Abstract
Solid pseudopapillary neoplasm (SPN) of the pancreas is a rare tumor of uncertain malignant potential, predominantly affecting young adult females. We report a case of clear cell variant of SPN, which was diagnosed by fine needle aspiration biopsy. The aspirate was highly cellular and exhibited delicate branching papillary structures with central capillaries covered with several layers of plasmacytoid tumor cells. Acinar and rosette-like formations, as well as single neoplastic cells were also observed. An unusual cytologic feature was the presence of large, clear cytoplasmic vacuoles. The diagnosis of SPN was confirmed by characteristic immunocytochemical staining pattern including nuclear staining for β-catenin, cytoplasmic staining for vimentin and lack of reactivity for cytokeratin.
Keywords: Clear cell, fine needle aspiration, pancreas, solid pseudopapillary neoplasm
INTRODUCTION
Solid pseudopapillary neoplasm (SPN) is an indolent epithelial tumor with a low malignant potential that occurs predominantly in females with a mean age of 28 years.[1] It is mainly localized in the body and tail of the pancreas.[2]
The clear cell variant of SPN is even more unusual with only six papers reported in the English literature.[1,3,4,5,6,7] We present the cytologic features of clear cell variant of SPN with the broad differential diagnosis and a review of past studies.
CASE REPORT
A 22-year-old woman was admitted to the hospital for abdominal pain. Ultrasonogram showed a solid/cystic mass, 2.6 cm in diameter, in the tail of the pancreas. Percutaneous ultrasound-guided fine needle aspiration biopsy (FNAB) was performed using a 22-gauge needle. Rapid onsite evaluation was performed using Diff-Quik-stained smears. Additional smears were fixed in ethanol and stained by the Papanicolaou method. The excess material was fixed in an alcohol-formalin solution for cell block preparation and paraffin sections were stained by hematoxylin and eosin. Immunocytochemical stains were performed on cell block sections.
The material was highly cellular and consisted of a monotonous population of loosely cohesive small to medium-sized, round to cuboidal epithelioid cells exhibiting high nuclear/cytoplasmic ratio, finely granular “salt and pepper” chromatin and nuclear grooves. Pseudopapillary structures with arborizing capillaries and metachromic core on Diff-Quik-stained smears were lined by epithelioid cells. Acinar and rosette-like formations, as well as single neoplastic cells were observed [Figure 1]. The background was clean, but scattered foamy histiocytes were present. Many tumor cells exhibited single or multiple clear cytoplasmic vacuoles, displacing the nucleus [Figures 2 and 3]. The vacuoles were well-defined and variable in size, producing coin-shaped empty spaces in the cytoplasm [Figures 4 and 5]. Necrosis and mitosis were not seen. Coin-shaped vacuoles were mimicking signet-ring appearance on the cell-block [Figures 6 and 7]. Periodic acid-schiff (PAS) and mucicarmine stains were negative on the cell block sections.
Figure 1.
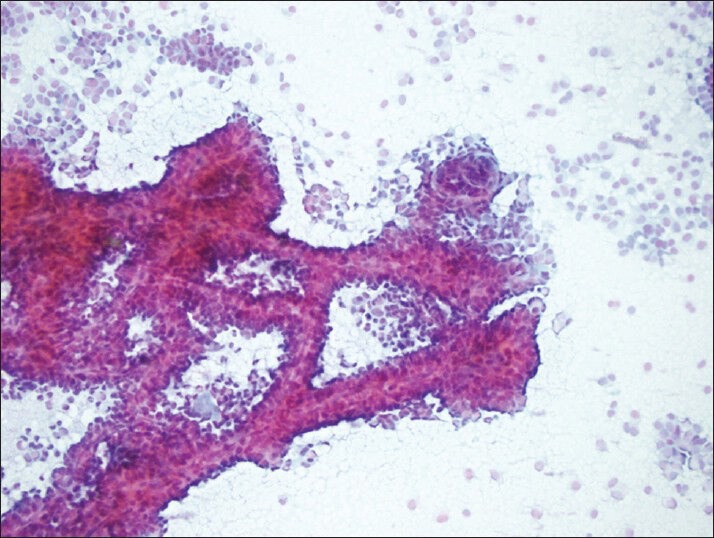
Typical complex-branching pseudopapillary structures surrounded with single and/or loosely cohesive neoplastic cells (PAP, smear)
Figure 2.
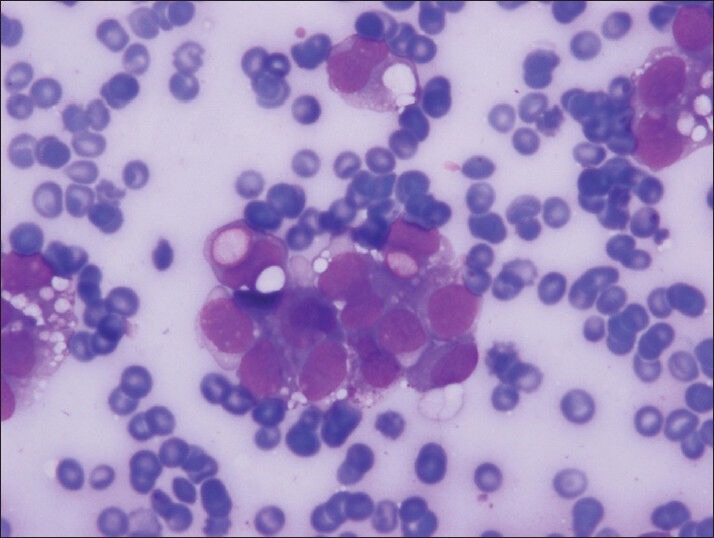
The clear vacuoles were often single but numerous clear vacuoles were also observed both in neoplastic groups and single, discohesive, plasmocytoid neoplastic cells (Diff-Quik, smears)
Figure 3.
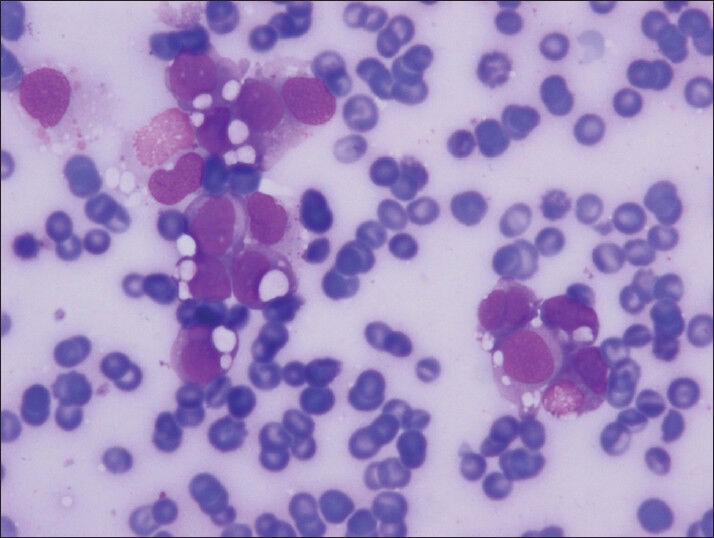
Small groups of cells with clear cell vacuoles in variable sized (Diff-Quik, smear)
Figure 4.
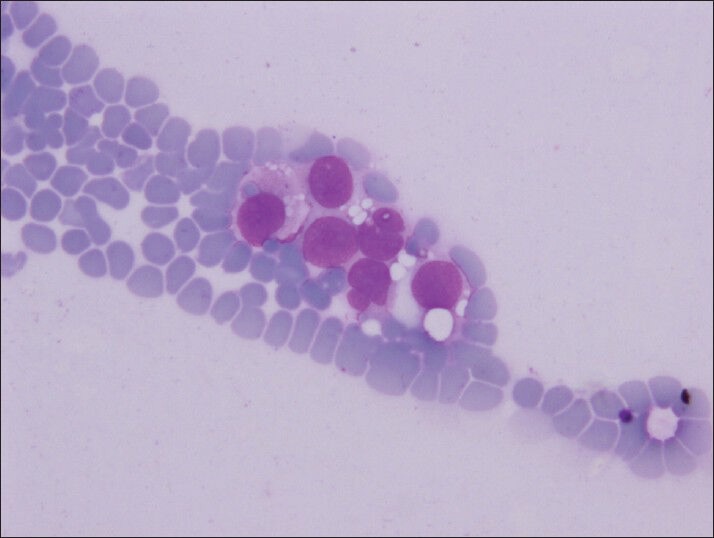
Intracytoplasmic vacuoles are well defined like coin–shaped (Diff-Quik, smears)
Figure 5.
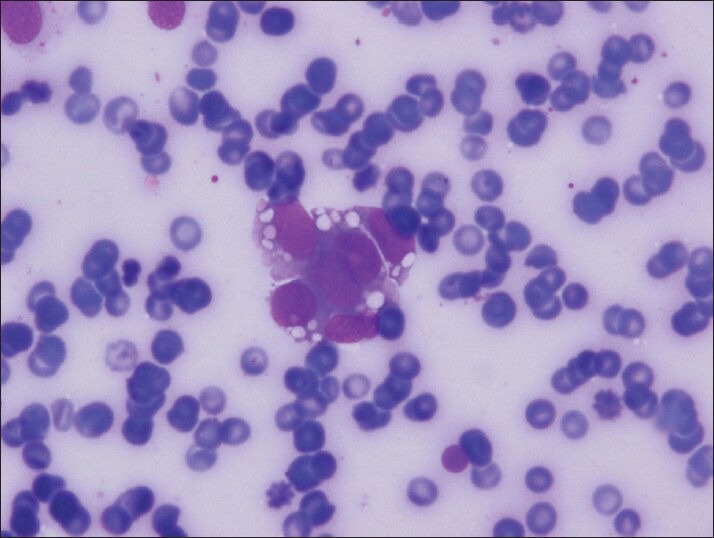
Vacuoles can be seen often and easily in Diff-Quik stained smears (Diff-Quik, smears)
Figure 6.
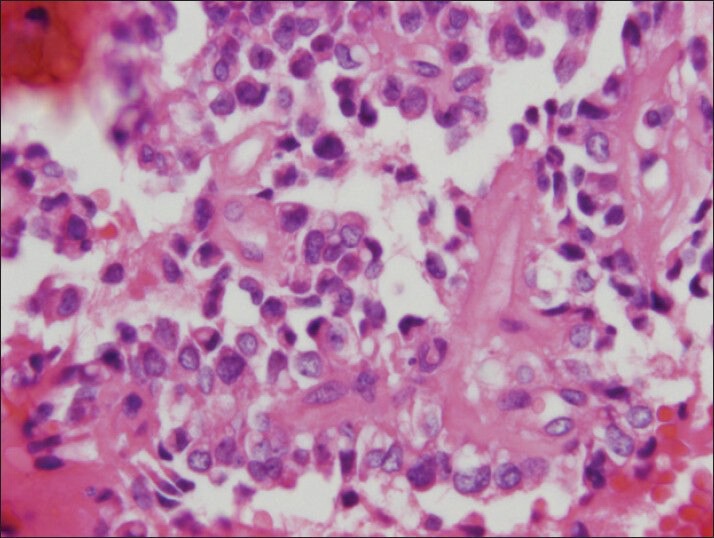
The vacuoles can be seen foamy to clear on cell block preparation (H and E, cell block)
Figure 7.
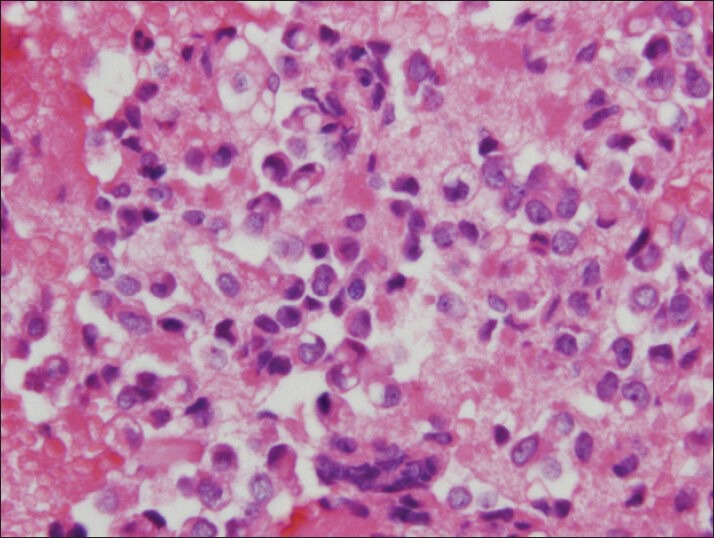
The vacuoles can mimick signet-ring appearance on cell block preparation (H and E, cell block)
Tumor cells showed strong nuclear staining for β-catenin and cytoplasmic staining for vimentin and CD10 [Figures 8–10]. The stains were negative for chromogranin A, synaptophysin and cytokeratin.
Figure 8.
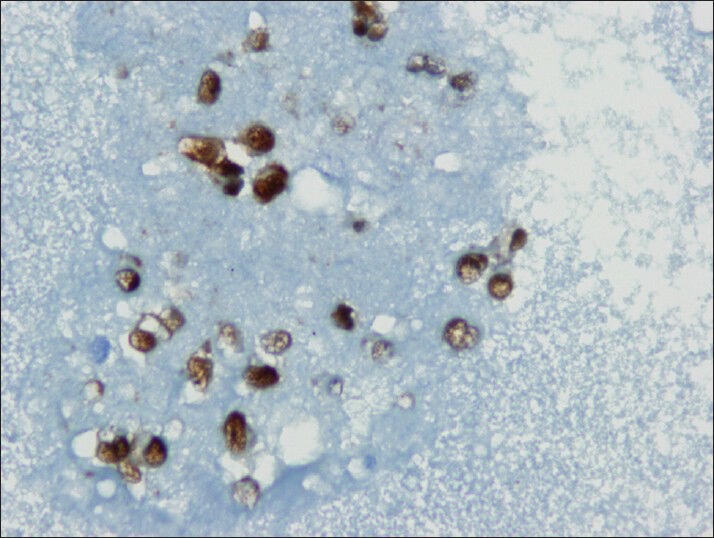
Neoplastic cells shows nuclear immuno reactivity with beta-catenin (cell block)
Figure 10.
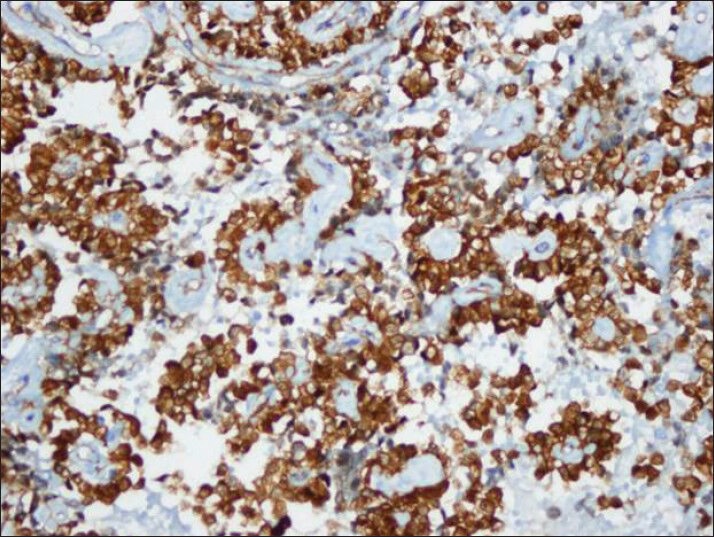
CD10 also showed strong cytoplasmic immuno reactivity similar to vimentin (cell block)
Figure 9.
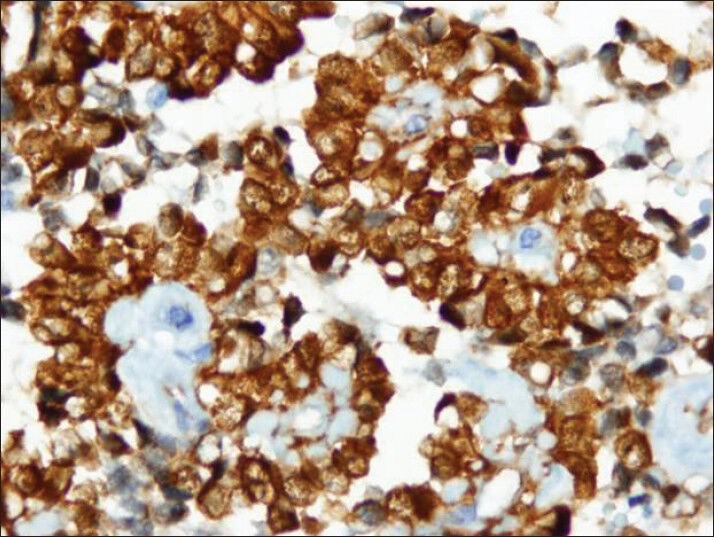
Strong cytoplasmic immuno reactivity with vimentin antibody (cell block)
DISCUSSION
SPN is a rare tumor, which accounts for 1-3% of all non-endocrine tumors of the pancreas. This tumor tends to occur in young adult females and is generally localized in the body and tail of the pancreas.[7,8] The pathogenesis of SPN is not well- understood. Many theories have been hypothesized for the origin of the tumor including small duct epithelium, acinar cells, endocrine cells, totipotential stem cells or primitive cells capable of both exocrine and endocrine differentiation along with genital ridge-related cells that being incorporated into the pancreas during early embryogenesis. Recent studies have suggested a neural crest origin for SPN.[9,10]
Massive necrosis results in cyst formation in these tumors, while viable tumor cells loosely adhere to the blood vessels, giving the appearance of pseudopapillary formation. SPN has diverse cytomorphological features. Differential diagnosis of SPN includes other solid, cystic and low grade malignant pancreatic neoplasms such as mucinous tumors, serous cystadenoma, neuroendocrine tumors, acinar cell carcinoma (ACC) and pancreatoblastoma (PB).[11,12,13] The combination of cytomorphologic features with pseudopapillary formation, myxohyaline change in vessels and cell degeneration produces various patterns such as acinar, trabecular, pseudorosette and single discohesive cells.[14] Accurate diagnosis can be achieved by careful attention to the cytomorphologic and architectural features. Immunocytochemistry is a highly useful tool in the differential diagnosis of this tumor.
In the present case, striking intracytoplasmic vacuoles were observed in addition to the characteristic cytomorphologic features of SPN. Clear cell dominated pattern SPN was first described in 2006 by Albores-Saavedra et al., as a distinct variant.[1] They demonstrated three cases of SPN with classic clinical features ranging in age from 26 to 32 years, in which the tumor cells had cytoplasmic vacuoles. Ultrastructurally, the cytoplasm contained a moderate amount of smooth and rough endoplasmic reticulum but no glycogen particles, lipid, mucin or neurosecretory granules. The authors stated that the vacuoles were formed by dilatation of the smooth endoplasmic reticulum and mitochondria. He acclaimed the need to be attentive particularly for endocrine tumors of the pancreas with clear cell morphology while diagnozing. The latter group of patients, which tend to be Von Hipple Lindau syndrome, also the clear cell cytoplasm in these tumors due to the presence of lipid droplets. Jhala et al., were first to report the cytologic features of the clear cell variant of SPN in 2007,[7] In a subsequent paper Jhala et al., compared the cytoplasmic vacuoles in pancreatic endocrine tumor (PEN) and SPN.[3] They concluded that the presence of large clear vacuoles was more in favor of SPN than PEN. Hav et al., and Tanino et al., reported one case each of this entity.[4,5] In a recent report, Zhao et al., emphasized the diagnostic challenges in the FNAB of this variant.[6]
Beside the classic mimics of SPN mentioned above, clear cell variant presents even broader differential diagnosis including primary and metastatic clear cell adenocarcinomas, metastatic renal cell carcinoma, oncocytoma, adrenal cortical tumors, ectopic adrenal tissue in the pancreas and metastatic balloon cell melanoma. Vacuoles can appear foamy in the Papanicolaou-stained smears and cell block sections, in contrast to the air-dried, Diff -Quik stained smears which show “coin-shaped” clear vacuoles. The foamy appearance can simulate oncocytoma or oncocytic tumors such as the conventional and chromophobe renal cell carcinoma, oncocytic adrenocortical neoplasms and extra adrenal oncocytic paragangliomas. Clear cell SPN can be distinguished from its mimics by careful attention to the clinical history, cytomorphologic and architectural features and the histological and immunocytochemical characteristics.
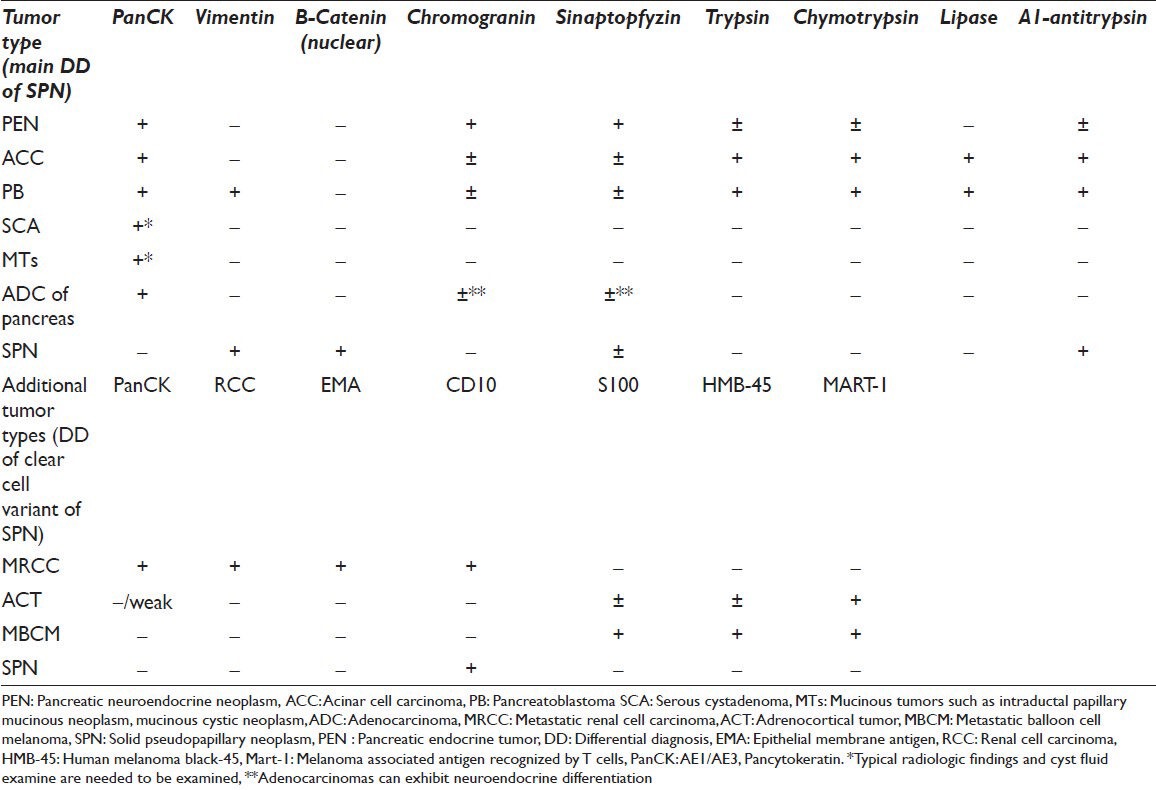
There may be still potential errors due to various presentations which result from the delphic origin of SPN. Immunocytochemistry can be the key in these scenarios, being cytokeratin negative and vimentin positive. Intense nuclear staining for β-catenin and focal positivity for alpha-1-antitripsin in PAS positive globules are highly useful. There are many additional stains in the literature such as CD10, progesterone receptors, cyclin D1, galectin3 etc., Neuroendocrine markers; particularly NSE, CD56 and synaptophysin can may be expressed by neoplastic cells of SPN, but Chromogranin A is uniformly negative, which along with the lack of epithelial markers help to differentiate this tumor from PEN. The combination of negative staining for cytokeratins and strong nuclear staining for β-catenin is also helpful in distinguishing this tumor from ACC and PB, which are well-known mimics of SPN.[2,3,7,12] The most helpful immunocytochemical stains in distinguishing clear cell SPN from other pancreatic neoplasms and extra-pancreatic clear cell tumors are demonstrated in Table 1.[2,6,12,13,15,16]
Table 1.
Immunophenotypic profile of classic type and clear cell variant of SPN including its common mimickers
To the best of our knowledge, only two previous papers have reported the FNAB features of clear cell SPN. We conclude that the clear cell variant of SPN can be diagnosed cytologically by careful attention to the cytomorphological criteria, combined with immunocytochemical stains.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All Authors attest to having no competing interests
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that they qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the study and takes public responsibility for appropriate portions of the content of this article. Each author acknowledges that this final version has been read and approved by them.
ETHICS STATEMENT BY ALL AUTHORS
As this is case report without identifiers, our institution does not require approval from Institutional Review Board (IRB) (or its equivalent). Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Footnotes
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/26/123785
Contributor Information
Sule Canberk, Email: sulecanberk@gmail.com.
Bilge Baskir Elcin, Email: baskirbilge@gmail.com.
Atay Uludokumaci, Email: atayuludokumaci@gmail.com.
Nesrin Uygun, Email: nesrinuygun@yahoo.com.
Fatih Gulsen, Email: fgulsen@yahoo.com.
REFERENCES
- 1.Albores-Saavedra J, Simpson KW, Bilello SJ. The clear cell variant of solid pseudopapillary tumor of the pancreas: A previously unrecognized pancreatic neoplasm. Am J Surg Pathol. 2006;30:1237–42. doi: 10.1097/01.pas.0000209849.97787.e0. [DOI] [PubMed] [Google Scholar]
- 2.Burford H, Baloch Z, Liu X, Jhala D, Siegal GP, Jhala N. E-cadherin/beta-catenin and CD10: A limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am J Clin Pathol. 2009;132:831–9. doi: 10.1309/AJCPVT8FCLFDTZWI. [DOI] [PubMed] [Google Scholar]
- 3.Jhala N, Siegal GP, Jhala D. Large, clear cytoplasmic vacuolation: An under-recognized cytologic clue to distinguish solid pseudopapillary neoplasms of the pancreas from pancreatic endocrine neoplasms on fine-needle aspiration. Cancer. 2008;114:249–54. doi: 10.1002/cncr.23595. [DOI] [PubMed] [Google Scholar]
- 4.Hav M, Lem D, Chhut SV, Kong R, Pauwels P, Cuvelier C, et al. Clear-cell variant of solid-pseudopapillary neoplasm of the pancreas: A case report and review of the literature. Malays J Pathol. 2009;31:137–41. [PubMed] [Google Scholar]
- 5.Tanino M, Kohsaka S, Kimura T, Tabu K, Nishihara H, Sawa H, et al. A case of clear cell variant of solid-pseudopapillary tumor of the pancreas in an adult male patient. Ann Diagn Pathol. 2012;16:134–40. doi: 10.1016/j.anndiagpath.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhao P, Debrito P, Ozdemirli M, Sidawy MK. Solid-pseudopapillary neoplasm of the pancreas: Awareness of unusual clinical presentations and morphology of the clear cell variant can prevent diagnostic errors. Diagn Cytopathol. 2013 doi: 10.1002/dc.22989. 00:00. [DOI] [PubMed] [Google Scholar]
- 7.Jhala N, Siegal GP, Jhala D. Fine-needle aspiration a powerful modality in the preoperative diagnosis of solid pseudopapillary neoplasm of the pancreas. Pathol Case Rev. 2007;12:170–6. [Google Scholar]
- 8.Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: A report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512–9. doi: 10.1097/01.pas.0000155159.28530.88. [DOI] [PubMed] [Google Scholar]
- 9.Kallichanda N, Tsai S, Stabile BE, Buslon V, Delgado DL, French SW. Histogenesis of solid pseudopapillary tumor of the pancreas: The case for the centroacinar cell of origin. Exp Mol Pathol. 2006;81:101–7. doi: 10.1016/j.yexmp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Jing W, Gulati P, Vargas H, French SW. Melanocytic differentiation in a solid pseudopapillary tumor of the pancreas. J Gastroenterol. 2004;39:579–83. doi: 10.1007/s00535-004-1346-5. [DOI] [PubMed] [Google Scholar]
- 11.Pailoor K, Kini H, Rau AR, Kumar Y. Cytological diagnosis of a rare case of solid pseudopapillary neoplasm of the pancreas. J Cytol. 2010;27:32–4. doi: 10.4103/0970-9371.66693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams AL, Siegal GP, Jhala NC. Solid pseudopapillary tumor of the pancreas: A review of salient clinical and pathologic features. Adv Anat Pathol. 2008;15:39–45. doi: 10.1097/PAP.0b013e31815e5237. [DOI] [PubMed] [Google Scholar]
- 13.Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: A surgical enigma? Ann Surg Oncol. 2002;9:35–40. doi: 10.1245/aso.2002.9.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Pettinato G, Di Vizio D, Manivel JC, Pambuccian SE, Somma P, Insabato L. Solid-pseudopapillary tumor of the pancreas: A neoplasm with distinct and highly characteristic cytological features. Diagn Cytopathol. 2002;27:325–34. doi: 10.1002/dc.10189. [DOI] [PubMed] [Google Scholar]
- 15.Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20(Suppl 1):S94–112. doi: 10.1038/modpathol.3800686. [DOI] [PubMed] [Google Scholar]
- 16.Stelow EB, Bardales RH, Stanley MW. Pitfalls in endoscopic ultrasound-guided fine-needle aspiration and how to avoid them. Adv Anat Pathol. 2005;12:62–73. doi: 10.1097/01.pap.0000155053.68496.ad. [DOI] [PubMed] [Google Scholar]


