Abstract
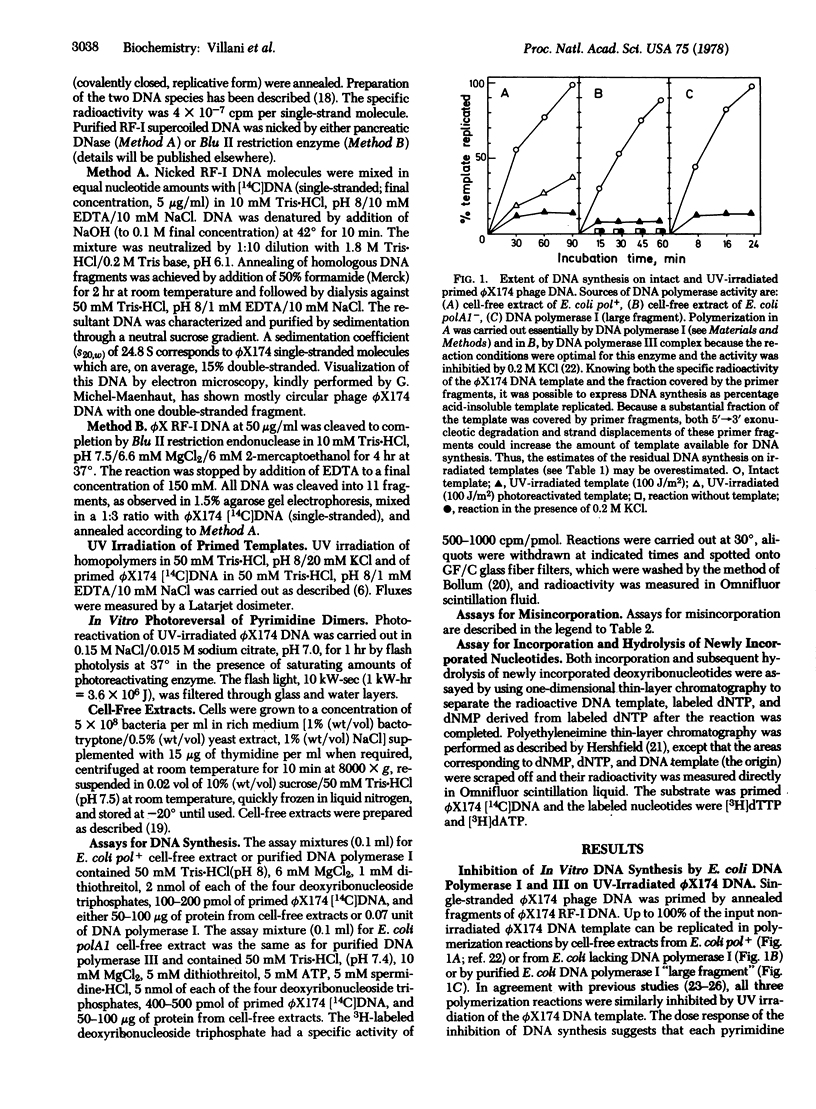
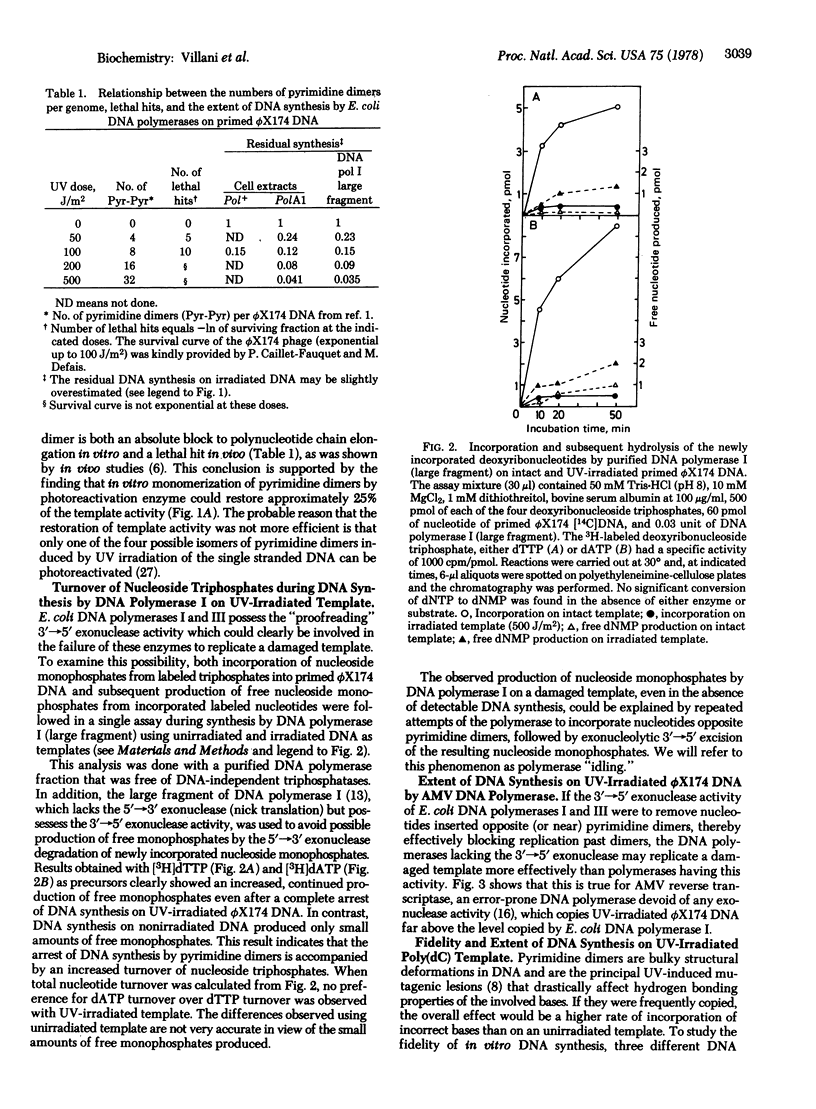
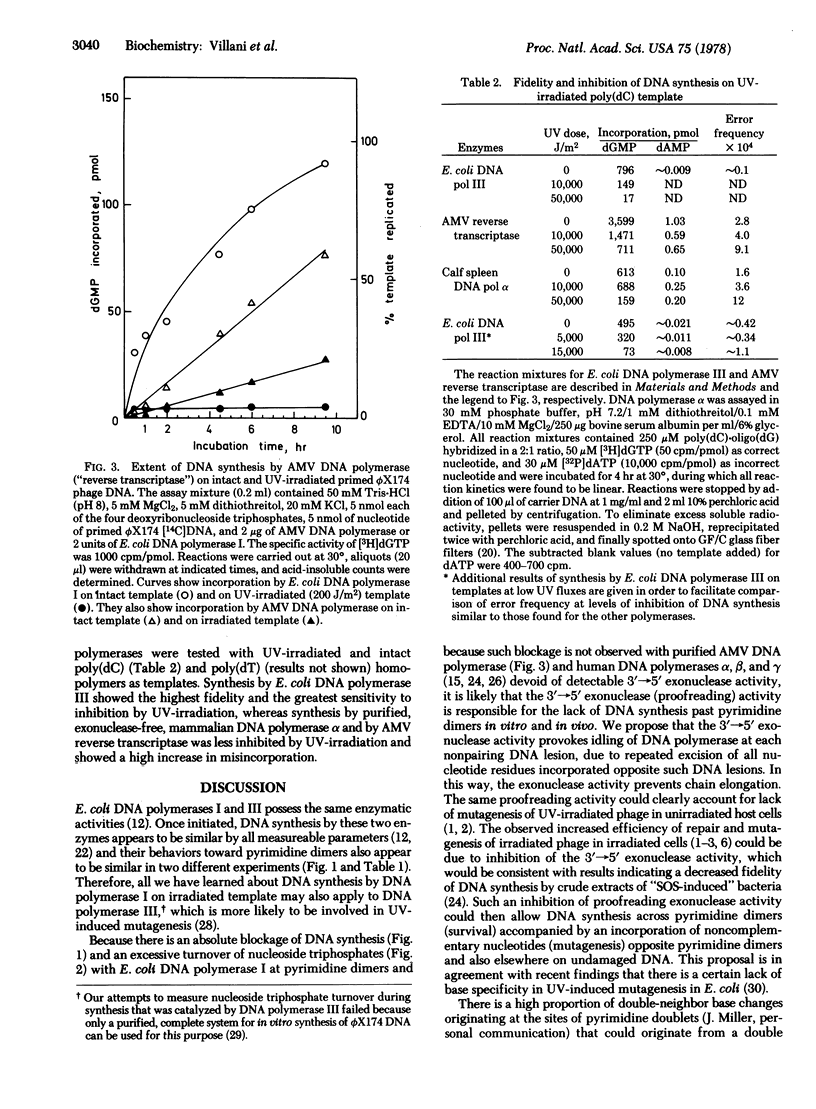
The effect of UV irradiation on the extent and fidelity of DNA synthesis in vitro was studied by using homopolymers and primed single-stranded ϕX174 phage DNA as substrates. Unfractionated and fractionated cell-free extracts from Escherichia coli pol+ and polA1 mutants as well as purified DNA polymerase I were used as sources of enzymatic activity. (DNA polymerases, as used here, refer to deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase, EC 2.7.7.7.) The extent of inhibition of DNA synthesis on UV-irradiated ϕX174 DNA suggested that pyrimidine dimers act as an absolute block for chain elongation by DNA polymerases I and III. Experiments with an irradiated poly(dC) template failed to detect incorporation of noncomplementary bases due to pyrimidine dimers. A large increase in the turnover of nucleoside triphosphates to free monophosphates during synthesis by DNA polymerase I on irradiated ϕX174 DNA has been observed. We propose that this nucleotide turnover is due to idling by DNA polymerase (i.e., incorporation and subsequent excision of nucleotides opposite UV photolesions, by the 3′→5′ “proofreading” exonuclease) thus preventing replication past pyrimidine dimers and the potentially mutagenic event that should result. In support of this hypothesis, DNA synthesis by DNA polymerase from avian myeloblastosis virus and by mammalian DNA polymerase α, both of which are devoid of any exonuclease activity, was found to be only partially inhibited, but not blocked, by UV irradiation of the template and accompanied by an increased incorporation of noncomplementary nucleotides. It is suggested that UV mutagenesis in bacteria requires an induced modification of the cellular DNA replication machinery, possibly an inhibition of the 3′→5′ exonuclease activity associated with DNA polymerases.
Keywords: pyrimidine dimers, DNA polymerases, proofreading exonuclease, misincorporation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battula N., Loeb L. A. On the fidelity of DNA replication. Lack of exodeoxyribonuclease activity and error-correcting function in avian myeloblastosis virus DNA polymerase. J Biol Chem. 1976 Feb 25;251(4):982–986. [PubMed] [Google Scholar]
- Ben-Hur E., Ben-Ishai R. Trans-syn thymine dimers in ultraviolet-irradiated denatured DNA: identification and photoreactivability. Biochim Biophys Acta. 1968 Aug 23;166(1):9–15. doi: 10.1016/0005-2787(68)90485-1. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. A role for single-strand breaks in bacteriophage phi-X174 genetic recombination. J Mol Biol. 1974 Sep 25;88(3):629–651. doi: 10.1016/0022-2836(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Bleichrodt J. F., Verheij W. S. Mutagenesis by ultraviolet radiation in bacteriophage phiX174: on the mutation stimulating processes induced by ultraviolet radiation in the host bacterium. Mol Gen Genet. 1974;135(1):19–27. doi: 10.1007/BF00433897. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. III. Requirement for a function of DNA polymerase III in ultraviolet-light mutagenesis. Mol Gen Genet. 1976 Feb 27;144(1):53–58. doi: 10.1007/BF00277304. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Defais M., Caillet-Fauquet P., Fox M. S., Radman M. Induction kinetics of mutagenic DNA repair activity in E. coli following ultraviolet irradiation. Mol Gen Genet. 1976 Oct 18;148(2):125–130. doi: 10.1007/BF00268375. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Nossal N. G. Control of mutation frequency by bacteriophage T4 DNA polymerase. II. Accuracy of nucleotide selection by the L88 mutator, CB120 antimutator, and wild type phage T4 DNA polymerases. J Biol Chem. 1976 Sep 10;251(17):5225–5232. [PubMed] [Google Scholar]
- Hershfield M. S. On the role of deoxyribonucleic acid polymerase in determining mutation rates. Characterization of the defect in the T4 deoxyribonucleic acid polymerase caused by the ts L88 mutation. J Biol Chem. 1973 Feb 25;248(4):1417–1423. [PubMed] [Google Scholar]
- Ichikawa-Ryo H., Kondo S. Indirect mutagenesis in phage lambda by ultraviolet preirradiation of host bacteria. J Mol Biol. 1975 Sep 5;97(1):77–92. doi: 10.1016/s0022-2836(75)80023-4. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Hinkle D. C., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Purification and properties. J Biol Chem. 1975 Jan 25;250(2):461–469. [PubMed] [Google Scholar]
- Livingston D. M., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Characterization of associated exonuclease activities. J Biol Chem. 1975 Jan 25;250(2):470–478. [PubMed] [Google Scholar]
- Lo K. Y., Bessman M. J. An antimutator deoxyribonucleic acid polymerase. I. Purification and properties of the enzyme. J Biol Chem. 1976 Apr 25;251(8):2475–2479. [PubMed] [Google Scholar]
- Masamune Y. Effect of ultraviolet irradiation of bacteriophage f1 DNA on its conversion to replicative form by extracts of Escherichia coli. Mol Gen Genet. 1976 Dec 22;149(3):335–345. doi: 10.1007/BF00268536. [DOI] [PubMed] [Google Scholar]
- Meyn M. S., Rossman T., Troll W. A protease inhibitor blocks SOS functions in Escherichia coli: antipain prevents lambda repressor inactivation, ultraviolet mutagenesis, and filamentous growth. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1152–1156. doi: 10.1073/pnas.74.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar R. K., Sinsheimer R. L. Nature of the complementary strands synthesized in vitro upon the single-stranded circular DNA of bacteriophage phiX174 after ultraviolet irradiation. Biophys J. 1971 Apr;11(4):355–369. doi: 10.1016/s0006-3495(71)86220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Radman M. An endonuclease from Escherichia coli that introduces single polynucleotide chain scissions in ultraviolet-irradiated DNA. J Biol Chem. 1976 Mar 10;251(5):1438–1445. [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Setlow P., Brutlag D., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. I. A proteolytic fragment containing the polymerase and 3' leads to 5' exonuclease functions. J Biol Chem. 1972 Jan 10;247(1):224–231. [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Wright M., Wickner S., Hurwitz J. Conversion of phiX174 and fd single-stranded DNA to replicative forms in extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3233–3237. doi: 10.1073/pnas.69.11.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


