability and HSV physiology, the role of surgical vein graft preparation in the development of IH in vitro, and the role of oxidative stress as a mechanistic contributor to vein graft dysfunction in HSV.
METHODS
HSV procurement
HSV samples were obtained after approval from the Institutional Review Boards of Vanderbilt University Medical Center and the Tennessee Valley Veterans Affairs Medical Center, Nashville, Tenn. HSV segments were collected after obtaining informed consent from patients undergoing coronary artery bypass grafting. Method of vein harvest (open or endoscopic) and graft preparation was at the discretion of the surgical team. Vein segments were collected immediately following surgical harvest (“unmanipulated” vein samples; UM) and were used immediately following collection for experiments described below. Additional vein segments were collected again later after a series of manipulations, including hydrostatic distention with a hand-held syringe, dotted or continuous marking with a surgical skin marker, and placement in heparinized plasmalyte (HP; 10 units heparin/mL plasmalyte) at room temperature for storage until implantation (“after manipulation” vein samples; AM; Fig 1), and were used immediately prior to implantation for the experiments described below. Veins were used for experimentation within 15 minutes of collection. When open harvest was employed, the vein was divided distally, cannulated, and crystalloid was intermittently infused to aid in side branch ligation. For all HSV used in this study, we obtained paired UM/AM samples, except where specified. All HSV used for the experiments described below was stored in HP at room temperature until experimentation. All AM-HSV segments procured for this study were small pieces removed from either end of the conduits used for revascularization and were collected at the time of arterial implantation. Areas of HSV subjected to clamp or crush injury were discarded. Therefore, all segments of AM-HSV we obtained were intended for use as part of bypass conduits and were obtained at the time of arterial implantation. The particular anatomic portion of HSV that was procured (ie, proximal vs distal) was, again, at the discretion of the surgical team and was not recorded for this study.
Fig 1.
Steps in surgical vein graft preparation and sources of tissue used in this investigation. Following human saphenous vein (HSV) harvest, vein graft preparation is performed. Ex vivo manipulations include hydrostatic distention to identify leaks and overcome spasm, marking with a surgical skin marker for orientation purposes, and repair of leaks. The conduit is then placed in a storage solution at room temperature where it undergoes a variable period of warm ischemia. These steps all precede eventual surgical implantation in the arterial circulation. For this investigation, HSV obtained immediately following harvest (“unmanipulated”; UM-HSV) was compared with HSV obtained following surgical vein graft preparation (“after manipulation”; AM-HSV).
Collection of clinical demographic variables
Along with prospective collection of HSV tissue, demographic variables of the source patients were prospectively collected, including age, gender, race, body mass index, medical comorbidities, preoperative laboratory values, preoperative medication regimen, and method of HSV harvest.
Physiological measurement of HSV smooth muscle functional viability
HSV samples were sectioned into 1-mm rings. These were weighed and their diameter was measured. HSV rings were suspended in a muscle bath containing a bicarbonate buffer as previously described.15 Smooth muscle viability was determined by contracting HSV with potassium chloride (KCl), which causes membrane depolarization and contraction of functionally viable smooth muscle.16 Rings were then washed to remove KCl and equilibrated in bicarbonate buffer for 30 minutes.6 The concentration of the physiologic agonist phenylephrine (PE) that induces submaximal contractile responses was determined by treating the tissue with increasing doses (0.01, 0.1, and 1 μM) of PE. Contractile response was defined as stress ([105 Newtons (N)/m2] = force (g) × 0.0987/area, where area is equal to the wet weight [(mg)/length (mm at maximal length)] divided by 1.055),17 which was calculated using the force (g) generated by the tissue. We have previously demonstrated that the production of force of less than 0.025 × 105 N/m2 in response to KCl correlates with diminished cellular viability as measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide live/dead assay.6
Physiological measurement of HSV smooth muscle-dependent vasorelaxation
HSV was prepared as described above except that the endothelium was gently mechanically denuded. Viable HSV was precontracted with PE and treated with escalating doses of sodium nitroprusside (SNP) necessary to achieve measurable vasorelaxation (10−8 to 10−6 M).
Physiological measurement of HSV endothelial-dependent vasorelaxation
HSV was prepared and tested as described above except that the endothelium was preserved. Viable HSV was precontracted with PE and then exposed to carbachol (CCH; 5 × 10−7 M), an acetylcholine analog, and the maximal relaxation response was determined.18
HSV immunohistochemical staining for CD31 and endothelial nitric oxide synthase (eNOS)
HSV segments were fixed in 10% formalin and sent to the Vanderbilt Translational Pathology Shared Resource for processing. Tissues were dehydrated with ethanol, embedded in paraffin, and immunostained using a Bond Max IHC stainer (Leica Biosystems GmbH, Nussloch, Germany). Heat-induced antigen retrieval was performed using their Epitope Retrieval 2 solution (Leica Biosystems GmbH) for 20 minutes. Slides were incubated with CD31 (NCL-CD31-1A10; Leica Microsystems, Buffalo Grove, Ill) at 1:100 dilution or eNOS (ab91205; Abcam, Inc, Cambridge, Mass) at 1:600 dilution for one hour. The Bond Polymer Refine detection system (Leica Biosystems GmbH) was used for visualization. Slides were then dehydrated, cleared, and coverslipped. The degree of endothelial staining for eNOS and CD31 was assessed using an Axiovert (Carl Zeiss SMT GmbH, Oberkochen, Germany) at 20× to 100× magnification. The intensity of staining was assessed by a blinded observer using a qualitative score from (1+) to (4+) based on the proportion of vein circumference with visible staining: (1+), <25% vein circumference; (2+), 25% to 49%; (3+), 50% to 74%; and (4+), 75% to 100%.
HSV physiologic measurements as a function of storage time
In order to investigate whether storage time had any influence on the physiology of UM vs AM-HSV, we measured physiology as a function of storage time. UM-HSV was procured as described above, sectioned into rings, and placed in HP at room temperature for 30 minutes, 1 hour, 2 hours, and 3 hours. At the end of incubation, HSV rings were suspended in a muscle bath in duplicate for measurement of contractile force, smooth muscle-dependent relaxation, and endothelial-dependent relaxation.
HSV organ culture
Additional UM-HSV and AM-HSV rings (3 mm in length) were cut, placed in eight-well chamber slides in duplicate, and maintained in RPMI 1640 medium with 30% FBS, 1% L-glutamine, and 1% penicillin/streptomycin for 14 days at 37°C/5% CO2 as previously described.19 After 14 days, tissue was fixed in formalin, imbedded in paraffin, and histologic sections were prepared and stained with Verhoeff-Van Gieson. Four-quadrant measurements were made of intimal and medial thickness of preculture and postculture rings by a blinded observer and intimal-to-medial ratio was calculated.
Measurement of reactive oxygen species in HSV
UM-HSV and AM-HSV were divided into 1-mm rings and immediately processed by the Vanderbilt Free Radical in Medicine Core for measurement of reactive oxygen species. Tissues were incubated for 30 minutes at 37°C in 1 mL of Krebs/HEPES buffer containing 50 μmol/L of dihydroethidium (DHE). Superoxide ( ) was measured using DHE and a high-performance liquid chromatography-based assay.20 The reaction of DHE with generates 2-hydroxyethidium. The 2-hydroxyethidium peak on high-performance liquid chromatography reflects the amount of formed in the tissue during the incubation and is expressed per milligram of protein.
Experimental induction of oxidative stress in HSV
UM-HSV was cut into 1-mm rings and placed in HP containing hydrogen peroxide (H2O2) concentrations of 100 μM, 1 mM, and 10 mM for 1 hour at room temperature. HSV physiological measurements were then performed in the muscle bath as described above.
Data analysis
Data is reported as mean ± standard error of the mean unless indicated otherwise. Paired two-tailed t-tests were conducted to assess the statistical significance of each experiment using GraphPad Prism software (La Jolla, Calif). P value of ≤.05 was considered statistically significant.
RESULTS
HSV collection and patient demographic variables
The demographic variables for the patients included in this analysis are listed in Table I. The demographics are typical for patients undergoing coronary revascularization. Unless stated otherwise, all experiments were performed with paired UM/AM samples from the same patients. Over half of the paired UM/AM-HSV samples were harvested endoscopically, and the remainder of the paired UM/AM-HSV samples were harvested utilizing conventional open harvest technique. Open vs endoscopic vein graft harvest did not produce any significant differences in the physiologic parameters we examined (data not shown). Angioscopy or valvulotomy were not utilized on any of the HSV collected for this study. Papaverine was not used during vein graft preparation.
Table I.
Preoperative clinical demographics of patients from whom HSV was procured for this study
| Age, years (mean ± SD) | 64.2 ± 11 |
| Gender, male | 78% |
| Body mass index (mean ± SD) | 30.0 ± 8 |
| Race: Caucasian/African American | 90%/10% |
| Endoscopic vein harvest | 57% |
| History of smoking | 65% |
| Hypertension | 91% |
| Number of antihypertensives (mean ± SD) | 1.9 ± 1.1 |
| Angiotensin-converting-enzyme inhibitor use | 49% |
| Beta blocker use | 68% |
| Antiplatelet drug use | 85% |
| Diabetes mellitus | 57% |
| Preoperative hemoglobin A1c (mean ± SD) | 6.7 ± 1.2 |
| Hyperlipidemia | 83% |
| Statin use | 72% |
| Left ventricular ejection fraction, mean | 53% |
| Chronic kidney disease | 57% |
| End stage renal disease, dialysis dependent | 4% |
| Peripheral vascular disease | 17% |
HSV, Human saphenous vein; SD, standard deviation.
HSV smooth muscle functional viability
UM-HSV generated significantly greater contractile force (0.13 ± 0.009 × 105 N/m2) compared with AM-HSV (0.05 ± 0.006 × 105; n = 47; P < .0001; Fig 2, A). All UM-HSV generated force of 0.025 × 105 N/m2 or greater, and therefore, all UM-HSV was functionally viable by our pre-established criteria.6 Of AM-HSV, 35 of 47 samples (74%) were viable. UM-HSV generated significantly greater contractile force in response to PE (0.08 ± 0.008 × 105 N/m2) compared with AM-HSV (0.04 ± 0.005 × 105; n = 41; P < .0001; Fig 2, B). These data suggest that surgical vein graft preparation causes injury that compromises smooth muscle viability.
Fig 2.
Physiologic measurements of human saphenous vein (HSV) smooth muscle and endothelial function before and after surgical vein graft preparation. Physiologic responses are significantly impaired in HSV obtained after manipulation (AM) compared with paired samples of unmanipulated (UM)-HSV; these include contractile response to potassium chloride (KCl; n = 47; P < .0001; A), contractile response to phenylephrine (PE; n = 41; P < .0001; B), smooth muscle-dependent relaxation in response to sodium nitroprusside (SNP; n = 34; P < .0001; C), and HSV endothelial function in response to carbachol (CCH; n = 36; P < .0001; D). *P < .05; **P < .01; and ***P < .001.
HSV smooth muscle-dependent vasorelaxation
UM-HSV generated significantly greater smooth muscle-dependent relaxation (62% ± 4%) compared with AM-HSV (31% ± 4%; n = 34; P < .0001; Fig 2, C). These data further suggest that surgical vein graft preparation causes injury that compromises smooth muscle viability.
HSV endothelial-dependent vasorelaxation
UM-HSV generated significantly greater endothelial-dependent relaxation (21% ± 3%) compared with AM-HSV (1% ± 2%; n = 36; P < .0001; Fig 2, D). These data suggest that surgical vein graft preparation causes injury that compromises endothelial viability. Given that that endothelium is the most fragile tissue component in HSV, these findings are not unexpected, and argue for less injurious means of surgical vein graft preparation in order to preserve this fragile monolayer.
Immunohistochemical staining for eNOS and CD31
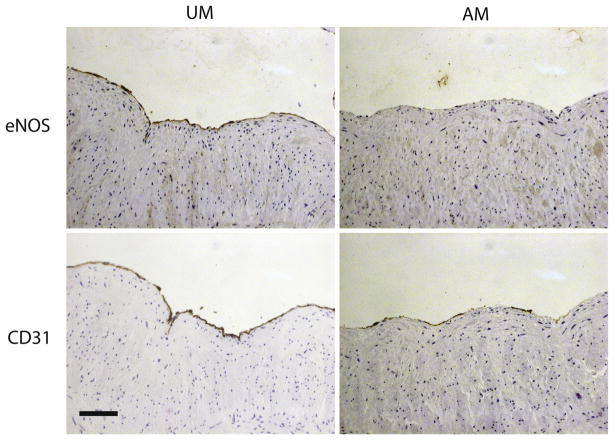
We observed 3+ or 4+ CD31 staining in UM-HSV (Table II). Surgical vein graft preparation was associated with diminished CD31 staining in eight of 11 samples of AM-HSV; endothelial coverage remained unchanged in the remaining three of 11 samples after surgical vein graft preparation. We observed more variability in UM-HSV eNOS staining. Among UM-HSV samples, nine of 11 had 3+ or 4+ eNOS staining, and two of 11 had 1+ or 2+ eNOS staining. Surgical vein graft preparation was associated with diminished eNOS staining in nine of 11 samples and this was more pronounced than loss of CD31 staining: two of 11 AM-HSV samples had 3+ staining; three of 11 samples had 2+ staining, and the remaining six of 11 had 1+ staining. Representative photomicrographs are illustrated in Fig 3. These data suggest that the diminished endothelial viability observed following surgical vein graft preparation results in part from endothelial denudation, and furthermore from loss of eNOS.
Table II.
Immunohistochemical staining for eNOS and CD31 in HSV obtained unmanipulated and after surgical manipulation
| HSV | UM-HSV eNOS staining | AM-HSV eNOS staining | UM-HSV CD31 staining | AM-HSV CD31 staining |
|---|---|---|---|---|
| 1 | +++ | +++ | +++ | ++++ |
| 2 | ++ | + | ++++ | ++++ |
| 3 | + | + | +++ | ++ |
| 4 | ++++ | +++ | ++++ | ++++ |
| 5 | +++ | ++ | ++++ | +++ |
| 6 | ++++ | ++ | ++++ | ++ |
| 7 | +++ | ++ | ++++ | +++ |
| 8 | +++ | + | ++++ | ++ |
| 9 | +++ | + | ++++ | ++ |
| 10 | +++ | + | ++++ | ++ |
| 11 | ++++ | + | ++++ | +++ |
AM-HSV, Human saphenous vein after surgical manipulation; eNOS, endothelial nitric oxide synthase; HSV, human saphenous vein; UM-HSV, unmanipulated human saphenous vein.
Fig 3.
Immunohistochemical staining of human saphenous vein (HSV) for endothelial nitric oxide synthase (eNOS) and CD31. Unmanipulated HSV (UM-HSV) exhibits continuous (4+) eNOS and CD31 staining. HSV obtained after surgical manipulation (AM-HSV) exhibits near absence of eNOS staining (1+) and patchy loss of endothelial coverage by CD31 staining (2+). Photomicrographs are representative of 11 HSV samples. The scale bar represents 100 μm.
HSV physiologic measurements as a function of storage time
We measured physiologic parameters in UM-HSV samples obtained from six patients. Duration of vein graft storage for up to 3 hours at room temperature in HP did not significantly impair or improve the physiologic variables tested (Fig 4). There was no significant change in contractile response to KCl (n = 6; P = NS; Fig 4, A), contractile response to PE (n = 6; P = NS; Fig 4, B), SNP-induced smooth muscle-dependent relaxation (n = 6; P = NS; Fig 4, C), or CCH-induced endothelial-dependent relaxation (n = 6; P = NS; Fig 4, D). These data suggest that the duration of time in a physiologic storage solution does not cause HSV dysfunction. Therefore, it must be the other components of surgical vein graft preparation – distension and marking – that contribute to vein graft dysfunction. These data suggest that the vein graft dysfunction we observed in AM-HSV was not due to the additional storage time in HP.
Fig 4.
Human saphenous vein (HSV) physiological measurements as a function of warm ischemia time in heparinized plasmalyte. Duration of warm ischemia for up to 3 hours does not influence smooth muscle contractile response to potassium chloride (KCl; n = 6; P = NS; A), smooth muscle contractile response to phenylephrine (PE; n = 6; P = NS; B), smooth muscle-dependent relaxation in response to sodium nitroprusside (SNP; n = 6; P = NS; C), or endothelial-dependent relaxation in response to carbachol (CCH; n = 6; P = NS; D).
HSV organ culture
After 2 weeks in organ culture, we observed an increase in intimal thickness of 22.81 ± 18.77 μm in UM-HSV, and intimal/medial ratio increased by 22% ± 27%. We observed an increase in intimal thickness by 38.53 ± 26.48 μm in AM-HSV, and intimal/medial ratio increased by 50% ± 37%. Compared with UM-HSV, AM-HSV developed a 69% increase in intimal thickness (n = 11; P = .043; Fig 5, A), and a 122% increase in intimal/medial ratio (n = 11; P = .015; Fig 5, B). These observations demonstrate that surgical vein graft preparation causes injury beyond that induced by harvest alone and that is sufficient to promote neo-intimal growth.
Fig 5.
Surgical vein graft preparation promotes development of intimal hyperplasia (IH) in organ culture. A, The increase in intimal thickness (μm) is significantly greater in human saphenous vein (HSV) obtained after surgical manipulation (AM) compared with HSV obtained unmanipulated (UM) after 2 weeks in organ culture (n = 11; P = .043). B, The percent increase in intimal to medial ratio (I/M ratio) is significantly greater in AM-HSV compared with UM-HSV (n = 11; P = .015). *P < .05; **P < .01; and ***P < .001.
Measurement of reactive oxygen species in HSV
Levels of 2-hydroxyethidium in UM-HSV were 227.6 ± 40.44 pmol/mg protein vs 382.5 ± 43.07 in AM-HSV (n = 4; P = .03; Fig 6), indicating increased generation of reactive oxygen species after surgical vein graft preparation. These observations demonstrate that surgical vein graft preparation causes additional reactive oxygen species generation beyond that induced by harvest alone.
Fig 6.

The generation of reactive oxygen species in human saphenous vein (HSV) is increased after undergoing surgical vein graft preparation. Levels of reactive oxygen species were assessed by measuring by the conversion of dihydroxyethidium (DHE) to 2-hydroxyethidium. Levels of 2-hydroxyethidium were significantly greater in HSV obtained after ex vivo surgical vein graft preparation injury compared with HSV immediately following surgical vein graft harvest (n = 4; P = .03). *P < .05; **P < .01; and ***P < .001. AM, After surgical manipulation; UM, unmanipulated.
Experimental induction of oxidative stress in HSV
We performed these experiments in UM-HSV samples obtained from six patients. One hour treatment of UM-HSV with H2O2 resulted in significant blunting of endothelial-dependent relaxation at concentrations of 1 mM and 10 mM, but not at 100 μM (Fig 7). Endothelial-dependent relaxation in UM-HSV was 27% ± 9% in comparison with 9% ± 5% in UM-HSV treated with 1 mM H2O2 (n = 6; P = .04) and 10% ± 4% in UM-HSV treated with 10 mM H2O2 (n = 5; P = .03). These data demonstrate that reactive oxygen species can cause endothelial dysfunction in HSV. Therefore, the presence of increased reactive oxygen species, such as that generated secondary to surgical vein graft preparation, serves as a secondary mediator of vein graft dysfunction.
Fig 7.
Experimental induction of oxidative stress causes endothelial dysfunction in human saphenous vein (HSV). Compared with unmanipulated (UM)-HSV, 1-hour treatment of UM-HSV with hydrogen peroxide (H2O2) caused a significant decline in endothelial-dependent relaxation when exposed to 1 mM H2O2 (n = 6; P = .04) and 10 mM H2O2 (n = 5; P = .03). *P < .05; **P < .01; and ***P < .001.
DISCUSSION
HSV is harvested, prepared, stored, and then reimplanted as a conduit to bypass arterial stenoses and occlusions. Therefore, HSV is an autotransplanted organ, but is not widely considered or treated as such. Surgical vein graft preparation causes successive injury at multiple levels prior to arterial implantation (Fig 1). Vein graft harvest is injurious secondary to hypoxia, stretch, and traction injury.6 After harvest, ex vivo vein graft preparation is performed on the “back table.” While methods of vein graft preparation remain surgeon-dependent, most distend the conduit with a hand-held syringe in order to identify leaks and overcome spasm. We have measured intraluminal pressures generated during gentle distension, and these uniformly exceed 750 mm Hg (data not shown), regardless of syringe size or perceived force placed on the syringe plunger. Conduit distension causes significant morphologic changes, including endothelial denudation and smooth muscle damage.7,21 Additionally, most surgeons mark the vein graft for orientation using a sterile surgical marking pen intended for use on the skin. We have recently demonstrated that this common practice causes smooth muscle and endothelial dysfunction secondary to toxic components in the ink, including isopropyl alcohol (a solvent).8 Isopropyl alcohol is converted to acetone in vivo,22 which increases oxidative stress by causing generation of reactive oxygen species.23 Finally, the conduit is placed in a storage solution for a variable duration. Normal saline is a commonly utilized storage solution and is an acidic, non-buffered solution with pH <6.0. Despite the non-physiologic properties of this storage solution, normal saline was utilized by 40% of centers (JH Alexander, MD, unpublished data, 2013) in the PREVENT IV trial.5 Vein preparation techniques have yet to be scientifically validated despite their widespread application.
Our results indicate that surgical vein graft preparation causes significant cellular dysfunction of the two principle HSV cell types: smooth muscle and endothelium (Fig 2, A–D). We have previously demonstrated that the production of force of less than 0.025 × 105 N/m2 in response to KCl correlates with diminished cellular viability as measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide live/dead assay.6 AM-HSV produced significantly diminished contractile force in response to KCl (Fig 2, A), and PE (Fig 2, B). Smooth muscle-dependent vasorelaxation in response to SNP (Fig 2, C) was also diminished. Therefore, surgical vein graft preparation causes additional vascular smooth muscle injury beyond that incurred during surgical vein graft harvest alone. To our knowledge, smooth muscle dysfunction has not been demonstrated to occur in HSV grafts this early ex vivo. These findings implicate surgical vein graft preparation as an important mediator of vein graft dysfunction.
The injury of potentially greatest consequence to the long-term success of the bypass conduit is the loss of endothelial function following surgical vein graft preparation (Fig 2, D). We observed a contractile response to CCH rather than relaxation in many of the AM-HSV samples. Acetylcholine (and its analog, CCH) act on vascular smooth muscle to cause vasoconstriction in tissue with endothelial injury or loss.24 These measurements of diminished to absent endothelial function correlated with loss of endothelial coverage on immunohistochemical staining for CD31, with superimposed and more pronounced loss of eNOS staining (Table II; Fig 3). These results suggest that, while harvest leads to mild endothelial denudation and loss of eNOS, these insults are magnified following ex vivo surgical vein graft preparation. A viable and intact endothelium serves multiple protective roles. Nitric oxide released by the endothelium promotes vasodilation and prevents platelet adhesion and thrombosis. In contrast, dysfunctional endothelium exerts prothrombotic properties25 and allows attachment of circulating platelets and leukocytes that secrete growth factors, a critical step in the development of the hyperplastic lesion.26 Endothelial denudation therefore increases the risk of thrombosis in the immediate postoperative period (<30 days), and may initiate further cascades of vascular injury and damage, leading to the development of IH in the short- and long-term lifespan of the conduit.
Surgically harvested HSV (UM-HSV) that does not undergo ex vivo surgical vein graft preparation exhibits preservation of physiologic function for up to 3 hours of storage in HP (10 U/mL) at room temperature (Fig 4). We have identified HP as a buffered physiologic storage solution that is superior to most other commonly utilized storage solutions for preservation of vein graft physiology (data not shown), and this solution has been universally adopted at our institution. These data demonstrate that the additional storage time in HP to which the AM-HSV segments were subjected in the course of our experimental design did not contribute to physiologic dysfunction. This suggests that the other manipulations occurring during this period, such as hydrostatic distention and marking, are the primary factors leading to smooth muscle and endothelial dysfunction (Fig 2, A–D).
Injury incurred during surgical vein graft preparation is a sufficient stimulus for the promotion of IH in organ culture, a well-validated in vitro model that replicates the smooth muscle proliferation, migration, and extracellular matrix deposition occurring in IH.19,27 We observed a significant increase in both intimal thickness and intimal/medial ratio in HSV subjected to surgical vein graft preparation (Fig 5). These data support the hypothesis that early vein graft injury is an important stimulus leading to IH. Importantly, this injury response can occur in the absence of the turbulent and pulsatile hemodynamic alterations associated with placement of a venous graft in arterial circulation and in the absence of growth factors released from circulating leukocytes and platelets, further implicating pre-existing tissue injury as an inciting agent in this process.
Our data indicate that cellular vein graft dysfunction is mediated in part by oxidative stress. Levels of reactive oxygen species nearly doubled in response to surgical vein graft preparation (Fig 6). Moreover, experimentally induced oxidative stress is a secondary mediator of endothelial dysfunction in UM-HSV (Fig 7). Therefore, reactive oxygen species are generated during vascular injury and serve as secondary mediators of tissue injury, particularly involving the endothelium.13 Even brief exposure to reactive oxygen species has been demonstrated to stimulate vascular smooth muscle cell proliferation in cultured cell lines persisting well beyond the time of exposure.28 The nicotinamide adenine dinucleotide phosphate oxidases are the most important source for superoxide production and they are activated by vascular injury and hypoxia.29,30 Oxidative stress enhances endothelial permeability and promotes leukocyte attachment.13 Binding and activation of neutrophils causes superoxide generation as part of the respiratory burst.31 Superoxide production can initiate a cascade of other reactive oxygen species (H2O2, hydroxyl radicals, and peroxynitrite), which indiscriminately react with and oxidize lipids, proteins, and DNA in the vicinity and cause cellular damage and death.13,30 Reactive oxygen species further react with and deplete NO, forming peroxynitrite.30 Therefore, oxidative stress is a plausible mechanistic contributor to the near-complete loss of measurable endothelial function (Fig 2, D) in HSV subjected to surgical vein graft preparation.
While oxidative stress has been implicated in the pathogenesis of multiple cardiovascular diseases, it remains to be demonstrated whether this contributes to vein graft dysfunction in human tissue. Our data demonstrate increased levels of reactive oxygen species induced very early following surgical vein graft preparation; these reactive oxygen species cause secondary injury to the vein graft. Reactive oxygen species have been demonstrated to initiate cellular responses with long-lasting effects persisting beyond the time of exposure. For example, it has been shown that very brief exposure to reactive oxygen species lasting only 10 minutes is a sufficient stimulus for vascular smooth muscle proliferation in cultured cell lines.28 It is not known yet whether avoiding vein graft injury alone is sufficient to prevent reactive oxygen species generation, or whether antioxidant agents have therapeutic efficacy. We have demonstrated that University of Wisconsin organ preservation solution (which contains the antioxidant glutathione) preserves vascular smooth muscle function significantly better than plasmalyte alone (data not shown). Furthermore, preliminary proteomics data point to a possible role of vascular smooth muscle apoptosis in vein graft failure. Whether this process is related to reactive oxygen species production is an area of future investigation.
Based on these data, the following recommendations should be considered. Conduit distention must be gentle and pressures must be limited to physiologic levels when possible. A simple but effective method for achieving this is to perform the proximal arterial anastomosis first, and then to revascularize and distend the conduit using arterial blood inflow. This method has the advantages of limiting conduit pressures to systemic arterial blood pressures, and shortening the period of “warm ischemia” during which the vein is otherwise stored at room temperature in hypoxic conditions. Marking the vein graft with surgical skin marking pens should be avoided. The toxic components in these pens cause significant vein graft dysfunction. When it is mandatory to mark the conduit (ie, during tunneling so as to avoid twisting), we urge clinicians to consider interrupted rather than continuous marking. A buffered physiologic storage solution such as plasmalyte, University of Wisconsin solution, or heparinized blood should be used to store the conduit. Saline should be avoided as a storage solution given its acidic and non-buffered properties.
This study has several limitations. First, collection of human tissue from different surgical teams introduced variability in the way the tissue was handled prior to experimentation. However, the advantage of this approach is that the AM-HSV segments obtained were sections from the actual vein grafts used to revascularize patients, and we obtained the tissue for experimentation immediately prior to arterial implantation. In addition, our data regarding IH was derived from an organ culture model possessing limitations, including the absence of hemodynamic alterations occurring in arterial circulation and the absence of circulating leukocytes and other circulating blood components that may modulate the development of IH. Future areas of investigation include further mechanistic work into the means by which reactive oxygen species induce vein graft failure, and also to investigate whether reactive oxygen species generation can be avoided using antioxidants or inhibitors of the nicotinamide adenine dinucleotide phosphate oxidases.
In summary, surgical vein graft preparation causes endothelial and smooth muscle dysfunction, diminished smooth muscle viability, production of reactive oxygen species, and IH. These results collectively argue for less injurious means of surgical vein graft preparation. The ex vivo component of vein graft preparation provides a unique therapeutic window for delivery of compounds, antioxidants, or drugs directly to the vein graft to prevent cellular dysfunction and injury. Preservation of cellular viability in transplanted organs is achieved in physiologic buffered storage solutions with antioxidants. Since vein grafts are autotransplanted organs, improved HSV preservation should be the focus of future practices of surgical vein graft preparation in order to enhance vein graft function and patency.
Clinical Relevance.
Approximately 1,000,000 aortocoronary and peripheral vascular reconstructions are performed annually using human saphenous vein grafts. However, outcomes from this procedure are limited by high rates of graft failure. The leading cause of vein graft failure is intimal hyperplasia. A multifactorial process, intimal hyperplasia is thought to arise at least in part due to vein graft injury. Significant trauma occurs to the graft during surgical harvest and subsequent preparation, significantly impairing cellular function and increasing oxidative stress. Efforts to reduce early vein graft injury during harvest and preparation may have the potential to reduce subsequent vein graft failure in patients.
Acknowledgments
This work was supported by NIH NRSA F32HL104965 (to M.J.O.), NCATS UL1 TR000445-06 (formerly NCRR UL1 RR024975-01) and NIH R01HL105731-01A1 (to J.C.F.), and NIH RO1HL70715 (to C.M.B.). This work was supported in part with resources and the use of facilities at the VA Tennessee Valley Healthcare System.
Footnotes
Author conflict of interest: none.
Presented in part at the 2012 American Heart Association Scientific Sessions, Los Angeles, Calif, November 3–7, 2012.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: MO, PK, JC, CB
Analysis and interpretation: MO, KH, IV, FL, PK, JC, CB
Data collection: MO, KH, IV, FL, PK, JC
Writing the article: MO, KH, CB
Critical revision of the article: MO, KH, IV, PK, JC, CB
Final approval of the article: MO, KH, IV, FL, PK, JC, CB
Statistical analysis: MO, KH, IV
Obtained funding: MO, JC, CB
Overall responsibility: CB
References
- 1.Clowes AW, Reidy MA. Prevention of stenosis after vascular reconstruction: pharmacologic control of intimal hyperplasia–a review. J Vasc Surg. 1991;13:885–91. doi: 10.1067/mva.1991.27929. [DOI] [PubMed] [Google Scholar]
- 2.LoGerfo FW, Quist WC, Cantelmo NL, Haudenschild CC. Integrity of vein grafts as a function of initial intimal and medial preservation. Circulation. 1983;68(3 Pt 2):II117–24. [PubMed] [Google Scholar]
- 3.Davies MG, Hagen PO. Pathophysiology of vein graft failure: a review. Eur J Vasc Endovasc Surg. 1995;9:7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 4.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–51. doi: 10.1016/j.jvs.2005.12.058. discussion: 751. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 6.Hocking KM, Brophy C, Rizvi SZ, Komalavilas P, Eagle S, Leacche M, et al. Detrimental effects of mechanical stretch on smooth muscle function in saphenous veins. J Vasc Surg. 2011;53:454–60. doi: 10.1016/j.jvs.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelini GD, Passani SL, Breckenridge IM, Newby AC. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc Res. 1987;21:902–7. doi: 10.1093/cvr/21.12.902. [DOI] [PubMed] [Google Scholar]
- 8.Eagle S, Brophy CM, Komalavilas P, Hocking K, Putumbaka G, Osgood M, et al. Surgical skin markers impair human saphenous vein graft smooth muscle and endothelial function. Am Surg. 2011;77:922–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Davies MG, Hagen PO. Influence of perioperative storage solutions on long-term vein graft function and morphology. Ann Vasc Surg. 1994;8:150–7. doi: 10.1007/BF02018863. [DOI] [PubMed] [Google Scholar]
- 10.Karayannacos PE, Hostetler JR, Bond MG, Kakos GS, Williams RA, Kilman JW, et al. Late failure in vein grafts: mediating factors in sub-endothelial fibromuscular hyperplasia. Ann Surg. 1978;187:183–8. doi: 10.1097/00000658-197802000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svendsen E, Dalen H, Moland J, Engedal H. A quantitative study of endothelial cell injury in aorto-coronary vein grafts. J Cardiovasc Surg (Torino) 1986;27:65–71. [PubMed] [Google Scholar]
- 12.Soyombo AA, Angelini GD, Bryan AJ, Newby AC. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovasc Res. 1993;27:1961–7. doi: 10.1093/cvr/27.11.1961. [DOI] [PubMed] [Google Scholar]
- 13.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–41. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 14.Weaver H, Shukla N, Ellinsworth D, Jeremy JY. Oxidative stress and vein graft failure: a focus on NADH oxidase, nitric oxide and eicosa-noids. Curr Opin Pharmacol. 2012;12:160–5. doi: 10.1016/j.coph.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Wingard CJ, Browne AK, Murphy RA. Dependence of force on length at constant cross-bridge phosphorylation in the swine carotid media. J Physiol. 1995;488(Pt 3):729–39. doi: 10.1113/jphysiol.1995.sp021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herlihy JT, Murphy RA. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973;33:275–83. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- 17.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension. 1998;31:1065–9. doi: 10.1161/01.hyp.31.5.1065. [DOI] [PubMed] [Google Scholar]
- 18.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 19.Tessier DJ, Komalavilas P, Liu B, Kent CK, Thresher JS, Dreiza CM, et al. Transduction of peptide analogs of the small heat shock-related protein HSP20 inhibits intimal hyperplasia. J Vasc Surg. 2004;40:106–14. doi: 10.1016/j.jvs.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–27. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy JH, Lever MJ, Addis BJ, Paneth M. Changes in vein inter-stitium following distension for aortocoronary bypass. J Cardiovasc Surg (Torino) 1989;30:992–5. [PubMed] [Google Scholar]
- 22.Kapp RW, Jr, Bevan C, Gardiner TH, Banton MI, Tyler TR, Wright GA. Isopropanol: summary of TSCA test rule studies and relevance to hazard identification. Regul Toxicol Pharmacol. 1996;23:183–92. doi: 10.1006/rtph.1996.0042. [DOI] [PubMed] [Google Scholar]
- 23.Dhar A, Desai K, Kazachmov M, Yu P, Wu L. Methylglyoxal production in vascular smooth muscle cells from different metabolic precursors. Metabolism. 2008;57:1211–20. doi: 10.1016/j.metabol.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Collier J, Vallance P. Biphasic response to acetylcholine in human veins in vivo: the role of the endothelium. Clin Sci (Lond) 1990;78:101–4. doi: 10.1042/cs0780101. [DOI] [PubMed] [Google Scholar]
- 25.Stern DM, Nawroth PP, Kisiel W, Handley D, Drillings M, Bartos J. A coagulation pathway on bovine aortic segments leading to generation of Factor Xa and thrombin. J Clin Invest. 1984;74:1910–21. doi: 10.1172/JCI111611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallitt EJ, Jevon M, Hornick PI. Therapeutics of vein graft intimal hyperplasia: 100 years on. Ann Thorac Surg. 2007;84:317–23. doi: 10.1016/j.athoracsur.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 27.Porter KE, Varty K, Jones L, Bell PR, London NJ. Human saphenous vein organ culture: a useful model of intimal hyperplasia? Eur J Vasc Endovasc Surg. 1996;11:48–58. doi: 10.1016/s1078-5884(96)80134-1. [DOI] [PubMed] [Google Scholar]
- 28.Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, et al. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–96. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 29.Brunssen C, Arsov A, Eickholt C, Leuner A, Langbein H, Brux M, et al. Hypoxia upregulates NADPH oxidase 4 by a HIF-independent mechanism in endothelial cells. Circulation. 2012;126:A15323. [Google Scholar]
- 30.Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther. 2008;120:254–91. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Jeremy JY, Yim AP, Wan S, Angelini GD. Oxidative stress, nitric oxide, and vascular disease. J Card Surg. 2002;17:324–7. doi: 10.1111/j.1540-8191.2001.tb01151.x. [DOI] [PubMed] [Google Scholar]