Abstract
The precise mechanism of heterotopic ossification caused by several types of tumours is largely unknown. However, recent studies have indicated that bone morphogenetic protein 2 (BMP2) is closely linked to the Wnt/β-catenin signaling pathway in this rare phenomenon of bone formation. We report a rare case of adrenal myelolipoma (ML) in a 27-year-old woman with heterotopic bone formation. Immunohistochemical findings showed BMP2 expression in the cytoplasm of tumour cells, as well as the matrix adjacent to newly developed bone tissue. In addition, β-catenin was prominent in the cytoplasm and nuclei of BMP2-positive tumour cells. To the best of our knowledge, this is the first report of adrenal ML showing heterotopic ossification with accelerated expression of both BMP2 and β-catenin. Our case findings indicate that BMP2 overexpression via aberrant canonical Wnt/β-catenin signaling may contribute to heterotopic bone formation occurring in adrenal ML.
Introduction
Formation of heterotopic bone in adrenal myelolipoma (ML) patients is extremely rare. Previous studies have shown that bone morphogenetic protein 2 (BMP2), known to be a primary inducer of bone formation, plays an important role in heterotopic ossification in several types of cancer.1–3 Interestingly, a more recent study found that coordination of BMP2 and Wnt/β-catenin signaling may be involved in the process of osteoplastic differentiation and subsequent bone formation,4 while β-catenin has also been reported to induce BMP2 expression in gastrointestinal cancer cells.5 We report a case of adrenal ML showing heterotopic bone formation with overexpression of BMP2 and β-catenin, indicating the possible involvement of BMP2 and the Wnt/β-catenin signaling pathway in heterotopic ossification.
Case report
A 27-year-old woman was referred to our hospital for an incidentally found left adrenal mass. She was otherwise in good physical condition (height 161 cm, body weight 48 kg) with normal blood pressure (108/69 mmHg). Laboratory examination and hormonal findings showed no abnormality. A computed tomography scan of her abdomen showed a 5.2 × 4.3 × 4.6-cm heterogeneous mass close to the left adrenal gland area, including adipose tissue and calcification. Based on the typical imaging findings and the hormonally inactive nature of the tumour, an adrenal ML was the most probable preoperative diagnosis. Because of the high risk of rupture and malignant potential, surgery with a tumorectomy was performed.
The excised specimen was a soft round mass sized 8.0 × 5.5 × 2.5 cm, with yellowish fatty tissue seen on the cut surface (Fig. 1, part A). The pathological diagnosis was an adrenal ML comprised of mature adipose tissue mixed with hematopoietic tissue (Fig. 1, part B). In addition, irregularly-shaped bone spicules were found surrounded by osteoblast-like cells, some of which had already undergone calcification (Fig. 1, part C).
Fig. 1.
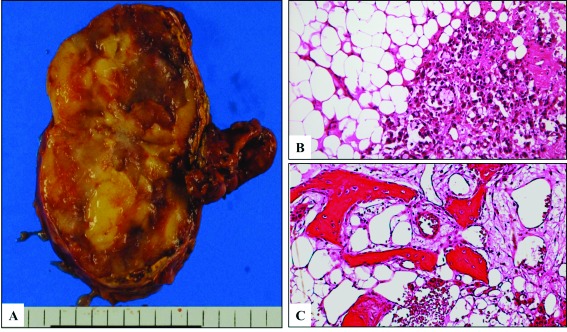
A: Gross appearance of resected specimen, which measured 5.2×4.3×4.6 cm in size and was composed of yellowish fatty tissue. B: Microscopic findings of the adrenal myelolipoma (ML) revealed mature adipose tissue mixed with hematopoietic tissue. Reduced from ×100. C: Ossification in the adrenal ML consisted of several irregular areas of immature bone with osteoblast-like cells. Reduced from ×100.
To clarify the mechanism of heterotopic ossification in this case of adrenal ML, immunohistochemical analysis was performed using anti-BMP2 (1:250, Abcam, Cambridge, MA) and anti-β-catenin (1:500, BD Biosciences, UK) antibodies. Positive staining for BMP2 was found in the matrix adjacent to the tumour cells and also in areas of developing bone formation with osteoblast-like cells (Fig. 2, part A). In addition, weak BMP2 expression was another interesting finding in the cytoplasm of the tumour cells (Fig. 2, part B), while positive β-catenin was a typical finding in the cytoplasm and/or nuclei of BMP2-positive tumour cells (Fig. 2, part C and D).
Fig. 2.
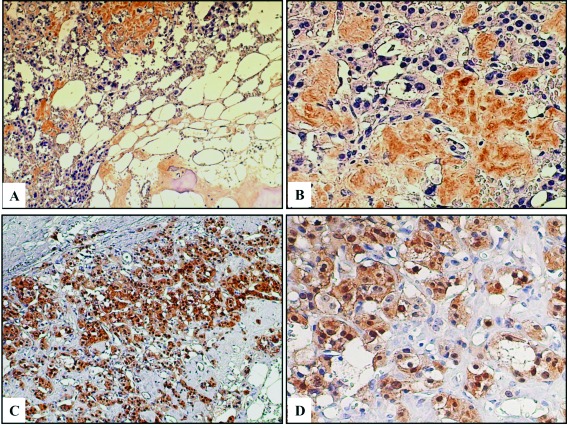
A: Positive immunohistochemical staining for BMP2 is observed in the matrix adjacent to tumour cells and the areas of bone formation Reduced from ×100. B: Weak staining for BMP2 is also seen in the cytoplasm of tumour cells. Reduced from ×200. C: Strong positive immunohistochemical staining for β-catenin is observed in BMP2 positive tumour cells. Reduced from ×100. D: Abnormal β-catenin staining is seen in the cytoplasm and/or nucleus of tumour cells.
Discussion
Heterotopic bone formation is an extremely rare phenomenon in ML patients, with only 6 reported cases of heterotopic ossification.6,7 Until recently, the precise mechanism underlying heterotopic ossification caused by several types of tumours was largely unknown. However, some reports have indicated that BMP2, a critical autocrine and paracrine growth factor that directs osteoblast differentiation and bone formation, plays an important role in heterotopic ossification.1–3 Komai and colleagues2 showed that heterotopic ossification may result from metaplasia of pluripotent stem cells into osteoblast cells induced by BMP2. Nevertheless, the exact molecular and cell biological associations of BMP2 with heterotopic ossification remain to be elucidated.
Wnt/β-catenin signaling plays significant roles in both embryonic development and tumorigenesis, and when activated induces differentiation of mesenchymal cells with pluripotential into osteoblast progenitors to become osteoblasts.4 In addition, this study4 clearly showed the cooperative regulation of BMP2 with Wnt/β-catenin signaling to induce osteoblastic differentiation and subsequent bone formation. Since activation of Wnt/β-catenin signaling induces and increases the expression of BMP2 and BMP target genes, respectively,4,5,8 the Wnt/β-catenin signaling pathway may be a candidate upstream activator of BMP2 expression in osteoblasts.4
In the present adrenal ML case, BMP2 expression was positive in the matrix adjacent to tumour cells, where bone spicules were also found with osteoblast-like cells. Furthermore, weak but positive BMP2 expression was found in the cytoplasm of the tumour cells, with β-catenin expression also identified in the cytoplasm and/or nuclei of BMP2-positive tumour cells. Based on these findings, we speculate that BMP2 overexpression in adrenal ML cases is closely related to an aberrant canonical Wnt/β-catenin signaling pathway, while BMP2 induction by the tumour cells may affect ectopic bone formation by transforming undifferentiated mesenchymal cells into osteoblast-like cells.
An adrenal ML is a relatively rare benign mesenchymal tumour, consisting of mature fat and bone marrow elements. Although several different pathogeneses have been postulated,9 recent cytogenetic analyses have revealed it to be a myeloid tumour arising from misplaced hematopoietic cells.7,10 In the present case, aberrant canonical Wnt/β-catenin signaling, typically found in variety of hematopoietic neoplasms,11 was shown to be involved. Thus, the pathogenesis of our case appears to be compatible with the dysregulation of Wnt/β-catenin signaling frequently found in hematopoietic tumours and also in line with the hypothesis stating that an adrenal ML is a myeloid tumour. Our findings will contribute to a better understanding of its pathogenesis.
Conclusion
We showed the possible involvement of BMP2 and the Wnt/β-catenin signaling pathway in heterotopic ossification. Our findings contribute to a better understanding of the mechanism of heterotopic bone formation and pathogenesis of adrenal ML.
Footnotes
Competing interests: Dr. Mitsui, Dr. Yasumoto, Dr. Hiraki, Dr. Arichi, Dr. Ishikawa, Dr. Harada, Dr. Maruyama, Dr. Shiina all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Imai N, Iwai A, Hatsuko S, et al. Expression of bone morphogenetic proteins in colon carcinoma with heterotopic ossification. Pathol Int. 2001;51:643–8. doi: 10.1046/j.1440-1827.2001.01243.x. [DOI] [PubMed] [Google Scholar]
- 2.Komai Y, Morimoto S, Saito K, et al. Possible involvement of bone morphogenetic protein 2 in heterotopic ossification in metastatic lesion from urothelial carcinoma of bladder. Int J Urol. 2006;13:1126–8. doi: 10.1111/j.1442-2042.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- 3.Kypson AP, Morphew E, Jones R, et al. Heterotopic ossification in rectal cancer: Rare finding with a novel proposed mechanism. J Surg Oncol. 2003;82:132–6. doi: 10.1002/jso.10181. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Oyajobi BO, Jarris SE, et al. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone. 2013;52:145–56. doi: 10.1016/j.bone.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JS, Crooks H, Dracheva T, et al. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744–8. [PubMed] [Google Scholar]
- 6.Rossi A, Insensate R. Bone tissue in adrenal myelolipoma: A case report. Tumori. 1998;84:90–3. doi: 10.1177/030089169808400121. [DOI] [PubMed] [Google Scholar]
- 7.Bishop E, Eble JN, Cheng L, et al. Adrenal myelolipomas show nonrandom X-chromosome inactivation in hematopoietic elements and fat: Support for a clonal origin of myelolipomas. Am J Surg Pathol. 2006;30:838–43. doi: 10.1097/01.pas.0000202044.05333.17. [DOI] [PubMed] [Google Scholar]
- 8.Hill TP, Taketo MM, Birchmeier W, et al. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development. 2006;133:1219–29. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- 9.Amin MB, Tickoo SK, Schultz D. Myelolipoma of the renal sinus. An unusual site for a rare extra-adrenal lesion. Arch Pathol Lab Med. 1999;123:631–4. doi: 10.5858/1999-123-0631-MOTRS. [DOI] [PubMed] [Google Scholar]
- 10.Chang KC, Chen PI, Huang ZH, et al. Adrenal myelolipoma with translocation. Cancer Genet Cytogenet. 2002;134:77–80. doi: 10.1016/S0165-4608(01)00592-1. [DOI] [PubMed] [Google Scholar]
- 11.Morgan RG, Pearn L, Liddiard K, et al. γ-Catenin is overexpressed in acute myeloid leukemia and promotes the stabilization and nuclear localization of β-catenin. Leukemia. 2013;27:336–43. doi: 10.1038/leu.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]


