Abstract
Many studies in different biological systems have revealed that 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) modulates signaling pathways triggered at the plasma membrane by agents such as Wnt, transforming growth factor (TGF)-β, epidermal growth factor (EGF), and others. In addition, 1α,25(OH)2D3 may affect gene expression by paracrine mechanisms that involve the regulation of cytokine or growth factor secretion by neighboring cells. Moreover, post-transcriptional and post-translational effects of 1α,25(OH)2D3 add to or overlap with its classical modulation of gene transcription rate. Together, these findings show that vitamin D receptor (VDR) cannot be considered only as a nuclear-acting, ligand-modulated transcription factor that binds to and controls the transcription of target genes. Instead, available data support the view that much of the complex biological activity of 1α,25(OH)2D3 resides in its capacity to interact with membrane-based signaling pathways and to modulate the expression and secretion of paracrine factors. Therefore, we propose that future research in the vitamin D field should focus on the interplay between 1α,25(OH)2D3 and agents that act at the plasma membrane, and on the analysis of intercellular communication. Global analyses such as RNA-Seq, transcriptomic arrays, and genome-wide ChIP are expected to dissect the interactions at the gene and molecular levels.
Keywords: 1α,25(OH)2D3; VDR; membrane-based signaling; Wnt; growth factors; cytokines; paracrine effects
Introduction
The active vitamin D metabolite 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) is a key regulator of gene expression in higher organisms. It modulates the activity of the vitamin D receptor (VDR), a member of the superfamily of nuclear hormone receptors that regulate gene transcription. Genome-wide chromatin immunoprecipitation studies have shown that VDR binds to hundreds of genome sites even in the absence of 1α,25(OH)2D3 and that ligand binding increases and partially changes these binding sites, which depend on the cell type and the duration of treatment (Carlberg and Campbell, 2013). While a subset of VDR binding sites may be responsible for the control of gene expression (VDREs or vitamin D response elements), others might be temporary anchorage places for a population of unliganded “dormant” VDR. According to the classical view, VDR binds DNA as heterodimers with a retinoid X receptor (RXRα, β, or γ) and, upon ligand binding, changes the transcription rate of neighboring genes.
However, many genes whose expression is altered by 1α,25(OH)2D3 do not contain VDREs. Putative mechanisms of this action include post-transcriptional regulation via changes in the levels of microRNAs that modulate the half-life and/or translation of their messenger RNAs (Thorne et al., 2011; Wang et al., 2011; Alvarez-Díaz et al., 2012; Kasiappan et al., 2012; Guan et al., 2013). Also, 1α,25(OH)2D3 may regulate genes post-translationally via changes in the phosphorylation or other modifications of proteins which affect their stability (Lin et al., 2003; Li et al., 2004), or through changes in the level or activity of proteases that target them (Alvarez-Díaz et al., 2010).
Increasing importance has recently been accorded to another mechanism of 1α,25(OH)2D3 action: the modulation of signaling pathways triggered by other agents at the plasma membrane. Indeed, a number of studies have shown that 1α,25(OH)2D3 modulates the effects of growth factors and cytokines by altering either their cytosolic signaling pathways or the activity of target transcription factors in the nucleus, or even in a paracrine fashion by inhibiting their synthesis and secretion by neighboring cells.
Here we review the available data on these non-classical, alternative mechanisms by which 1α,25(OH)2D3 modulates gene expression. Notably, for specific genes such as c-MYC, both direct transcriptional and indirect modes of regulation by 1α,25(OH)2D3 have been described (Pan and Simpson, 1999; Pálmer et al., 2001; Toropainen et al., 2010; Salehi-Tabar et al., 2012).
Interaction of 1α,25(OH)2D3 with Wnt, hedgehog, and notch pathways
Wnt, Hedgehog, and Notch signaling pathways, which have long been known to play crucial roles during development, are now considered critical for many tumorigenic processes in which they function abnormally due to mutation and/or changes in expression of components.
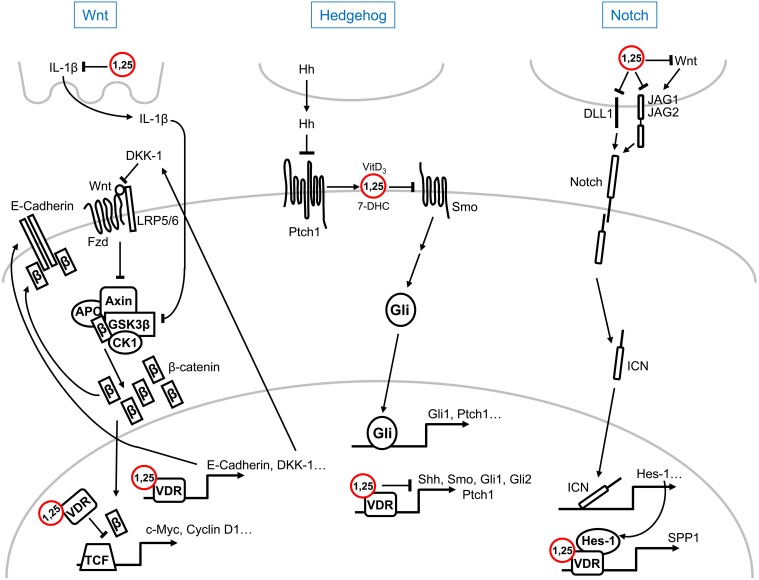
Wnt factors activate several signaling pathways upon binding to different plasma membrane receptors: the canonical or Wnt/β-catenin and the non-canonical (planar polarity, Ca2+…) pathways (Clevers and Nusse, 2012). Activation of the Wnt/β-catenin pathway by mutation of APC or AXIN tumor suppressor genes or of CTNNB1/β-catenin oncogene together with changes in the expression of a number of regulatory genes (SFRPs, DICKKOPF (DKK)s…) is a hallmark of most colorectal cancers and of a variable proportion of several other malignancies (Clevers and Nusse, 2012). A series of studies report that 1α,25(OH)2D3 antagonizes Wnt/β-catenin signaling in colon cancer cells by several mechanisms: the reduction of transcriptionally active β-catenin/T-cell factor complexes, the induction of β-catenin relocation from the nucleus toward the adherens junctions structures at the plasma membrane, and the increase in the level of the Wnt inhibitor DKK-1 (Pálmer et al., 2001; Shah et al., 2006; Aguilera et al., 2007) (Figure 1). In this way, the pathway endpoint, i.e., the activation of β-catenin target genes, is attenuated by 1α,25(OH)2D3 (Pálmer et al., 2001). Emphasizing the importance of this action, an additional indirect mechanism of Wnt/β-catenin antagonism in colon cancer has been proposed involving IL-1β, which will be reviewed in section 1α,25(OH)2D3 and Cytokines. Although 1,25(OH)2D3 inhibits β-catenin/TCF transcriptional activity in colon and other cancer cells, the upregulation of the Wnt/β-catenin pathway by either ligand-activated or unliganded VDR has been described in osteoblasts and keratinocytes, where it promotes bone formation and hair follicle differentiation, respectively (Larriba et al., 2013). However, the results reported in keratinocytes are controversial: while VDR enhances Wnt signaling through direct binding to Lymphocyte Enhancer-binding Factor (LEF)-1 independently of ligand and β-catenin (Luderer et al., 2011), ligand-activated VDR is believed to inhibit Wnt/β-catenin signaling (Bikle, 2011; Jiang et al., 2012).
Figure 1.
Schematic representation of the multilevel crosstalk of 1α,25(OH)2D3 (1,25) with Wnt, Hedgehog, and Notch signaling pathways. For simplicity, only main components and regulators of the pathways are shown. Explanations, details, and references can be found in the text.
Inhibition of Hedgehog (Hh) signaling by vitamin D compounds has also been suggested. In a study combining experiments in zebrafish, the yeast Pichia pastori and mouse fibroblasts, secreted vitamin D3, or its precursor 7-dehydrocholesterol (7-DHC), was shown to mediate the paracrine inhibition of Smoothened (Smo) by Patched (Ptch)1 which leads to pathway inactivation (Bijlsma et al., 2006). In the model proposed, which includes the binding of vitamin D3 to Smo at high (micromolar) concentrations, Hh ligands activate the pathway by blocking the induction of the secretion of vitamin D3/7-DHC by Ptch1 (Bijlsma et al., 2006) (Figure 1). The Hh pathway is aberrantly activated in basal cell carcinoma, the most frequent human tumor type. Interestingly, 1α,25(OH)2D3 inhibits proliferation and induces differentiation of mouse basal cell carcinomas and embryonal rhabdomyosarcomas with an activated Hh pathway due to Ptch1 deletion (Uhmann et al., 2011, 2012). As in the previous study, 1α,25(OH)2D3 acts at the level of Smo in a VDR-independent manner (Figure 1). Curiously, Tang et al. found that vitamin D3 inhibits Hh and cell proliferation more effectively than 7-DHC, 25(OH)D3, or 1α,25(OH)2D3 in murine basal cell carcinoma cells (Tang et al., 2011). Vitamin D3 also inhibits proliferation and Hh pathway through inactivation of Smo in cultured mouse pancreatic adenocarcinoma cells, but has no antitumor activity in vivo (Brüggemann et al., 2010). A common concern in all these studies is the high concentration of vitamin D3 required to observe the reported effects. Research in Vdr-deficient mice and in mouse skin explants has shown that lack of VDR increases the expression of several components of the Hh pathway such as Shh, Smo, Gli1, Gli2, and Ptch1, while 1α,25(OH)2D3 suppresses their expression (Bikle et al., 2013) (Figure 1). However, the interaction between 1α,25(OH)2D3 and Hh signaling in human skin remains to be elucidated.
Few studies link vitamin D with Notch signaling. Differentiation of human osteoblasts with vitamin D3 and dexamethasone distinctly affects the expression of Notch receptor family members (Schnabel et al., 2002). In rodent osteoblasts, the transcription factor Hes-1, which is an effector of the Notch pathway, enhances the induction of SPP1/osteopontin transcription by 1α,25(OH)2D3, indicating the collaboration of 1α,25(OH)2D3 and Notch pathways in bone remodeling (Shen and Christakos, 2005) (Figure 1). Transcriptomic analyses in human RWPE1 immortalized non-tumorigenic prostate cells showed the reduction of the RNA levels of the NOTCH ligands JAGGED (JAG)1, JAG2, and Delta-like (DLL)1 by 1α,25(OH)2D3 (Kovalenko et al., 2010) (Figure 1). By contrast, no changes in the expression of NOTCH-1 and JAG1 were detected in cultured human keratinocytes upon 1α,25(OH)2D3 treatment (Reichrath and Reichrath, 2012). As JAG1 transcription and, consequently, Notch signaling are upregulated by Wnt/β-catenin in colorectal cancer cells (Rodilla et al., 2009), the repressive effect of 1α,25(OH)2D3 on the Notch pathway in this system may be secondary to the antagonism of the Wnt/β-catenin pathway (Figure 1).
Interplay of 1α,25(OH)2D3 with agents that trigger signaling pathways via plasma membrane kinase receptors
There is mutual antagonism between 1α,25(OH)2D3 and epidermal growth factor (EGF), a potent mitogen, in primary colon epithelial cells and in established colon (Caco-2) and breast (T47D) tumor cell lines. This is based on the cross-inhibition of the expression of their respective receptors, VDR and EGFR (Tong et al., 1998, 1999). However, it is a cell type-dependent effect as EGF increases VDR in the rat small intestine and 1α,25(OH)2D3 increases EGFR in BT-20 breast cancer cells (Bruns et al., 1989; Desprez et al., 1991). In addition, 1α,25(OH)2D3 inhibits EGFR signaling by increasing the level of E-cadherin protein at the plasma membrane, which downregulates EGFR (Pálmer et al., 2001; Andl and Rustgi, 2005), and by decreasing that of SPROUTY-2, a cytosolic protein that reduces EGFR ubiquitination, internalization and degradation (Cabrita and Christofori, 2008; Barbáchano et al., 2010).
Transforming growth factor (TGF)-β has opposite roles in carcinogenesis: it inhibits proliferation of normal epithelial cells, but it later induces epithelial-mesenchymal transition, immunosuppression and metastasis (Pickup et al., 2013). 1α,25(OH)2D3 induces the expression of the type I TGF-β receptor and both agents, 1α,25(OH)2D3 and TGF-β, cooperate in Caco-2 cell growth inhibition (Chen et al., 2002; Pálmer et al., 2003). Moreover, Smad3, a mediator of TGF-β signaling, is a co-activator of VDR and contributes to gene regulation by 1α,25(OH)2D3 (Yanagisawa et al., 1999), an effect that is abrogated by Smad7 in transfected COS-7 cells (Yanagi et al., 1999). Reinforcing the interaction between both signaling pathways, 1α,25(OH)2D3 induces the expression of Smad anchor for receptor activation (SARA) (Pálmer et al., 2003), which maintains the epithelial phenotype by recruiting Smads 2/3 to the activated TGF-β receptors and regulates endocytic trafficking of EGFR and other proteins (Tang et al., 2011; Kostaras et al., 2013). Notably, a recent study of R. M. Evans' group has revealed a genome-wide overlap of VDR and Smad3 binding sites that is responsible for the abrogation by VDR ligands of the TGF-β1-mediated activation of hepatic stellate cells during liver fibrosis (Ding et al., 2013). These authors show that TGF-β1 signaling redistributes VDR-binding sites in the genome and facilitates VDR binding at Smad3 profibrotic target genes. Upon ligand activation, VDR binding at coregulated genes decreases Smad3 occupancy at these sites, causing inhibition of fibrosis (Ding et al., 2013). This is a regulatory feed-back mechanism in which VDR ligands limit the fibrotic process and so ensure an appropriate non-pathological tissue response. Given the crucial roles of TGF-β in carcinogenesis, future studies should examine whether vitamin D compounds play similar roles in the maintenance of epithelial integrity opposing the onset of carcinomas.
1α,25(OH)2D3 and TGF-β interact also in bone. Curiously, in rat (UMR 106 and ROS 17/2.8) and human (MG-63) osteoblastic cells TGF-β increases VDR expression but inhibits the stimulation of osteocalcin and osteopontin transcription and RNA levels by 1α,25(OH)2D3 (Staal et al., 1994). TGF-β exerts this inhibitory effect by reducing the binding of VDR-RXR complexes to VDREs localized in the promoter of these genes without affecting the nuclear availability of VDR at least in ROS 17/2.8 cells (Staal et al., 1996). In contrast to the stimulation of osteocalcin synthesis in human and rat cells, 1α,25(OH)2D3 decreases osteocalcin production in mouse fetal long bone cultures and neonatal osteoblastic MC3T3 cells while stimulating bone resorption (Staal et al., 1998). This bone resorption action of 1α,25(OH)2D3 is dose-dependently inhibited by TGF-β (Staal et al., 1998).
A complex, cell type-, context- and sometimes age-dependent relation exists between 1α,25(OH)2D3 and insulin-like growth factors (IGF)-I and II. For instance, in C2C12 myoblasts 1α,25(OH)2D3 decreases IGF-I expression while it increases that of IGF-II (Garcia et al., 2011). In HT29 colon carcinoma cells several vitamin D compounds inhibit the secretion of IGF-II thus attenuating its cell proliferation activity (Oh et al., 2001). In addition, 1α,25(OH)2D3 blocks the mitogenic activity of insulin and IGF-I in MCF7 breast cancer cells, at least in part due to the inhibition of c-FOS upregulation (Vink-van Wijngaarden et al., 1996). 1α,25(OH)2D3 and IGF-I have also opposite effects on mouse long bones: IGF-I increases osteocalcin production, which is completely blocked by 1α,25(OH)2D3, and inhibits the enhancement of bone resorption caused by 1α,25(OH)2D3 (Staal et al., 1998). Furthermore, 1α,25(OH)2D3 variably regulates the expression of several IGF binding proteins (IGFBPs), a group of molecules with pleiotropic actions that transport IGFs and also modulate cell survival/apoptosis: 1α,25(OH)2D3 induces IGFBP3 expression in SW480-ADH colon carcinoma, SaOS-2 osteosarcoma, PC3 prostate cancer, MCF7 breast carcinoma and MCF-10A normal mammary cells (Pálmer et al., 2003; Matilainen et al., 2005; Malinen et al., 2011; Brosseau et al., 2013), IGFBP6 in SaOS-2, SW480-ADH and colon carcinoma HT29 cells (Oh et al., 2001; Pálmer et al., 2003; Matilainen et al., 2005), IGFBP1 and IGFBP5 in SaOS-2 and PC3 cells, and IGFBP4 in SaOS-2 cells (Matilainen et al., 2005). Conversely, 1α,25(OH)2D3 represses IGFBP4 in HT29 and SW480-ADH cells, and IGFBP2 in HT29 cells (Oh et al., 2001; Pálmer et al., 2003). In ovarian cells, 1α,25(OH)2D3 alone induces IGFBP1 production but, conversely, it enhances the inhibitory effect of insulin (Parikh et al., 2010). Curiously, recent studies show that IGFBP3 interacts with VDR (Li et al., 2013) and that IGFBP6 binds VDR and blocks the induction of osteoblast differentiation by 1α,25(OH)2D3 (Cui et al., 2011).
Cell type-dependent effects of 1α,25(OH)2D3 have also been described for hepatocyte growth factor (HGF) signaling. 1α,25(OH)2D3 activates the HGF gene promoter and induces HGF expression and secretion in rat NRK-49F renal interstitial fibroblasts (Li et al., 2005) and in human keloid fibroblasts (Zhang et al., 2011). Consistently with these results, vitamin D deficiency reduces HGF and HGF receptor/c-Met expression during liver regeneration in rats (Goupil et al., 1997). Conversely, 1α,25(OH)2D3 decreases the level of HGF RNA in human HL-60 promyelocitic leukemia cells (Inaba et al., 1993), smooth muscle cells (Shalhoub et al., 2010) and MG-63 osteosarcoma cells (Chattopadhyay et al., 2003). Moreover, the expression of c-Met is inhibited by 1α,25(OH)2D3 in human MHCC97 hepatocellular cell line (Wu et al., 2007). Curiously, 1α,25(OH)2D3 and HGF cooperate to increase osteogenic differentiation of human bone marrow stem cells and maturation of chondrocyte progenitor cells (Grumbles et al., 1996; D'Ippolito et al., 2002; Chen et al., 2011, 2012). Also, 1α,25(OH)2D3 and HGF additively inhibit proliferation of androgen-unresponsive prostate cancer cells (Qadan et al., 2000).
In concordance with its regulatory role in the organism, 1α,25(OH)2D3 favors physiological and homeostatic angiogenesis but inhibits angiogenesis in pathological conditions. Thus, 1α,25(OH)2D3 promotes myogenic differentiation of C2C12 cells by increasing the expression of two key angiogenic factors: vascular endothelial growth factor (VEGF) and fibroblast growth factor-1 (Garcia et al., 2013). In addition, 1α,25(OH)2D3 stimulates pro-angiogenic properties of endothelial progenitor cells by increasing VEGF levels (Grundmann et al., 2012). 1α,25(OH)2D3 also upregulates VEGF expression in osteoblast-like cells but not in breast cancer cells (Schlaeppi et al., 1997). Likewise, ED-71, a vitamin D analog, enhances VEGF expression and promotes angiogenesis in a murine bone marrow ablation model (Okuda et al., 2007). Indeed, increased production of VEGF in vascular smooth muscle cells results from the activation of a VDRE present in the VEGF gene promoter (Cardus et al., 2009). By contrast, 1α,25(OH)2D3 downregulates hypoxia-inducible factor (HIF)-1 and VEGF protein expression in several human colon, prostate and breast cancer cell lines (Ben-Shoshan et al., 2007), decreases VEGF production by human lumbar annulus cells (Gruber et al., 2008), and protects against diabetic retinopathy in rats by inhibiting VEGF expression in the retina (Ren et al., 2012).
1α,25(OH)2D3 also modulates the activity of signaling pathways mediated by other types of plasma membrane receptors such us G protein-coupled receptors. Shen et al. found that 1α,25(OH)2D3 suppresses the expression of parathyroid hormone-related protein (PTHrP) in prostate cancer cells via a negative VDRE localized within the non-coding region of the gene, thus antagonizing the induction of cell proliferation and of the expression of the pro-invasive integrin α6β4 exerted by PTHrP signaling (Shen et al., 2007).
1α,25(OH)2D3 and cytokines
The anti-inflammatory and immunomodulatory actions, and thus some of the anticancer and antimicrobial effects of 1α,25(OH)2D3, are mediated by the regulation of cytokine production and/or through the control of their receptors or downstream signaling pathways. Globally, 1α,25(OH)2D3 contributes to the autocrine and paracrine control of innate and adaptative immune responses (Adorini and Penna, 2008).
1α,25(OH)2D3 regulates the function of antigen-presenting cells and T-lymphocytes. It inhibits Th1 cells differentiation and, therefore, the secretion of Th1-type cytokines, enhances the development of Th2 cells, and induces tolerogenic monocytes and dendritic cells. IL-4 and IL-10 are among the commonly increased cytokines, while IL-1, IL-2, IL-6, IL-17, tumor necrosis factor (TNF)-α and interferon (IFN)-γ are decreased (Adorini and Penna, 2008).
Mechanistically, ligand-activated VDR directly downregulates the expression of IL-10, IL-2, and IL-12B in lipopolysaccharide-treated human monocytes (THP-1) through its binding to VDREs located in the genomic regions of these genes and the recruitment of the co-repressor NCOR/SMRT and histone deacetylases (Matilainen et al., 2010a,b; Gynther et al., 2011). Remarkably, IL-10 is downregulated by short 1α,25(OH)2D3 treatment (8 h) but upregulated at late time points (48 h) (Matilainen et al., 2010a). In addition, direct VDR binding to a single VDRE mediates the upregulation of IL-8 gene by 1α,25(OH)2D3 in undifferentiated and differentiated THP-1 cells (Ryynänen and Carlberg, 2013).
1α,25(OH)2D3 also changes the expression of target genes in immune cells by repressing crucial transcription factors such as nuclear factor kappa B (NFkB) and signaling pathways such as Janus kinase-signal transducer and activator of transcription (JAK-STAT) (Yu et al., 1995; Muthian et al., 2006; Geldmeyer-Hilt et al., 2011). 1α,25(OH)2D3 also represses NFκB activity in fibroblasts and adipocytes (Harant et al., 1998; Mutt et al., 2012), and fibroblasts lacking VDR have increased NFκB activity (Sun et al., 2006). Direct (increase in IκBα expression and reduction of nuclear translocation of p65) and indirect (upregulation of IGFBP3 and clusterin) mechanisms contribute to the inhibition of NFκB activation (Krishnan and Feldman, 2010). D. Feldman's group has reported that, in addition to inhibiting NFκB, the anti-inflammatory effects of 1α,25(OH)2D3 in prostate cancer cells include the reduction of pro-inflammatory prostaglandins (PG) production via suppression of ciclooxygenase-2, downregulation of PG receptors, and upregulation of 15-hydroxyprostaglandin dehydrogenase, which inactivates PGs (Krishnan and Feldman, 2010). Moreover, 1α,25(OH)2D3 decreases the synthesis of pro-inflammatory IL-6 through the inactivation of p38 kinase due to the upregulation of the mitogen kinase phosphatase (MKP)5 and the blockade of TNF-α (Krishnan and Feldman, 2010). In Jurkat cells, the repression of IL-2 gene by 1α,25(OH)2D3 is at least partially due to the blockade of NFATp/AP-1 complex formation at a positive regulatory NFAT-1 site, which is bound by VDR-RXR heterodimers (Alroy et al., 1995).
1α,25(OH)2D3 reduces the secretion of interleukin (IL)1-β in THP macrophages by blocking the activation of STAT1 (Kaler et al., 2009). As IL1-β activates the Wnt/β-catenin pathway in colon carcinoma cells via inhibition of GSK3β activity and subsequent stabilization and nuclear translocation of β-catenin, this mechanism may contribute to the antagonism of Wnt signaling by 1α,25(OH)2D3 (Kaler et al., 2009) (Figure 1). Curiously, IL-1α is believed to be upregulated and to mediate the antiproliferative effects of 1α,25(OH)2D3 in prostate progenitor/stem cells (Maund et al., 2011).
In human osteoblasts, 1α,25(OH)2D3 completely overrules the inhibitory effect of IFN-β on mineralization. This dominant effect on osteoblast differentiation and bone formation is reflected in the downregulation of IFN-related and -regulated genes by 1α,25(OH)2D3 (Woeckel et al., 2012). Concomitantly, 1α,25(OH)2D3 also induces activin A, a strong inhibitor of mineralization, and represses follistatin, the natural antagonist of activin A, to ensure a fine-tuned regulation of the mineralization process (Woeckel et al., 2013b).
Recent findings have underscored the complexity of 1α,25(OH)2D3 action and its role in the antimicrobial response as part of innate and adaptative immunity. Thus, activation of macrophage Toll-like receptors (TLRs) by intracellular bacteria such as Mycobacterium tuberculosis upregulates VDR and CYP27B1 genes that allow the induction of the antimicrobial peptide cathelicidin by 1α,25(OH)2D3 (Liu et al., 2006). In monocytes, TLR activation triggers induction of defensin β4 (DEFB4) gene requiring the cooperation between IL-1β and 1α,25(OH)2D3, which is explained by the presence of one VDRE and two IL-1β-activatable NFkB sites in the DEFB4 promoter (Liu et al., 2009). In addition, 1α,25(OH)2D3 is required for the antimicrobial effect of IFN-γ in human macrophages (Fabri et al., 2011). Moreover, by inducing the expression of TLR2 and CD14 receptors and cathelicidin, 1α,25(OH)2D3 mediates the effect of TGF-β favoring the response to microbial infection and wound injury by keratinocytes (Schauber et al., 2007). These findings show also unexpected cooperation of 1α,25(OH)2D3 with agents (IL-1β, TGF-β, IFN-γ) that are antagonistic in other cell types.
Interplay of 1α,25(OH)2D3/VDR with transcription factors
Liganded or unliganded VDR interacts with or regulates the expression of a number of transcription factors that are downstream effectors of different signaling pathways (Table 1). An interesting example is the upregulation by 1α,25(OH)2D3 of CDKN1B/p27Kip1, a cell cycle regulator gene which lacks VDREs. 1α,25(OH)2D3 was first shown to induce CDKN1B transcription by stimulating the binding of Sp1 and NF-Y transcription factors to the CDKN1B promoter in the myelomonocytic U937 cell line (Inoue et al., 1999). Later, direct VDR-Sp1 interaction at the promoter Sp1 sites was described as responsible for this effect (Huang et al., 2004). In addition to the enhancement of transcription, 1α,25(OH)2D3 increases the stability of p27Kip1 protein by repressing p45Skp2, an F-box protein, through the induction of VDR-Sp1 complexes that together with histone deacetylase 1 are recruited to Sp1 sites at the p45Skp2 gene promoter (Lin et al., 2003; Li et al., 2004; Huang and Hung, 2006).
Table 1.
Interplay between VDR and other transcription factors.
| Transcription factor | Biological effect | References |
|---|---|---|
| Sp1/NF-Y | Potentiation | Inoue et al., 1999; Huang et al., 2004 |
| AP-1/NFAT1 | Repression | Towers et al., 1999 |
| AP-1 | Activation | Chen et al., 1999 |
| CREB | Repression | Yuan et al., 2007 |
| FOXO3a, FOXO4 | Activation | An et al., 2010 |
| p53 | Mutual repression | Stambolsky et al., 2010; Chen et al., 2013 |
| PPAR-α/δ | Activation | Sertznig et al., 2009a,b |
| PPAR-γ | Variable | Alimirah et al., 2012; Woeckel et al., 2013a |
| RAR | Variable | Jiménez-Lara and Aranda, 1999; Tavera-Mendoza et al., 2006; Anand et al., 2008; Ng et al., 2010 |
| ER | Downregulation | Krishnan et al., 2010; Swami et al., 2013 |
| AR | Crossregulation | Zhao et al., 1999; Ting et al., 2005 |
| PIT-1 | Downregulation | Seoane and Pérez-Fernández, 2006 |
The granulocyte-macrophage colony-stimulating factor (GM-CSF) gene is another example of unusual regulation by 1α,25(OH)2D3. Ligand-activated VDR represses GM-CSF through a composite DNA element recognized by Jun-Fos heterodimers (AP-1) and nuclear factor of activated T-cells (NFAT)1 (Towers et al., 1999). In the absence of RXR, VDR binds to c-Jun and stabilizes AP-1 bound to DNA, which outcompetes NFAT1 and decreases GM-CSF transcription. In Caco-2 cells, 1α,25(OH)2D3 stimulates AP-1 via activation of protein kinase C-α, ERK and JNK leading to cell differentiation (Chen et al., 1999).
In renal cells, 1α,25(OH)2D3 suppresses renin gene expression by blocking the cyclic AMP response element (CRE) through direct binding of VDR to CRE-binding protein (CREB) and so, inhibiting the binding of CREB to the CRE (Yuan et al., 2007). By a complex mechanism, 1α,25(OH)2D3 also regulates several Forkhead box (FOX) transcription factors. Ligand-activated VDR binds FOXO3a and FOXO4 together with their regulators, sirtuin 1 deacetylase and protein phosphatase 1, inducing deacetylation and dephosphorylation of FOXO proteins, thereby activating these (An et al., 2010). In the case of the p53 tumor suppressor protein a mutual regulation takes place: while mutated p53 interacts physically with VDR and changes VDR-target genes, converting 1α,25(OH)2D3 from a pro-apoptotic into an anti-apoptotic agent (Stambolsky et al., 2010), 1α,25(OH)2D3 activates the promoter of Mdm2 in a p53-dependent fashion promoting the expression of this negative regulator of p53 protein stability and function (Chen et al., 2013).
Multiple interplays between 1α,25(OH)2D3/VDR and other nuclear receptor ligands have been described. Among them, crosstalk between liganded VDR and peroxisome proliferator-activated receptor (PPAR)-α/δ in melanoma cells (Sertznig et al., 2009a,b) that may involve the stimulation of PPAR-δ expression by 1α,25(OH)2D3 (Dunlop et al., 2005). A synergistic action of 1α,25(OH)2D3 and rosiglitazone, a PPAR-γ ligand, has been shown during osteoblast-mediated mineralization (Woeckel et al., 2013a), while in human T47D breast cancer cells PPAR-γ binds VDR and represses its transcriptional activity, possibly also by competing for RXR heterodimerization (Alimirah et al., 2012). Titration out of common co-activators, but not of RXR, may be the mechanism by which ligand-bound VDR represses retinoic acid receptor (RAR) transactivation in GH4C1 pituitary cells (Jiménez-Lara and Aranda, 1999). The relation between 1α,25(OH)2D3 and retinoic acid is however complex, as cooperative effects on target genes and cellular outcome (proliferation inhibition and differentiation) have been described in other systems (Tavera-Mendoza et al., 2006; Anand et al., 2008; Ng et al., 2010). As for estrogen receptor (ER), D. Feldman's group has shown that 1α,25(OH)2D3 exerts a multilevel protective effect against breast cancer that includes the inhibition of estrogen synthesis through the direct and indirect repression of aromatase (CYP19) and the downregulation of ER-α expression through two VDREs in its promoter region (Krishnan et al., 2010; Swami et al., 2013). Likewise, there is a complex and unresolved relationship between 1α,25(OH)2D3 and androgen receptor (AR) synthesis and signaling. 1α,25(OH)2D3 induces AR in LNCaP cells (Zhao et al., 1999) while AR reduces VDR transcriptional activity (Ting et al., 2005), perhaps in some cells by a mechanism mediated by prohibitin (Mooso et al., 2010). In addition, 1α,25(OH)2D3 inhibits glucuronidation and so, inactivation of androgen in prostate cancer cells through the repression of UDP-glucuronosyltransferases (UGT) 2B15 and 2B17, which is counterintuitive given the growth promoting action of androgen and the antiproliferative effect of 1α,25(OH)2D3 in prostate cancer cells (Kaeding et al., 2008). In human bladder, 1α,25(OH)2D3 and analogs inhibit cell proliferation promoted by androgen and keratinocyte growth factor and induce apoptosis at least in part by repressing Bcl-2 expression (Crescioli et al., 2005).
Pituitary transcription factor (Pit)-1 activates growth hormone and prolactin genes in the anterior pituitary and also in breast cancer cells (Seoane and Pérez-Fernández, 2006). In MCF7 cells, VDR homodimers bind the PIT-1 promoter and inhibit its expression in the presence of 1α,25(OH)2D3 without involvement of RXR (Seoane and Pérez-Fernández, 2006).
Conclusions
The available evidence shows that the classical view of VDR only as a nuclear-acting ligand-modulated transcription factor that regulates the rate of transcription of those genes to which it binds is outdated. Instead, VDR and its ligand constitute a multilevel main regulator of gene expression in higher cells acting directly or indirectly, and via a variety of different mechanisms, on many signaling pathways. Some of them are triggered from the plasma membrane by paracrine or endocrine agents, and 1α,25(OH)2D3 interacts at different levels: membrane receptors, cytosolic signaling molecules or effector nuclear transcription factors. In most cases 1α,25(OH)2D3 action is mediated by nuclear VDR but in a few others this is unclear and non-canonical VDR-independent or extranuclear effects have been proposed. Available studies show that 1α,25(OH)2D3 and these signaling pathways interact variably and with distinct outcomes in a cell/tissue-specific fashion and sometimes also differentially between normal and malignant cells.
Perspectives
The increasingly recognized importance of its non-cell autonomous actions has widened the scope of the study of 1α,25(OH)2D3. On the one hand, an in-depth study of the interplay between 1α,25(OH)2D3 and other agents, which seems to be cell-specific in terms of biological outcome, is necessary to elucidate the possibilities of combined therapies using vitamin D compounds and inhibitors or activators of a variety of signaling pathways. On the other hand, several of these interactions take place at the intercellular level. By using high-throughput techniques and genome-wide analyses, we expect to be able to identify secreted paracrine and intracellular mediators of the interaction between 1α,25(OH)2D3 and other signaling pathways responsible for the regulatory actions of 1α,25(OH)2D3 in the organism. Future research should aim to discern how vitamin D compounds modulate tissue and organ physiology and how they may be used to treat pathological processes such as infections, autoimmune disorders, or cancer.
Author contributions
María Jesús Larriba, José Manuel González-Sancho, Félix Bonilla, and Alberto Muñoz wrote the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Robin Rycroft for his valuable assistance in the preparation of the English manuscript. The work in the authors' laboratory is supported by Ministerio de Economía y Competitividad of Spain (SAF2010-18302, BFU2010-19659), Fondo Europeo de Desarrollo Regional-Instituto de Salud Carlos III (RD12/0036/0021, RD12/0036/0041), and Comunidad de Madrid (S2010/BMD-2344, Colomics2).
References
- Adorini L., Penna G. (2008). Control of autoimmune diseases by the vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 4, 404–412 10.1038/ncprheum0855 [DOI] [PubMed] [Google Scholar]
- Aguilera O., Peña C., García J. M., Larriba M. J., Ordóñez-Morán P., Navarro D., et al. (2007). The Wnt antagonist DICKKOPF-1 gene is induced by 1α,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 28, 1877–1884 10.1093/carcin/bgm094 [DOI] [PubMed] [Google Scholar]
- Alimirah F., Peng X., Yuan L., Mehta R. R., von Knethen A., Choubey D., et al. (2012). Crosstalk between the peroxisome proliferator-activated receptor γ (PPARγ) and the vitamin D receptor (VDR) in human breast cancer cells: PPARγ binds to VDR and inhibits 1α,25-dihydroxyvitamin D3 mediated transactivation. Exp. Cell Res. 318, 2490–2497 10.1016/j.yexcr.2012.07.020 [DOI] [PubMed] [Google Scholar]
- Alroy I., Towers T. L., Freedman L. P. (1995). Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol. Cell. Biol. 15, 5789–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Díaz S., Larriba M. J., López-Otín C., Muñoz A. (2010). Vitamin D: proteases, protease inhibitors and cancer. Cell Cycle 9, 32–37 10.4161/cc.9.1.10266 [DOI] [PubMed] [Google Scholar]
- Alvarez-Díaz S., Valle N., Ferrer-Mayorga G., Lombardía L., Herrera M., Domínguez O., et al. (2012). MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum. Mol. Genet. 21, 2157–2165 10.1093/hmg/dds031 [DOI] [PubMed] [Google Scholar]
- An B. S., Tavera-Mendoza L. E., Dimitrov V., Wang X., Calderon M. R., Wang H. J., et al. (2010). Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol. Cell. Biol. 30, 4890–4900 10.1128/MCB.00180-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P. K., Kaul D., Sharma M. (2008). Synergistic action of vitamin D and retinoic acid restricts invasion of macrophages by pathogenic mycobacteria. J. Microbiol. Immunol. Infect. 41, 17–25 [PubMed] [Google Scholar]
- Andl C. D., Rustgi A. K. (2005). No one-way street: cross-talk between E-cadherin and receptor tyrosine kinase (RTK) signaling: a mechanism to regulate RTK activity. Cancer Biol. Ther. 4, 28–31 10.4161/cbt.4.1.1431 [DOI] [PubMed] [Google Scholar]
- Barbáchano A., Ordóñez-Morán P., García J. M., Sánchez A., Pereira F., Larriba M. J., et al. (2010). SPROUTY-2 and E-cadherin regulate reciprocally and dictate colon cancer cell tumourigenicity. Oncogene 29, 4800–4813 10.1038/onc.2010.225 [DOI] [PubMed] [Google Scholar]
- Ben-Shoshan M., Amir S., Dang D. T., Dang L. H., Weisman Y., Mabjeesh N. J. (2007). 1α,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 6, 1433–1439 10.1158/1535-7163.MCT-06-0677 [DOI] [PubMed] [Google Scholar]
- Bijlsma M. F., Spek C. A., Zivkovic D., van de Water S., Rezaee F., Peppelenbosch M. P. (2006). Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 4:e232 10.1371/journal.pbio.0040232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D. (2011). The vitamin D receptor: a tumor suppressor in skin. Discov. Med. 11, 7–17 [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Elalieh H., Welsh J., Oh D., Cleaver J., Teichert A. (2013). Protective role of vitamin D signaling in skin cancer formation. J. Steroid Biochem. Mol. Biol. 136, 271–279 10.1016/j.jsbmb.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosseau C., Pirianov G., Colston K. W. (2013). Role of insulin-like growth factor binding protein-3 in 1,25-dihydroxyvitamin-D3-induced breast cancer cell apoptosis. Int. J. Cell Biol. 2013, 960378 10.1155/2013/960378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann L. W., Queiroz K. C., Zamani K., van Straaten A., Spek C. A., Bijlsma M. F. (2010). Assessing the efficacy of the hedgehog pathway inhibitor vitamin D3 in a murine xenograft model for pancreatic cancer. Cancer Biol. Ther. 10, 79–88 10.4161/cbt.10.1.12165 [DOI] [PubMed] [Google Scholar]
- Bruns D. E., Krishnan A. V., Feldman D., Gray R. W., Christakos S., Hirsch G. N., et al. (1989). Epidermal growth factor increases intestinal calbindin-D9k and 1,25-dihydroxyvitamin D receptors in neonatal rats. Endocrinology 125, 478–485 10.1210/endo-125-1-478 [DOI] [PubMed] [Google Scholar]
- Cabrita M. A., Christofori G. (2008). Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis 11, 53–62 10.1007/s10456-008-9089-1 [DOI] [PubMed] [Google Scholar]
- Cardus A., Panizo S., Encinas M., Dolcet X., Gallego C., Aldea M., et al. (2009). 1,25-dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis 204, 85–89 10.1016/j.atherosclerosis.2008.08.020 [DOI] [PubMed] [Google Scholar]
- Carlberg C., Campbell M. J. (2013). Vitamin D receptor signaling mechanisms: integrated actions of a well-defined transcription factor. Steroids 78, 127–136 10.1016/j.steroids.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay N., MacLeod R. J., Tfelt-Hansen J., Brown E. M. (2003). 1α,25(OH)2-vitamin D3 inhibits HGF synthesis and secretion from MG-63 human osteosarcoma cells. Am. J. Physiol. Endocrinol. Metab. 284, E219–E227 10.1152/ajpendo.00247.2002 [DOI] [PubMed] [Google Scholar]
- Chen A., Davis B. H., Bissonnette M., Scaglione-Sewell B., Brasitus T. A. (1999). 1,25-Dihydroxyvitamin D3 stimulates activator protein-1-dependent Caco-2 cell differentiation. J. Biol. Chem. 274, 35505–35513 10.1074/jbc.274.50.35505 [DOI] [PubMed] [Google Scholar]
- Chen A., Davis B. H., Sitrin M. D., Brasitus T. A., Bissonnette M. (2002). Transforming growth factor-β 1 signaling contributes to Caco-2 cell growth inhibition induced by 1,25(OH)2D3. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G864–G874 10.1152/ajpgi.00524.2001 [DOI] [PubMed] [Google Scholar]
- Chen H., Reed G., Guardia J., Lakhan S., Couture O., Hays E., et al. (2013). Vitamin D directly regulates Mdm2 gene expression in osteoblasts. Biochem. Biophys. Res. Commun. 430, 370–374 10.1016/j.bbrc.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Aenlle K. K., Curtis K. M., Roos B. A., Howard G. A. (2012). Hepatocyte growth factor (HGF) and 1,25-dihydroxyvitamin D together stimulate human bone marrow-derived stem cells toward the osteogenic phenotype by HGF-induced up-regulation of VDR. Bone 51, 69–77 10.1016/j.bone.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Chen K., Perez-Stable C., D'Ippolito G., Schiller P. C., Roos B. A., Howard G. A. (2011). Human bone marrow-derived stem cell proliferation is inhibited by hepatocyte growth factor via increasing the cell cycle inhibitors p53, p21 and p27. Bone 49, 1194–1204 10.1016/j.bone.2011.08.023 [DOI] [PubMed] [Google Scholar]
- Clevers H., Nusse R. (2012). Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Crescioli C., Morelli A., Adorini L., Ferruzzi P., Luconi M., Vannelli G. B., et al. (2005). Human bladder as a novel target for vitamin D receptor ligands. J. Clin. Endocrinol. Metab. 90, 962–972 10.1210/jc.2004-1496 [DOI] [PubMed] [Google Scholar]
- Cui J., Ma C., Qiu J., Ma X., Wang X., Chen H., et al. (2011). A novel interaction between insulin-like growth factor binding protein-6 and the vitamin D receptor inhibits the role of vitamin D3 in osteoblast differentiation. Mol. Cell. Endocrinol. 338, 84–92 10.1016/j.mce.2011.03.011 [DOI] [PubMed] [Google Scholar]
- Desprez P. Y., Poujol D., Falette N., Lefebvre M. F., Saez S. (1991). 1,25-Dihydroxyvitamin D3 increases epidermal growth factor receptor gene expression in BT-20 breast carcinoma cells. Biochem. Biophys. Res. Commun. 176, 1–6 10.1016/0006-291X(91)90880-G [DOI] [PubMed] [Google Scholar]
- Ding N., Yu R. T., Subramaniam N., Sherman M. H., Wilson C., Rao R., et al. (2013). A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 153, 601–613 10.1016/j.cell.2013.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ippolito G., Schiller P. C., Perez-stable C., Balkan W., Roos B. A., Howard G. A. (2002). Cooperative actions of hepatocyte growth factor and 1,25-dihydroxyvitamin D3 in osteoblastic differentiation of human vertebral bone marrow stromal cells. Bone 31, 269–275 10.1016/S8756-3282(02)00820-7 [DOI] [PubMed] [Google Scholar]
- Dunlop T. W., Vaisanen S., Frank C., Molnar F., Sinkkonen L., Carlberg C. (2005). The human peroxisome proliferator-activated receptor δ gene is a primary target of 1α,25-dihydroxyvitamin D3 and its nuclear receptor. J. Mol. Biol. 349, 248–260 10.1016/j.jmb.2005.03.060 [DOI] [PubMed] [Google Scholar]
- Fabri M., Stenger S., Shin D. M., Yuk J. M., Liu P. T., Realegeno S., et al. (2011). Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 3, 104ra102 10.1126/scitranslmed.3003045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. A., Ferrini M. G., Norris K. C., Artaza J. N. (2013). 1,25(OH)2vitamin D3 enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C2C12 skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 133, 1–11 10.1016/j.jsbmb.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. A., King K. K., Ferrini M. G., Norris K. C., Artaza J. N. (2011). 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 152, 2976–2986 10.1210/en.2011-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmeyer-Hilt K., Heine G., Hartmann B., Baumgrass R., Radbruch A., Worm M. (2011). 1,25-dihydroxyvitamin D3 impairs NF-κB activation in human naïve B cells. Biochem. Biophys. Res. Commun. 407, 699–702 10.1016/j.bbrc.2011.03.078 [DOI] [PubMed] [Google Scholar]
- Goupil D., Ethier C., Zarnegar R., Gascon-Barre M. (1997). Hepatic expression of regeneration marker genes following partial hepatectomy in the rat. Influence of 1,25-dihydroxyvitamin D3 in hypocalcemia. J. Hepatol. 26, 659–668 [DOI] [PubMed] [Google Scholar]
- Gruber H. E., Hoelscher G., Ingram J. A., Chow Y., Loeffler B., Hanley E. N., Jr. (2008). 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine 33, 755–765 10.1097/BRS.0b013e3181695d59 [DOI] [PubMed] [Google Scholar]
- Grumbles R. M., Howell D. S., Wenger L., Altman R. D., Howard G. A., Roos B. A. (1996). Hepatocyte growth factor and its actions in growth plate chondrocytes. Bone 19, 255–261 10.1016/8756-3282(96)00180-9 [DOI] [PubMed] [Google Scholar]
- Grundmann M., Haidar M., Placzko S., Niendorf R., Darashchonak N., Hubel C. A., et al. (2012). Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am. J. Physiol. Cell Physiol. 303, C954–C962 10.1152/ajpcell.00030.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H., Liu C., Chen Z., Wang L., Li C., Zhao J., et al. (2013). 1,25-Dihydroxyvitamin D3 up-regulates expression of hsa-let-7a-2 through the interaction of VDR/VDRE in human lung cancer A549 cells. Gene 522, 142–146 10.1016/j.gene.2013.03.065 [DOI] [PubMed] [Google Scholar]
- Gynther P., Toropainen S., Matilainen J. M., Seuter S., Carlberg C., Väisänen S. (2011). Mechanism of 1α,25-dihydroxyvitamin D3-dependent repression of interleukin-12B. Biochim. Biophys. Acta 1813, 810–818 10.1016/j.bbamcr.2011.01.037 [DOI] [PubMed] [Google Scholar]
- Harant H., Wolff B., Lindley I. J. (1998). 1α,25-Dihydroxyvitamin D3 decreases DNA binding of nuclear factor-κB in human fibroblasts. FEBS Lett. 436, 329–334 10.1016/S0014-5793(98)01153-3 [DOI] [PubMed] [Google Scholar]
- Huang Y.-C., Chen J.-Y., Hung W.-C. (2004). Vitamin D3 receptor/Sp1 complex is required for the induction of p27KIP1 expression by vitamin D3. Oncogene 23, 4856–4861 10.1038/sj.onc.1207621 [DOI] [PubMed] [Google Scholar]
- Huang Y.-C., Hung W.-C. (2006). 1,25-Dihydroxyvitamin D3 transcriptionally represses p45Skp2 expression via the Sp1 sites in human prostate cancer cells. J. Cell. Physiol. 209, 363–369 10.1002/jcp.20741 [DOI] [PubMed] [Google Scholar]
- Inaba M., Koyama H., Hino M., Okuno S., Terada M., Nishizawa Y., et al. (1993). Regulation of release of hepatocyte growth factor from human promyelocytic leukemia cells, HL-60, by 1,25-dihydroxyvitamin D3, 12-O-tetradecanoylphorbol 13-acetate, and dibutyryl cyclic adenosine monophosphate. Blood 82, 53–59 [PubMed] [Google Scholar]
- Inoue T., Kamiyama J., Sakai T. (1999). Sp1 and NF-Y synergistically mediate the effect of vitamin D3 in the p27KIP1 gene promoter that lacks vitamin D response elements. J. Biol. Chem. 274, 32309–32317 10.1074/jbc.274.45.32309 [DOI] [PubMed] [Google Scholar]
- Jiang Y. J., Teichert A. E., Fong F., Oda Y., Bikle D. D. (2012). 1α,25(OH)2-Dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the β-catenin pathway. J. Steroid Biochem. Mol. Biol. 136, 229–232 10.1016/j.jsbmb.2012.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Lara A. M., Aranda A. (1999). Vitamin D represses retinoic acid-dependent transactivation of the retinoic acid receptor-β2 promoter: the AF-2 domain of the vitamin D receptor is required for transrepression. Endocrinology 140, 2898–2907 10.1210/endo.140.6.6770 [DOI] [PubMed] [Google Scholar]
- Kaeding J., Belanger J., Caron P., Verreault M., Belanger A., Barbier O. (2008). Calcitrol (1α,25-dihydroxyvitamin D3) inhibits androgen glucuronidation in prostate cancer cells. Mol. Cancer Ther. 7, 380–390 10.1158/1535-7163.MCT-07-0455 [DOI] [PubMed] [Google Scholar]
- Kaler P., Augenlicht L., Klampfer L. (2009). Macrophage-derived IL-1β stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene 28, 3892–3902 10.1038/onc.2009.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiappan R., Shen Z., Tse A. K., Jinwal U., Tang J., Lungchukiet P., et al. (2012). 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J. Biol. Chem. 287, 41297–41309 10.1074/jbc.M112.407189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostaras E., Sflomos G., Pedersen N. M., Stenmark H., Fotsis T., Murphy C. (2013). SARA and RNF11 interact with each other and ESCRT-0 core proteins and regulate degradative EGFR trafficking. Oncogene 32, 5220–5232 10.1038/onc.2012.554 [DOI] [PubMed] [Google Scholar]
- Kovalenko P. L., Zhang Z., Cui M., Clinton S. K., Fleet J. C. (2010). 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genomics 11:26 10.1186/1471-2164-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. V., Feldman D. (2010). Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr. Relat. Cancer 17, R19–R38 10.1677/ERC-09-0139 [DOI] [PubMed] [Google Scholar]
- Krishnan A. V., Swami S., Peng L., Wang J., Moreno J., Feldman D. (2010). Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology 151, 32–42 10.1210/en.2009-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriba M. J., González-Sancho J. M., Barbáchano A., Niell N., Ferrer-Mayorga G., Muñoz A. (2013). Vitamin D is a multilevel repressor of Wnt/β-catenin signaling in cancer cells. Cancers 5, 1242–1260 10.3390/cancers5041242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Jin D., Fu S., Mei G., Zhou J., Lei L., et al. (2013). Insulin-like growth factor binding protein-3 modulates osteoblast differentiation via interaction with vitamin D receptor. Biochem. Biophys. Res. Commun. 436, 632–637 10.1016/j.bbrc.2013.04.111 [DOI] [PubMed] [Google Scholar]
- Li P., Li C., Zhao X., Zhang X., Nicosia S. V., Bai W. (2004). p27Kip1 stabilization and G1 arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J. Biol. Chem. 279, 25260–25267 10.1074/jbc.M311052200 [DOI] [PubMed] [Google Scholar]
- Li Y., Spataro B. C., Yang J., Dai C., Liu Y. (2005). 1,25-dihydroxyvitamin D inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int. 68, 1500–1510 10.1111/j.1523-1755.2005.00562.x [DOI] [PubMed] [Google Scholar]
- Lin R., Wang T. T., Miller W. H., White J. H. (2003). Inhibition of F-Box protein p45SKP2 expression and stabilization of cyclin-dependent kinase inhibitor p27KIP1 in vitamin D analog-treated cancer cells. Endocrinology 144, 749–753 10.1210/en.2002-0026 [DOI] [PubMed] [Google Scholar]
- Liu P. T., Schenk M., Walker V. P., Dempsey P. W., Kanchanapoomi M., Wheelwright M., et al. (2009). Convergence of IL-1β and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS ONE 4:e5810 10.1371/journal.pone.0005810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. T., Stenger S., Li H., Wenzel L., Tan B. H., Krutzik S. R., et al. (2006). Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- Luderer H. F., Gori F., Demay M. B. (2011). Lymphoid enhancer-binding factor-1 (LEF1) interacts with the DNA-binding domain of the vitamin D receptor. J. Biol. Chem. 286, 18444–18451 10.1074/jbc.M110.188219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen M., Ryynänen J., Heinäniemi M., Väisänen S., Carlberg C. (2011). Cyclical regulation of the insulin-like growth factor binding protein 3 gene in response to 1α,25-dihydroxyvitamin D3. Nucleic Acids Res. 39, 502–512 10.1093/nar/gkq820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilainen J. M., Husso T., Toropainen S., Seuter S., Turunen M. P., Gynther P., et al. (2010a). Primary effect of 1α,25(OH)2D3 on IL-10 expression in monocytes is short-term down-regulation. Biochim. Biophys. Acta 1803, 1276–1286 10.1016/j.bbamcr.2010.07.009 [DOI] [PubMed] [Google Scholar]
- Matilainen J. M., Räsänen A., Gynther P., Väisänen S. (2010b). The genes encoding cytokines IL-2, IL-10 and IL-12B are primary 1α,25(OH)2D3 target genes. J. Steroid Biochem. Mol. Biol. 121, 142–145 10.1016/j.jsbmb.2010.03.020 [DOI] [PubMed] [Google Scholar]
- Matilainen M., Malinen M., Saavalainen K., Carlberg C. (2005). Regulation of multiple insulin-like growth factor binding protein genes by 1α,25-dihydroxyvitamin D3. Nucleic Acids Res. 33, 5521–5532 10.1093/nar/gki872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maund S. L., Barclay W. W., Hover L. D., Axanova L. S., Sui G., Hipp J. D., et al. (2011). Interleukin-1α mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer Res. 71, 5276–5286 10.1158/0008-5472.CAN-10-2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooso B., Madhav A., Johnson S., Roy M., Moore M. E., Moy C., et al. (2010). Androgen Receptor regulation of Vitamin D Receptor in response of castration-resistant prostate cancer cells to 1α-Hydroxyvitamin D5 - a calcitriol analog. Genes Cancer 1, 927–940 10.1177/1947601910385450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthian G., Raikwar H. P., Rajasingh J., Bright J. J. (2006). 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNγ axis leading to Th1 response in experimental allergic encephalomyelitis. J. Neurosci. Res. 83, 1299–1309 10.1002/jnr.20826 [DOI] [PubMed] [Google Scholar]
- Mutt S. J., Karhu T., Lehtonen S., Lehenkari P., Carlberg C., Saarnio J., et al. (2012). Inhibition of cytokine secretion from adipocytes by 1,25-dihydroxyvitamin D3 via the NF-κB pathway. FASEB J. 26, 4400–4407 10.1096/fj.12-210880 [DOI] [PubMed] [Google Scholar]
- Ng K. Y., Ma M. T., Leung K. K., Leung P. S. (2010). Vitamin D and vitamin A receptor expression and the proliferative effects of ligand activation of these receptors on the development of pancreatic progenitor cells derived from human fetal pancreas. Stem Cell Rev. 7, 53–63 10.1007/s12015-010-9146-1 [DOI] [PubMed] [Google Scholar]
- Oh Y. S., Kim E. J., Schaffer B. S., Kang Y. H., Binderup L., MacDonald R. G., et al. (2001). Synthetic low-calcaemic vitamin D3 analogues inhibit secretion of insulin-like growth factor II and stimulate production of insulin-like growth factor-binding protein-6 in conjunction with growth suppression of HT-29 colon cancer cells. Mol. Cell. Endocrinol. 183, 141–149 10.1016/S0303-7207(01)00598-6 [DOI] [PubMed] [Google Scholar]
- Okuda N., Takeda S., Shinomiya K., Muneta T., Itoh S., Noda M., et al. (2007). ED-71, a novel vitamin D analog, promotes bone formation and angiogenesis and inhibits bone resorption after bone marrow ablation. Bone 40, 281–292 10.1016/j.bone.2006.08.017 [DOI] [PubMed] [Google Scholar]
- Pálmer H. G., González-Sancho J. M., Espada J., Berciano M. T., Puig I., Baulida J., et al. (2001). Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J. Cell Biol. 154, 369–387 10.1083/jcb.200102028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pálmer H. G., Sánchez-Carbayo M., Ordóñez-Morán P., Larriba M. J., Cordón-Cardó C., Muñoz A. (2003). Genetic signatures of differentiation induced by 1α,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 63, 7799–7806 [PubMed] [Google Scholar]
- Pan Q., Simpson R. U. (1999). c-Myc intron element-binding proteins are required for 1,25-dihydroxyvitamin D3 regulation of c-myc during HL-60 cell differentiation and the involvement of HOXB4. J. Biol. Chem. 274, 8437–8444 10.1074/jbc.274.13.8437 [DOI] [PubMed] [Google Scholar]
- Parikh G., Varadinova M., Suwandhi P., Araki T., Rosenwaks Z., Poretsky L., et al. (2010). Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm. Metab. Res. 42, 754–757 10.1055/s-0030-1262837 [DOI] [PubMed] [Google Scholar]
- Pickup M., Novitskiy S., Moses H. L. (2013). The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer 13, 788–799 10.1038/nrc3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadan L. R., Perez-Stable C. M., Schwall R. H., Burnstein K. L., Ostenson R. C., Howard G. A., et al. (2000). Hepatocyte growth factor and vitamin D cooperatively inhibit androgen-unresponsive prostate cancer cell lines. Endocrinology 141, 2567–2573 10.1210/endo.141.7.7546 [DOI] [PubMed] [Google Scholar]
- Reichrath S., Reichrath J. (2012). No evidence for induction of key components of the Notch signaling pathway (Notch-1, Jagged-1) by treatment with UV-B, 1,25(OH)2D3, and/or epigenetic drugs (TSA, 5-Aza) in human keratinocytes in vitro. Dermatoendocrinology 4, 44–52 10.4161/derm.19027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Li W., Zhao Q., Ma L., Zhu J. (2012). The impact of 1,25-dihydroxyvitamin D3 on the expressions of vascular endothelial growth factor and transforming growth factor-β1 in the retinas of rats with diabetes. Diabetes Res. Clin. Pract. 98, 474–480 10.1016/j.diabres.2012.09.028 [DOI] [PubMed] [Google Scholar]
- Rodilla V., Villanueva A., Obrador-Hevia A., Robert-Moreno A., Fernandez-Majada V., Grilli A., et al. (2009). Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. U.S.A. 106, 6315–6320 10.1073/pnas.0813221106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryynänen J., Carlberg C. (2013). Primary 1,25-dihydroxyvitamin D3 response of the interleukin 8 gene cluster in human monocyte- and macrophage-like cells. PLoS ONE 8:e78170 10.1371/journal.pone.0078170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi-Tabar R., Nguyen-Yamamoto L., Tavera-Mendoza L. E., Quail T., Dimitrov V., An B. S., et al. (2012). Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc. Natl. Acad. Sci. U.S.A. 109, 18827–18832 10.1073/pnas.1210037109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J., Dorschner R. A., Coda A. B., Buchau A. S., Liu P. T., Kiken D., et al. (2007). Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 117, 803–811 10.1172/JCI30142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeppi J. M., Gutzwiller S., Finkenzeller G., Fournier B. (1997). 1,25-Dihydroxyvitamin D3 induces the expression of vascular endothelial growth factor in osteoblastic cells. Endocr. Res. 23, 213–229 [DOI] [PubMed] [Google Scholar]
- Schnabel M., Fichtel I., Gotzen L., Schlegel J. (2002). Differential expression of Notch genes in human osteoblastic cells. Int. J. Mol. Med. 9, 229–232 [PubMed] [Google Scholar]
- Seoane S., Pérez-Fernández R. (2006). The vitamin D receptor represses transcription of the pituitary transcription factor Pit-1 gene without involvement of the retinoid X receptor. Mol. Endocrinol. 20, 735–748 10.1210/me.2005-0253 [DOI] [PubMed] [Google Scholar]
- Sertznig P., Dunlop T., Seifert M., Tilgen W., Reichrath J. (2009a). Cross-talk between vitamin D receptor (VDR)- and peroxisome proliferator-activated receptor (PPAR)-signaling in melanoma cells. Anticancer Res. 29, 3647–3658 [PubMed] [Google Scholar]
- Sertznig P., Seifert M., Tilgen W., Reichrath J. (2009b). Activation of vitamin D receptor (VDR)- and peroxisome proliferator-activated receptor (PPAR)-signaling pathways through 1,25(OH)2D3 in melanoma cell lines and other skin-derived cell lines. Dermatoendocrinology 1, 232–238 10.4161/derm.1.4.9629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Islam M. N., Dakshanamurthy S., Rizvi I., Rao M., Herrell R., et al. (2006). The molecular basis of vitamin D receptor and β-catenin crossregulation. Mol. Cell 21, 799–809 10.1016/j.molcel.2006.01.037 [DOI] [PubMed] [Google Scholar]
- Shalhoub V., Shatzen E. M., Ward S. C., Young J. I., Boedigheimer M., Twehues L., et al. (2010). Chondro/osteoblastic and cardiovascular gene modulation in human artery smooth muscle cells that calcify in the presence of phosphate and calcitriol or paricalcitol. J. Cell. Biochem. 111, 911–921 10.1002/jcb.22779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q., Christakos S. (2005). The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J. Biol. Chem. 280, 40589–40598 10.1074/jbc.M504166200 [DOI] [PubMed] [Google Scholar]
- Shen X., Mula R. V. R., Li J., Weigel N. L., Falzon M. (2007). PTHrP contributes to the anti-proliferative and integrin α6β4-regulating effects of 1,25-dihydroxyvitamin D3. Steroids 72, 930–938 10.1016/j.steroids.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal A., Birkenhäger J. C., Pols H. A. P., Buurman C. J., Vink-van Wijngaarden T., Kleinekoort W. M., et al. (1994). Transforming growth factor β-induced dissociation between vitamin D receptor level and 1,25-dihydroxyvitamin D3 action in osteoblast-like cells. Bone Miner. 26, 27–42 10.1016/S0169-6009(08)80160-2 [DOI] [PubMed] [Google Scholar]
- Staal A., Geertsma-Kleinekoort W. M. C., Van Den Bemd G. J. C. M., Buurman C. J., Birkenhäger J. C., Pols H. A. P., et al. (1998). Regulation of osteocalcin production and bone resorption by 1,25-dihydroxyvitamin D3 in mouse long bones: interaction with the bone-derived growth factors TGF-β and IGF-I. J. Bone Miner. Res. 13, 36–43 10.1359/jbmr.1998.13.1.36 [DOI] [PubMed] [Google Scholar]
- Staal A., Van Wijnen A. J., Desai R. K., Pols H. A. P., Birkenhäger J. C., Deluca H. F., et al. (1996). Antagonistic effects of transforming growth factor-β on vitamin D3 enhancement of osteocalcin and osteopontin transcription: reduced interactions of vitamin D receptor/retinoid X receptor complexes with vitamin E response elements. Endocrinology 137, 2001–2011 10.1210/endo.137.5.8612541 [DOI] [PubMed] [Google Scholar]
- Stambolsky P., Tabach Y., Fontemaggi G., Weisz L., Maor-Aloni R., Siegfried Z., et al. (2010). Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell 17, 273–285 10.1016/j.ccr.2009.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Kong J., Duan Y., Szeto F. L., Liao A., Madara J. L., et al. (2006). Increased NF-κB activity in fibroblasts lacking the vitamin D receptor. Am. J. Physiol. Endocrinol. Metab. 291, E315–E322 10.1152/ajpendo.00590.2005 [DOI] [PubMed] [Google Scholar]
- Swami S., Krishnan A. V., Peng L., Lundqvist J., Feldman D. (2013). Transrepression of the estrogen receptor promoter by calcitriol in human breast cancer cells via two negative vitamin D response elements. Endocr. Relat. Cancer 20, 565–577 10.1530/ERC-12-0281 [DOI] [PubMed] [Google Scholar]
- Tang J. Y., Xiao T. Z., Oda Y., Chang K. S., Shpall E., Wu A., et al. (2011). Vitamin D3 inhibits hedgehog signaling and proliferation in murine basal cell carcinomas. Cancer Prev. Res. 4, 744–751 10.1158/1940-6207.CAPR-10-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. B., Ling G. H., Sun L., Liu F. Y. (2010). Smad anchor for receptor activation (SARA) in TGF-β signaling. Front. Biosci. 2, 857–860 10.2741/E147 [DOI] [PubMed] [Google Scholar]
- Tavera-Mendoza L., Wang T. T., Lallemant B., Zhang R., Nagai Y., Bourdeau V., et al. (2006). Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Rep. 7, 180–185 10.1038/sj.embor.7400594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. L., Maguire O., Doig C. L., Battaglia S., Fehr L., Sucheston L. E., et al. (2011). Epigenetic control of a VDR-governed feed-forward loop that regulates p21waf1/cip1 expression and function in non-malignant prostate cells. Nucleic Acids Res. 39, 2045–2056 10.1093/nar/gkq875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting H. J., Bao B. Y., Hsu C. L., Lee Y. F. (2005). Androgen-receptor coregulators mediate the suppressive effect of androgen signals on vitamin D receptor activity. Endocrine 26, 1–9 10.1385/ENDO:26:1:001 [DOI] [PubMed] [Google Scholar]
- Tong W.-M., Hofer H., Ellinger A., Peterlik M., Cross H. S. (1999). Mechanism of antimitogenic action of vitamin D in human colon carcinoma cells: relevance for suppression of epidermal growth factor-stimulated cell growth. Oncol. Res. 11, 77–84 [PubMed] [Google Scholar]
- Tong W.-M., Kállay E., Hofer H., Hulla W., Manhardt T., Peterlik M., et al. (1998). Growth regulation of human colon cancer cells by epidermal growth factor and 1,25-dihydroxyvitamin D3 is mediated by mutual modulation of receptor expression. Eur. J. Cancer 34, 2119–2125 10.1016/S0959-8049(98)00267-6 [DOI] [PubMed] [Google Scholar]
- Toropainen S., Väisänen S., Heikkinen S., Carlberg C. (2010). The down-regulation of the human MYC gene by the nuclear hormone 1α,25-dihydroxyvitamin D3 is associated with cycling of corepressors and histone deacetylases. J. Mol. Biol. 400, 284–294 10.1016/j.jmb.2010.05.031 [DOI] [PubMed] [Google Scholar]
- Towers T. L., Staeva T. P., Freedman L. P. (1999). A two-hit mechanism for vitamin D3-mediated transcriptional repression of the granulocyte-macrophage colony-stimulating factor gene: vitamin D receptor competes for DNA binding with NFAT1 and stabilizes c-Jun. Mol. Cell. Biol. 19, 4191–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhmann A., Niemann H., Lammering B., Henkel C., Hess I., Nitzki F., et al. (2011). Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol. Cancer Ther. 10, 2179–2188 10.1158/1535-7163.MCT-11-0422 [DOI] [PubMed] [Google Scholar]
- Uhmann A., Niemann H., Lammering B., Henkel C., Hess I., Rosenberger A., et al. (2012). Calcitriol inhibits Hedgehog signaling and induces vitamin D receptor signaling and differentiation in the patched mouse model of embryonal rhabdomyosarcoma. Sarcoma 2012, 357040 10.1155/2012/357040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink-van Wijngaarden T., Pols H. A. P., Buurman C. J., Birkenhäger J. C., van Leeuwen J. P. T. M. (1996). Inhibition of insulin- and insulin-like growth factor-I-stimulated growth of human breast cancer cells by 1,25-dihydroxyvitamin D3 and the vitamin D3 analogue EB1089. Eur. J. Cancer 32A, 842–848 10.1016/0959-8049(95)00647-8 [DOI] [PubMed] [Google Scholar]
- Wang W. L., Chatterjee N., Chittur S. V., Welsh J., Tenniswood M. P. (2011). Effects of 1α,25 dihydroxyvitamin D3 and testosterone on miRNA and mRNA expression in LNCaP cells. Mol. Cancer 10, 58 10.1186/1476-4598-10-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeckel V. J., Bruedigam C., Koedam M., Chiba H., van der Eerden B. C. J., van Leeuwen J. P. T. M. (2013a). 1α,25-dihydroxyvitamin D3 and rosiglitazone synergistically enhance osteoblast-mediated mineralization. Gene 512, 438–443 10.1016/j.gene.2012.07.051 [DOI] [PubMed] [Google Scholar]
- Woeckel V. J., Koedam M., van de Peppel J., Chiba H., van der Eerden B. C. J., van Leeuwen J. P. T. M. (2012). Evidence of vitamin D and interferon-β cross-talk in human osteoblasts with 1α,25-dihydroxyvitamin D3 being dominant over interferon-β in stimulating mineralization. J. Cell. Physiol. 227, 3258–3266 10.1002/jcp.24020 [DOI] [PubMed] [Google Scholar]
- Woeckel V. J., van der Eerden B. C. J., Schreuders-Koedam M., Eijken M., Van Leeuwen J. P. T. M. (2013b). 1α,25-dihydroxyvitamin D3 stimulates activin A production to fine-tune osteoblast-induced mineralization. J. Cell. Physiol. 228, 2167–2174 10.1002/jcp.24388 [DOI] [PubMed] [Google Scholar]
- Wu F. S., Zheng S. S., Wu L. J., Teng L. S., Ma Z. M., Zhao W. H., et al. (2007). Calcitriol inhibits the growth of MHCC97 heptocellular cell lines by down-modulating c-met and ERK expressions. Liver Int. 27, 700–707 10.1111/j.1478-3231.2007.01487.x [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Suzawa M., Kawabata M., Miyazono K., Yanagisawa J., Kato S. (1999). Positive and negative modulation of vitamin D receptor function by transforming growth factor-β signaling through Smad proteins. J. Biol. Chem. 274, 12971–12974 [DOI] [PubMed] [Google Scholar]
- Yanagisawa J., Yanagi Y., Masuhiro Y., Suzawa M., Watanabe M., Kashiwagi K., et al. (1999). Convergence of transforming growth factor-β and vitamin D signaling pathways on SMAD transcriptional coactivators. Science 283, 1317–1321 [DOI] [PubMed] [Google Scholar]
- Yu X. P., Bellido T., Manolagas S. C. (1995). Down-regulation of NF-κB protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U.S.A. 92, 10990–10994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Pan W., Kong J., Zheng W., Szeto F. L., Wong K. E., et al. (2007). 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J. Biol. Chem. 282, 29821–29830 10.1074/jbc.M705495200 [DOI] [PubMed] [Google Scholar]
- Zhang G. Y., Cheng T., Luan Q., Liao T., Nie C. L., Zheng X., et al. (2011). Vitamin D: a novel therapeutic approach for keloid, an in vitro analysis. Br. J. Dermatol. 164, 729–737 10.1111/j.1365-2133.2010.10130.x [DOI] [PubMed] [Google Scholar]
- Zhao X. Y., Ly L. H., Peehl D. M., Feldman D. (1999). Induction of androgen receptor by 1α,25-dihydroxyvitamin D3 and 9-cis retinoic acid in LNCaP human prostate cancer cells. Endocrinology 140, 1205–1212 10.1210/endo.140.3.6561 [DOI] [PubMed] [Google Scholar]