Abstract
Adipose tissue is a major metabolic organ, and it has been traditionally classified as either white adipose tissue (WAT) or brown adipose tissue (BAT). WAT and BAT are characterized by different anatomical locations, morphological structures, functions, and regulations. WAT and BAT are both involved in energy balance. WAT is mainly involved in the storage and mobilization of energy in the form of triglycerides, whereas BAT specializes in dissipating energy as heat during cold- or diet-induced thermogenesis. Recently, brown-like adipocytes were discovered in WAT. These brown-like adipocytes that appear in WAT are called beige or brite adipocytes. Interestingly, these beige/brite cells resemble white fat cells in the basal state, but they respond to thermogenic stimuli with increased levels of thermogenic genes and increased respiration rates. In addition, beige/brite cells have a gene expression pattern distinct from that of either white or brown fat cells. The current epidemic of obesity has increased the interest in studying adipocyte formation (adipogenesis), especially in beige/brite cells. This review summarizes the developmental process of adipose tissues that originate from the mesenchymal stem cells and the features of these three different types of adipocytes.
Keywords: White adipocytes, Brown adipocytes, Beige/brite adipocytes, Mesenchymal stem cells, Adipogenesis, Thermogenesis, Browning
Core tip: Here, we summarize the characteristic differences of the white, brown and beige adipocytes derived from mesenchymal stem cells, including their anatomical location. In particular, we focus on the newly discovered brown-like adipocytes called beige/brite adipocytes. A deeper understanding of the molecular mechanism of these adipocytes may provide clues for overcoming obesity and its associated metabolic diseases.
INTRODUCTION
Obesity is a worldwide challenge and not unique to any one country. Furthermore, obesity is closely connected to many metabolic diseases. Essentially, obesity and overweight are caused by the energy imbalance between the calories consumed and calories expended. Adipose tissue, which is composed mostly of adipocytes, is a major endocrine organ and plays a key role in energy homeostasis. Two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), have been identified[1]. In practice, obesity does not depend on body weight but depends on either the number of white adipocytes or the amount of WAT. WAT functions primarily to store excess energy in the form of triglycerides (TGs). In contrast, BAT oxidizes fuels and dissipates energy in the form of heat, which suggests that BAT plays a natural anti-obesity role. Therefore, a deeper understanding of the regulation mechanisms of adipose tissues can potentially open the way to treating obesity-associated metabolic diseases. In this review, we describe the recent advances in studying the characteristics of white, brown, and beige/brite adipocytes (a third class of adipocytes). Additionally, we review the molecular mechanisms involved in the development of adipocytes and suggest possible future therapeutic approaches.
ANATOMICAL LOCATIONS OF ADIPOSE TISSUES
Adipose tissue depots are distinguished by their different anatomical locations. WAT is distributed throughout the body, and there are two representative types: visceral WAT (vWAT) and subcutaneous WAT (sWAT). vWAT is distributed around organs and provides protective padding. sWAT is located under the skin and provides insulation from heat or cold. vWAT, or abdominal fat, is located inside the peritoneum and is distributed around internal organs (e.g., stomach, liver, intestines, and kidneys). Depending on the location, vWAT is sub-classified roughly into mesenteric, retroperitoneal, perigonadal and omental adipose tissue. Mesenteric adipose tissue resembles a web that supports the intestines, and the paired perigonadal adipose tissue is attached to the uterus and ovaries in females and the epididymis and testis in males. The paired retroperitoneal depots are found along the dorsal wall of the abdomen. Lastly, omental depots are located around the stomach and spleen and extend into the ventral abdomen (Figure 1A). The locations of sWAT differ from those of vWAT; sWAT is located inside the abdominal cavity and can be found underneath the skin as well as in the intramuscular fat that is interspersed amongst skeletal muscles. A typical example of sWAT is inguinal WAT, which is found anterior to the upper site of the hind limbs and underneath the skin. In humans, sWAT is typically distributed around the hips, thighs, and buttocks (Figure 1B).
Figure 1.
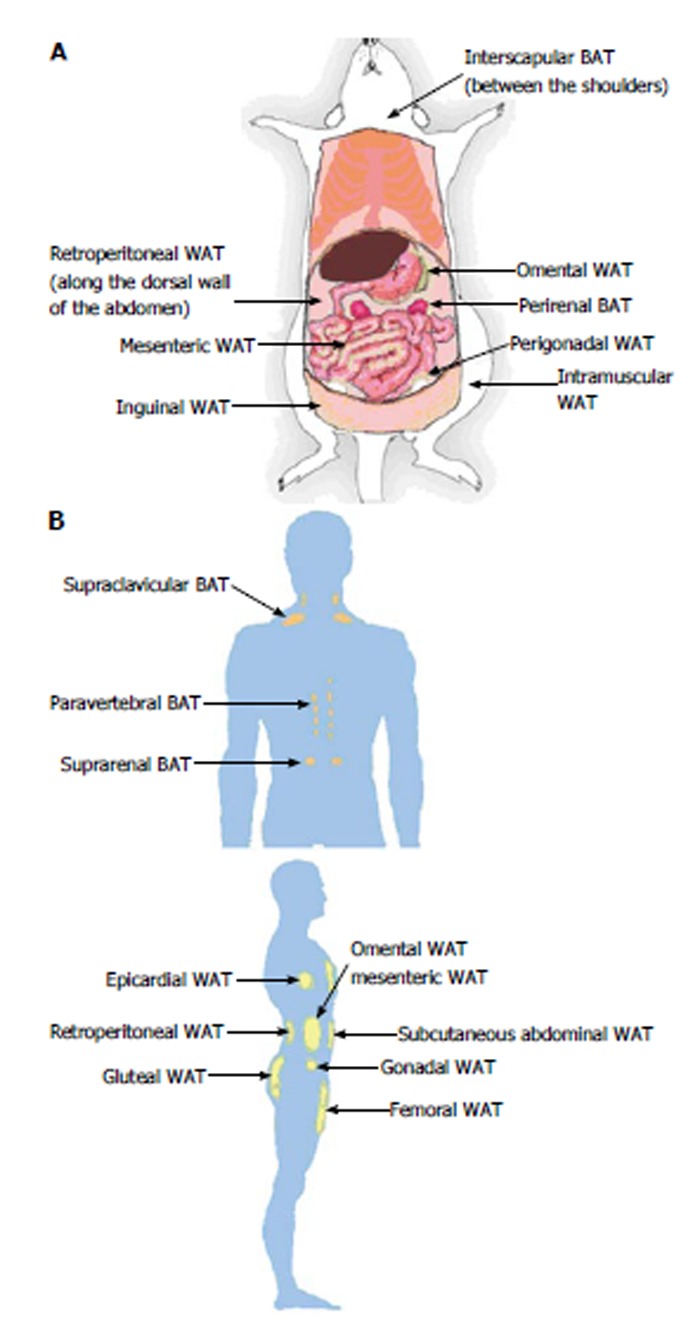
Locations of adipose tissue depots in a mouse (A) and an adult human (B). A: Subcutaneous (inguinal and intramuscular), visceral (mesenteric, omental, perigonadal and retroperitoneal) and brown (interscapular and perirenal) adipose tissue depots are shown in a mouse model; B: Subcutaneous (abdominal, femoral and gluteal), visceral (epicardial, gonadal, mesenteric, omental and retroperitoneal) and brown (paravertebral, supraclavicular and suprarenal) adipose tissue depots are shown in a human model. WAT: White adipose tissue; BAT: Brown adipose tissue.
Because beige/brite adipocyte cells were recently defined[2], brown adipocytes are sometimes termed “classical”, “constitutive”, or “developmentally programmed” brown adipocytes to distinguish them from brown-like cells in WAT. Classical brown fat is primarily distributed around interscapular BAT (iBAT), axillary, paravertebral, and perirenal sites. The most classical brown fat depots are located in interscapular (in the upper back region) and perirenal (around the kidney) sites in rodents and large mammals. iBAT is distributed subcutaneously between the shoulders and can be easily removed. In contrast, it is difficult to selectively remove perirenal BAT from the whole pad without removing the kidney. In humans, small areas of iBAT are found in the thorax region (supraclavicular), chest and abdomen[3]. In humans and other large mammalian species, BAT was traditionally thought to be restricted to the neonatal and early childhood periods[3,4]. However, positron emission tomography (PET) scanning technology was recently adapted for detecting metabolically active sites for oncology diagnosis; this application is based on the uptake of radiolabeled non-metabolizable glucose derivatives. The results obtained from a scanning experiment using PET to analyze BAT clearly demonstrated that active BAT is present in adult humans at discrete anatomical sites, especially in the upper trunk, such as cervical, supraclavicular, paravertebral, pericardial, and to some extent, mediastinal and mesenteric areas[5-8] (Figure 1B).
Recently, a new type of brown-like adipocyte was discovered that shows distinct gene expression patterns from those of white or brown adipocytes. These novel brown-like cells that reside within WAT, especially inguinal WAT, were termed beige/brite adipocytes or inducible brown adipocytes[2]. Adult human neck fat depots are composed of classical BAT, and these depots have the molecular features of classical BAT. However, unexpectedly and interestingly, some studies analyzed the gene expressed in the BAT areas of neonate and adult humans and found beige/brite cell-selective genes[9]. In contrast, Cypess et al[10] identified and more precisely analyzed the anatomical sites of adult human BAT around neck fat depots. The researchers isolated samples of neck fat from superficial and deep depots and then compared the gene expression patterns. The results showed that human superficial neck fat had an expression pattern similar to that of mouse sWAT; however, the expression pattern from human deep neck fat was more similar to that of mouse iBAT. Overall, these reports indicate that more extensive analysis is necessary in human BAT studies. Finding beige/brite cells, which were once roughly classified as BAT, requires us to now further distinguish BAT as either classical BAT or beige/brite adipose tissue. It is highly probable that the tissue that was previously assumed to be BAT in some of the above mentioned studies may in fact be beige/brite adipose tissue.
FEATURES AND FUNCTIONS OF ADIPOCYTES
Traditionally, two different types of adipose tissues, WAT and BAT, have been identified in human and other mammals. These adipose tissues have different colors, morphology, metabolic functions, biochemical features, and gene expression patterns. WAT is the main storage organ of energy in the form of lipids for the organism, whereas BAT plays a role in regulating body temperature by generating heat via the consumption of stored energy.
WAT generally constitutes as much as 20% of the body weight of normal adult humans. The development of WAT begins in utero but primarily occurs after birth when specialized fat storage cells are needed to provide fuel during fasting periods. WAT is normally characterized by an ivory or yellowish color as well as unilocular/large lipid droplets. The primary function of WAT is to store excess energy as TGs to regulate energy homeostasis. Although the expression of uncoupling protein 1 (UCP1), which is known to be a unique selective marker of BAT[11], is nearly undetectable, the isoform UCP2 has been reported to be expressed in parts of WAT[12]. Furthermore, some genes, such as those for Adiponectin, Resistin[13], LPL, and G3PDH[14], are known selective markers of WAT (Table 1).
Table 1.
Differences amongst the three types of adipocytes
| Brown | White | Beige (Brown-like) | |
| Location | Interscapular, perirenal, axillary, paravertebral | Inguinal (sWAT), mesenteric, retroperitoneal, perigonadal, omental (vWAT) | Within inguinal WAT, other sWAT? |
| Morphology | Multilocular/small lipid droplets | Unilocular/large lipid droplets | Unilocular large/multiple small lipi droplets |
| Function | Heat production | Storage of energy as triglycerides | Adaptive thermogenesis |
| Mitochondria | (+++) | (+) | Upon stimulation (++) |
| Iron content | Abundant | Low | Upon stimulation (Abundant) |
| Correlation with insulin resistance | Negative | Positive | Negative |
| UCP1 | (+++) | Nearly undetectable | Upon stimulation (++) |
| Vascularization/Capillaries | Abundant | Low | Cold stimulation led to increase of angiogenesis in sWAT[66] |
| α-, β-Adrenergic receptors | β3 (+++) | β3 (++), α2 (+) | β3/α2? |
| Obesity | Negative effect | Positive effect | Negative effect |
| Enriched markers | UCP1, Eva1, Pdk4, Ebf3, Hspb7[2,9] | Ang, Resistin[13] LPL, G3PDH[14] | Tmem26, Tbx1[2], Cited1[9], Shox2[67] |
| Activators | Cold, thyroid hormone, thiazolidinediones, FGF21, Bmp7, Bmp8b, natriuretic peptide | HFD | Cold, thiazolidinediones, natriuretic peptide, FGF21, irisin, catecholamines, β-adrenergic receptor agonists |
WAT: White adipose tissue; vWAT: Visceral WAT; sWAT: Subcutaneous WAT; UCP1: Uncoupling protein 1; FGF21: Fibroblast growth factor-21; HFD: High fat diet.
Mitochondria play an essential role in adipose tissue because mature adipocytes require a large amount of ATP to maintain processes such as lipolysis, β-oxidation of fatty acids, and fatty acid synthesis. Mature brown adipocytes have a relatively high mitochondrial content and contain a specialized mitochondrial protein called UCP1[15]. Lipolysis occurs during cold exposure, which activates sympathetic nervous system signaling in brown adipocytes; the resulting free fatty acids are used to generate heat using the UCP1 protein. Therefore, in comparison to white adipocytes, brown adipocytes have significantly higher levels of mitochondria that contain red-brownish iron and consequently appear brown in color. They also contain many multilocular/small lipid droplets. As mentioned above, the main function of BAT is to regulate the non-shivering thermogenesis that dissipates energy as heat in response to cold exposure[16-18]. The thermogenic process of brown adipocytes is activated by UCP1, also known as thermogenin, in their mitochondria. The UCP1 expressed in the inner membrane of mitochondria is mainly regulated by adrenergic signaling through sympathetic innervations, and this signaling is responsible for the production of heat via the respiratory uncoupling reaction. UCP1 causes a proton leak across the inner membrane of mitochondria, thereby converting chemical energy into the heat. UCP1 is responsible for the main function of BAT and is a representative marker of brown adipocytes[15,18]. Additionally, BAT is highly vascularized and innervated, which likely allows BAT to respond to sympathetic nerve activity and dissipate the generated heat throughout the body through blood vessels. In addition to UCP1, Eva1, Pdk4, Ebf3, and Hspb7 have also been reported to be BAT-specific markers[3,4] (Table 1).
Previous evidences have supported the idea that white and brown adipocytes coexist within the same depot, which suggests that white adipocytes transdifferentiate into brown adipocytes via several factors that normally regulate BAT development or activity[19-21]. However, a new type of brown-like adipocyte within WAT called beige/brite cells was recently discovered, and this transdifferentiation process is referred to as the “browning” or “britening” of WAT. Researchers have also reported the differential expression of several genes that can be used to distinguish beige/brite adipocytes from brown adipocytes. These genes encode proteins with very distinct cellular functions, including transcription factors (e.g., Tbx15), metabolism-related proteins (e.g., Slc27a1), and proteins associated with inflammatory pathways (e.g., CD40 and CD137)[2,9,22]. Interestingly, beige/brite adipocytes have the characteristics of both white and brown adipocytes. They display unilocular/large lipid morphology as well as gene expression patterns similar to those of white adipocytes during basal states. However, upon cold stimulation, beige/brite adipocytes change into an “intermediate cell morphology” in which multilocular lipid droplets surround large ones; this change ultimately results in UCP1 expression and a transformation into the multilocular/small lipid morphology characteristic of brown adipocytes[2,9,22]. Moreover, the inducible browning processes are reversible reactions. In other words, when mice were rewarmed at room temperature after cold stimulation, the former beige/brite adipocytes re-converted into white adipocytes with decreased expression of brown-selective marker genes approximately 6 wk after the warm adaptation[23]. This result suggests that browning and whitening are reversible processes and depend on environmental conditions.
It is unclear whether the beige/brite fat cells arise through the transdifferentiation of pre-existing white adipocytes or by de novo adipogenesis from a subgroup of precursor cells. Previously, several reports suggested that beige/brite adipocytes arise from pre-existing white adipocytes. Himms-Hagen et al[19] observed that mature adipocytes transform into beige/brite adipocytes without dividing, and Cinti[24] showed that large unilocular white adipocytes convert into beige/brite adipocytes in response to cold or 3-adrenergic agonists. However, new research has recently shown conflicting results. During the writing of this paper, Wang et al[25] suggested that most beige/brite adipocytes stem from a subgroup of precursors in WAT. In that study, the researchers developed a system for inducible, permanent labeling of mature adipocytes. Although cold induced the formation of beige/brite adipocytes, the researchers observed large areas of beige/brite fat cells with multiple small lipid droplets that were not labeled in the subcutaneous white fat.
DIFFERENTIATION OF ADIPOCYTES
BAT develops and differentiates before birth because its function is to protect a newborn against cold. In contrast, the formation of WAT commences shortly after birth. Mesenchymal stem cells (MSCs), which are multipotent stem cells, become adipoblasts and subsequently differentiate into preadipocytes. Under certain types of stimulation, preadipocytes are converted into mature adipocytes in the final phase of differentiation[26].
The initial phase of adipogenesis is characterized by the proliferation of preadipocytes. Preadipocytes progress through multiple rounds of mitosis until they reach growth arrest, the G1 phase of the cell cycle. At this point, the preadipocytes must re-enter the cell cycle, undergo mitotic clonal expansion until they eventually exit the cell cycle, acquire the metabolic features of mature adipocytes, change their morphology, and accumulate cytoplasmic TGs[27]. Mature adipocytes are believed to have lost the ability to divide following the completion of terminal differentiation[28]. Thus, inducing differentiation in cells isolated from the stromal vascular fraction of adipose tissue depots requires the specific contents of a “differentiation cocktail”. The differentiation induction cocktail contains fetal bovine serum, insulin, dexamethasone (a glucocorticoid), and 3-isobutyl-1-methylxanthine (IBMX). Insulin is an adipogenesis-inducing hormone that promotes cell cycle reentry and synchronous cell division (mitotic clonal expansion). This process is dependent on the induction of two members of CCAAT/enhancer-binding protein (C/EBP) family: C/EBP-β and C/EBP-δ. Dexamethasone treatment is important for inducing differentiation because it activates the transcription factor C/EBP-β. IBMX is a phosphodiesterase inhibitor that increases intracellular cyclic AMP (cAMP) levels, leading to the activation of the transcription factor C/EBP-δ.
Both white and brown adipocytes originate from the mesoderm, but they are believed to be derived from different precursor cells (Figure 2). MSCs can be committed to either an adipogenic lineage of Myf5-negative cells or a myogenic lineage of Myf5-positive cells[29,30]. Myf5 is known to be a key myogenic regulatory factor. White adipocytes are derived from the adipogenic lineage, whereas brown adipocytes are derived from the myogenic lineage. Although the adipocytes originate from different lineages, the subsequent adipogenic differentiation shares common transcriptional cascades that mainly involve peroxisome proliferator-activated receptor-γ (PPAR-γ), the dominant regulator of fat cell development, and C/EBPs[26,28].
Figure 2.
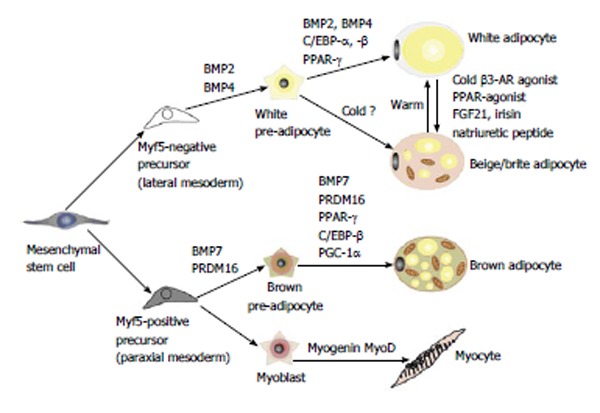
Differentiation into white, beige or brown adipocytes. Previously, white and brown adipocytes were thought to be derived from the same precursor cell. However, recent studies demonstrated that brown fat shares a progenitor cell (Myf5+) with skeletal muscle and not with white adipocytes. The Myf5+ precursors are induced to transform into mature brown adipocytes by bone morphogenetic protein 7 (BMP7), peroxisome proliferator-activated receptor-γ (PPAR-γ) and CCAAT/enhancer-binding proteins (C/EBPs) in cooperation with the transcriptional co-regulator PR domain-containing 16 (PRDM16) and PGC-1α. White adipocytes can also be transformed to brown-like adipocytes, called beige/brite adipocytes, by cold exposure, a β-adrenergic agonist or a PPAR-γ agonist. AR: adrenergic receptor; FGF21: Fibroblast growth factor 21; PGC-1α: Peroxisome proliferator activated receptor gamma coactivator 1 alpha.
Differentiation into white adipocytes from progenitor cells
C/EBP family members are important for adipocyte differentiation, whereby the early induction of C/EBP-β and C/EBP-δ leads to the induction of C/EBP-α and PPAR-γ. Immediately after the induction of differentiation, the cAMP response element binding protein becomes phosphorylated and then induces the expression of C/EBP-β[31]. In a relatively early stage of differentiation, mitogen- activated protein kinase and GSK3β phosphorylate C/EBP-β, which induces the dimerization of two monomers of C/EBP-β, thereby creating a DNA-binding domain. The binding of C/EBP-β to DNA allows preadipocytes to re-enter the cell cycle, that is, C/EBP-β plays a role in mitotic clonal expansion. Furthermore, the functions of C/EBP-β and C/EBP-δ may be redundant[32]. A knockout of C/EBP-β in mice has little effect on adipose tissue accumulation, whereas C/EBP-β and C/EBP-δ double-knockout mice show considerably reduced adipose tissue accumulation. The binding of C/EBP-β to DNA leads to increased levels of C/EBP-α and PPAR-γ, which act together as transcriptional activators[26,33]. C/EBP-α functions to maintain PPAR-γ expression. Upon expression, PPAR-γ and C/EBP-α exert positive feedback on each other, and this stage is regarded as a key step in acquiring the adipocyte phenotype in mature adipocytes. In addition, PPAR-γ is essential for regulating gene transcription to promote and maintain the differentiated state of adipocytes (i.e., lipid metabolism, glucose metabolism, and insulin sensitivity). The dominant negative form of PPAR-γ leads to de-differentiation and the loss of lipid accumulation in differentiated 3T3-L1 cells[34]. Furthermore, the absence of C/EBP-α in mice impairs the development of WAT, but interestingly, it has no effect on BAT. Thus, some researchers have speculated that the lack of C/EBP-α can be compensated for in brown fat development by C/EBP-β[35].
Differentiation of into brown adipocytes from progenitor cells
In contrast to white adipocytes, brown adipocytes originate from the myogenic lineage of Myf5-positive progenitor cells. The differentiation of brown preadipocytes into brown adipocytes is controlled by transforming growth factor-β family proteins, such as bone morphogenetic protein (BMP)-7[36] and myostatin[37]. However, Wnt signaling is known to suppress the differentiation of the preadipocytes into brown adipocytes[38]. C/EBP-β and PR domain containing 16 (PRDM16) have been shown to act as key transcriptional factors in the differentiation of brown adipocytes[32,39,40]. When PRDM16 was suppressed in brown precursor cells using an shRNA system, the cells differentiated into skeletal muscle cells. Additionally, the myoblasts that ectopically expressed PRDM16 were converted into brown fat cells[40]. PRDM16, together with C/EBP-β, operates as a critical switch factor in determining the fate of BAT from the myogenic lineage[41]. In the Myf5-positive myogenic lineage, the PRDM16 and C/EBP-β transcriptional complex induces the expression of PPAR-γ and peroxisome proliferator activated receptor gamma coactivator 1 alpha (PGC-1α), which subsequently induces the differentiation of brown adipocytes[42]. In particular, PGC-1α also cooperates with PPAR-γ and PPAR-α and regulates mitochondrial biogenesis and oxidative metabolism[43,44]. In addition, C/EBP-β has been reported to be a key transcriptional activator of UCP1 expression and the thermogenesis process[32,41]. Interestingly, overexpression of C/EBP-β alone induces a brown fat cell-like phenotype in white adipocytes[45].
Formation of beige/brite adipocytes (Browning)
After the completion of adipocyte differentiation, some differential processes are sometimes still observed. Interestingly, white adipose depots have the ability to switch between energy storage and expenditure. Thus, these depots can shift from a WAT phenotype to a BAT-like phenotype in terms of features such as morphology, gene expression pattern, and mitochondrial respiratory activity under some specific stimuli[46]. As mentioned above, this induction of the brown adipocyte-like phenotype in WAT is called “browning” and the beige/brite cells of WAT are capable of this transformation. The beige/brite cells in WAT are derived from precursor cells that are different from classical brown adipocytes and are closer to the white adipocyte cell lineage[47]. These beige/brite cells show a white adipocyte-like phenotype, including large lipid droplets and the lack of UCP1 expression, under basal conditions. However, in response to certain stimuli (cold exposure[21] or β3-adrenergic activators[19]), beige/brite cells transform into cells having BAT-like characteristics, such as multilocular/small lipid droplets and UCP1 expression.
Recently, in an in vivo lineage-tracing study using transgenic mice[23], brown and beige adipocytes were either transiently or permanently labeled, thereby allowing the tracing of current and past UCP1-expressing cells. After the first cold stimulation, the beige/brite adipocytes expressed both the permanent and transient labels in inguinal WAT. Additionally, when returned to warm conditions, the former beige/brite adipocytes were permanently retained but lost the transient label. The second round of cold stimulation resulted in the re-browning of the whitened former beige/brite adipocytes, as well as the formation of new beige/brite adipocytes within inguinal white fat depots. This experiment strongly suggests that inter-conversion between white and beige/brite adipocytes is possible. Considering these results, we speculate that beige/brite cells can regulate the adaptive thermogenesis against cold in sWAT because the primary function of BAT is non-shivering thermogenesis. In general, classical BAT protects an organism from decreasing temperatures during the neonatal period when the organism is not yet sufficiently capable of adapting to a change in environment; in adults, classical BAT is still present and increases energy expenditure in response to cold or an excess energy state. We think that the classical BAT has already been set up to control energy homeostasis and is thus a fixed mechanism. Meanwhile, beige/brite cells provide a more flexible means to regulate body temperature and energy balance.
Several factors that can lead to WAT browning have been reported. One of strong inducer of beige/brite cells is cold exposure. Chronic cold exposure induces remarkable changes in metabolism as well as gene expression. In addition, it stimulates the differentiation of precursors into beige/brite adipocytes within one week of exposure[23]. Although recent report assumed that a cool temperature (27-33 °C, in vitro cells) can directly activate the thermogenic gene process in a cell-autonomous manner in sWAT but not in classical BAT, the detailed mechanism is not yet clear. Traditionally, it has been accepted that thermogenic activity is regulated by a canonical β-adrenergic receptor pathway via the sympathetic nervous system. Catecholamines, such as norepinephrine, activate β-adrenergic receptor (there are three subtypes, 1, 2, and 3, in humans, but mainly 3 and 1 are involved) that are coupled to a G-protein and increase the intracellular cAMP level. In a subsequent process, this signal leads to fatty acid mobilization and induces the UCP1 expression in mitochondria related to non-shivering thermogenesis. Thus, catecholamines or β-adrenergic receptor agonists mimic the majority of thermogenic effects, as demonstrated using CL316243[48-51]. Other agents, such as the PPAR-γ activator thiazolidinediones, can also promote WAT browning[52]. In addition, multiple novel nonadrenergic soluble molecules that are capable of inducing BAT activity and WAT browning have been identified[53]. Although some of these molecules act indirectly by modulating sympathetic activation and the subsequent noradrenergic pathways, several agents [e.g., fibroblast growth factor-21 (FGF21) and the cardiac peptides (ANP/BNP)] appear to have direct effects on brown adipocytes and the browning process[54-56]. Recently, the Spiegelman group identified irisin[57], a novel hormonal factor that converts white fat into the more thermogenic beige fat. Irisin is secreted and released from muscle during exercise and appears to affect the browning process in WAT but not classical BAT activation (Figure 3). Other stimuli are able to enhance the recruitment of beige cells; these stimuli include prostaglandins, which are locally generated by cyclooxygenase-2-mediated production, Bmp8b, the transcription factor FOXC2, and cyclic guanosine monophosphate [24,58-60]. A recent study suggested that the overexpression of BMP-4 promotes the browning of WAT[61].
Figure 3.
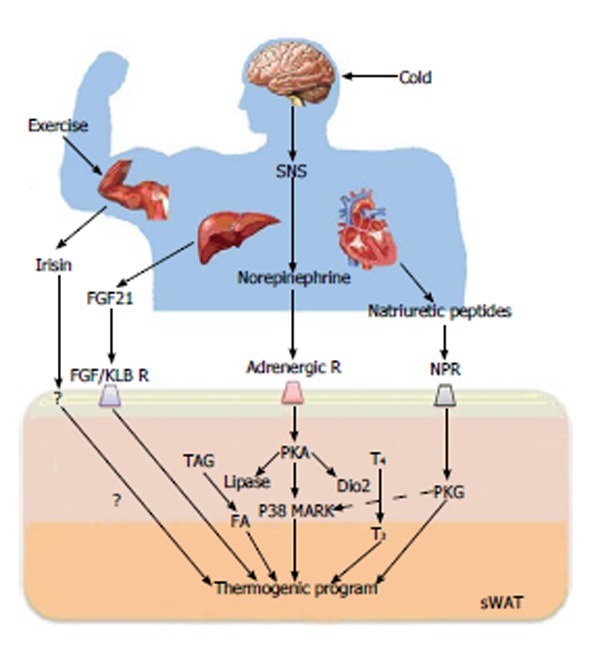
Key regulators of the browning process and their action mechanisms. Browning is induced by sympathetic nervous system (SNS)-independent or SNS-dependent signals. These signals sometimes synergistically or competitively influence the activation of browning of subcutaneous white adipose tissue (sWAT). Irisin is a newly discovered myokine and is released by skeletal muscle during exercise. Irisin induces the browning process of sWAT. Fibroblast growth factor-21 (FGF21), a hormonal factor from the liver, directly activates the thermogenic process via interaction with the FGF receptor/β-Klotho (KLB) complex. The norepinephrine secreted by the SNS in response to thermogenic stimuli induces the activation of adrenergic receptor(s). The adrenergic receptor-mediated signal increases the level of intracellular cAMP and activates cAMP-dependent protein kinase A (PKA). Subsequently, PKA activates p38 MAP kinase (p38 MAPK) and 5’-deiodinase 2 (Dio2), which catalyzes the conversion of thyroxine (T4) into the active form 3,5,3’-tri-iodothyronine (T3). Then, it ultimately induces the gene process for thermogenic activation. Natriuretic peptides (NPs) originating from the heart activate the thermogenic process through binding to the NP receptor, activation of protein kinase G (PKG) and the subsequent activation of p38 MAPK and NPR. NPR: Natriuretic peptides receptor; TAG: Triacylglycerol; FA: Fatty acid.
THERAPEUTIC POTENTIAL
sWAT and BAT have intrinsic beneficial metabolic properties, whereas vWAT is the main cause of insulin resistance and type II diabetes mellitus. Obesity and its related metabolic diseases are worldwide challenges. Many strategies to address the problems have been attempted, but there are still no clear solutions. Recently, however, the rediscovery of BAT in human adults led to many investigations of BAT for anti-obesity treatments. Some of the experimental evidence suggests that BAT could be a new therapeutic tool as well as a precise regulator of energy homeostasis. People who have adapted to cold environments show some resistance to the development of diabetes, possibly due to the maintenance of a larger amounts of BAT[62]. In addition, the extent of human BAT activity in patients is inversely associated with obesity, age and type II diabetes[63]. In mouse experiments, the mouse strains with higher thermogenic gene expression in WAT depots tended to be more resistant to obesity and insulin resistance than those with lower levels[64]. Based on these results, many molecules (such as irisin[57], FGF21[55], and natriuretic peptides[56]) that induce BAT activation or WAT browning have been studied as potential drugs. Of course, these molecules also create side effects; nevertheless, these molecules may be an important key to address many challenges if the side effects can be mitigated.
CONCLUSION
WAT is an important endocrine organ that maintains body homeostasis by storing excess energy and secreting hormones. However, the excessive accumulation of fat in the organ causes obesity and obesity-associated metabolic disorders. Thus, developing treatments for obesity is important for maintaining public health. Interestingly, a potential solution to the problem of obesity-associated diseases has been found in brown fat, a type of adipose tissue that dissipates energy through a thermogenesis process. Previous studies showed that activated BAT is inversely correlated with BMI[65], adipose tissue mass and insulin resistance. Thus, BAT is one of the best targets for creating strategies to treat obesity and obesity-associated diseases. However, the transdifferentiation of white adipocytes into brown adipocytes is difficult because each type of adipose tissue is derived from a different progenitor lineage. The recent discovery of beige/brite adipocytes within WAT that are derived from the same lineage provides the possibility to overcome this challenge. Moreover, beige/brite cells are distributed throughout the human body, and they are highly activated in response to a variety of factors, including endogenous hormones. Therefore, WAT browning as well as BAT activation may contribute to an important strategy for treating obesity. A deeper understanding of the biological mechanisms that regulate the conversion within adipocytes will help in developing browning-inducing strategies for suppressing obesity.
ACKNOWLEDGMENTS
We are grateful to Professor Dae-Sik Lim for useful discussion and critical advice, and we appreciate the continuous encouragement and helpful guidance of Drs. Sung Goo Park, Baek Soo Han and Sang Chul Lee. In addition, we thank Ms. Hyeryung Choi and Min Jeong Son for insightful comments. We also thank Mr. Moon Gull Lee for assistance in making figures.
Footnotes
P- Reviewers: Scarfi S, Wakao H S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
References
- 1.Bae KH, Kim WK, Lee SC. Involvement of protein tyrosine phosphatases in adipogenesis: new anti-obesity targets. BMB Rep. 2012;45:700–706. doi: 10.5483/BMBRep.2012.45.12.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lean ME. Brown adipose tissue in humans. Proc Nutr Soc. 1989;48:243–256. doi: 10.1079/pns19890036. [DOI] [PubMed] [Google Scholar]
- 4.Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 5.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 6.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 7.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 8.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl. 1978;32:89–93. doi: 10.1007/978-3-0348-5559-4_9. [DOI] [PubMed] [Google Scholar]
- 12.Mahadik SR, Lele RD, Saranath D, Seth A, Parikh V. Uncoupling protein-2 (UCP2) gene expression in subcutaneous and omental adipose tissue of Asian Indians: Relationship to adiponectin and parameters of metabolic syndrome. Adipocyte. 2012;1:101–107. doi: 10.4161/adip.19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bełtowski J. Adiponectin and resistin--new hormones of white adipose tissue. Med Sci Monit. 2003;9:RA55–RA61. [PubMed] [Google Scholar]
- 14.Dani C, Amri EZ, Bertrand B, Enerback S, Bjursell G, Grimaldi P, Ailhaud G. Expression and regulation of pOb24 and lipoprotein lipase genes during adipose conversion. J Cell Biochem. 1990;43:103–110. doi: 10.1002/jcb.240430202. [DOI] [PubMed] [Google Scholar]
- 15.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 17.Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 18.Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab. 2006;291:E350–E357. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- 19.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 20.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 22.Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19–E31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 23.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 24.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab. 2009;297:E977–E986. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 25.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 27.Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- 28.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 29.Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JW, Tang QQ, Vinson C, Lane MD. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA. 2004;101:43–47. doi: 10.1073/pnas.0307229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- 34.Linhart HG, Ishimura-Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, Bick RJ, Darlington GJ. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc Natl Acad Sci USA. 2001;98:12532–12537. doi: 10.1073/pnas.211416898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmona MC, Iglesias R, Obregón MJ, Darlington GJ, Villarroya F, Giralt M. Mitochondrial biogenesis and thyroid status maturation in brown fat require CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277:21489–21498. doi: 10.1074/jbc.M201710200. [DOI] [PubMed] [Google Scholar]
- 36.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim WK, Choi HR, Park SG, Ko Y, Bae KH, Lee SC. Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated β-catenin stabilization. Int J Biochem Cell Biol. 2012;44:327–334. doi: 10.1016/j.biocel.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Kang S, Bajnok L, Longo KA, Petersen RK, Hansen JB, Kristiansen K, MacDougald OA. Effects of Wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1alpha. Mol Cell Biol. 2005;25:1272–1282. doi: 10.1128/MCB.25.4.1272-1282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 is an inducer of C/EBPβ required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab. 2011;14:658–670. doi: 10.1016/j.cmet.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Townsend K, Tseng YH. Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte. 2012;1:13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–1493. doi: 10.1074/jbc.M006246200. [DOI] [PubMed] [Google Scholar]
- 45.Karamanlidis G, Karamitri A, Docherty K, Hazlerigg DG, Lomax MA. C/EBPbeta reprograms white 3T3-L1 preadipocytes to a Brown adipocyte pattern of gene expression. J Biol Chem. 2007;282:24660–24669. doi: 10.1074/jbc.M703101200. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown. Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 1990;4:2890–2898. [PubMed] [Google Scholar]
- 49.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993;34:1057–1091. [PubMed] [Google Scholar]
- 50.Giacobino JP. Beta 3-adrenoceptor: an update. Eur J Endocrinol. 1995;132:377–385. doi: 10.1530/eje.0.1320377. [DOI] [PubMed] [Google Scholar]
- 51.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 52.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 54.Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X, Enerbäck S, Smith U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res. 2003;11:1182–1191. doi: 10.1038/oby.2003.163. [DOI] [PubMed] [Google Scholar]
- 60.Jennissen K, Siegel F, Liebig-Gonglach M, Hermann MR, Kipschull S, van Dooren S, Kunz WS, Fässler R, Pfeifer A. A VASP-Rac-soluble guanylyl cyclase pathway controls cGMP production in adipocytes. Sci Signal. 2012;5:ra62. doi: 10.1126/scisignal.2002867. [DOI] [PubMed] [Google Scholar]
- 61.Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dayaratne DA. Impact of ecology on development of NIDDM. Med Hypotheses. 2010;74:986–988. doi: 10.1016/j.mehy.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 63.Lee P, Swarbrick MM, Ho KK. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev. 2013;34:413–438. doi: 10.1210/er.2012-1081. [DOI] [PubMed] [Google Scholar]
- 64.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W, Wang Q, Zhang M, Xu M, Gu W, Qi L, Li B, Ning G. Brown adipose tissue activation is inversely related with central obesity and metabolic parameters in adult human. Endocrine Abstracts. 2012;29:OC12.5. doi: 10.1371/journal.pone.0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]


