Abstract
Background:
Spontaneous intracranial hypotension is an uncommon clinical entity. Heritable connective tissue disorders (HCTD), such as Marfan syndrome, are frequently implicated as an underlying cause, due to dural structural weaknesses that predispose patients to spontaneous cerebrospinal fluid (CSF) leak. Due to the high prevalence of multi-system disease in HCTD, diagnosis and treatment are often complicated.
Case Description:
We present a 58-year-old female with Marfan syndrome on anticoagulation for a mechanical aortic valve replacement who came to medical attention with severe, acute-onset headache following a straining episode. Noninvasive magnetic resonance (MR) myelography confirmed thoracic CSF extravasations and multiple lumbar diverticula. The patient was treated conservatively and her symptoms resolved.
Conclusion:
We discuss the common presentation, diagnostic tools, and treatment options for spontaneous CSF leaks in patients with Marfan syndrome or related HCTD with an emphasis on noninvasive modalities and a review of the major radiographic criteria used to diagnose dural abnormalities, such as dural ectasia.
Keywords: Magnetic resonance myelography, Marfan syndrome, spontaneous intracranial hypotension
INTRODUCTION
Spontaneous intracranial hypotension (SIH) is caused by cerebrospinal fluid (CSF) leak, which leads to significant volume loss from the subarachnoid space and intracranial pressure changes. The characteristic clinical presentation is orthostatic headache, frequently accompanied by nausea, dizziness, neck stiffness, upper extremity radicular pain, cranial nerve palsies, tinnitus or decreased hearing, blurry vision, or chronic headaches.[24,26] In patients who can tolerate lumbar puncture (LP), a CSF opening pressure of less than 60 mm H2O is a common finding.[26]
Although a precipitating etiology is often difficult to identify, retrospective studies have estimated that between 16% and 38% of SIH patients have an underlying heritable connective tissue disorder (HCTD), such as Marfan syndrome, Ehlers–Danlos syndrome, Loeys–Dietz syndrome, or benign joint hypermobility syndrome.[3,10,15,18,33] An association between SIH and HCTD was also identified by two prospective analyses, which observed evidence of HCTD in 18% and 66% of 50- and 18-patient cohorts with SIH, respectively.[21,25]
The underlying pathophysiology predisposing HCTD patients toward CSF leak remains incompletely understood, although recent pathologic and genetic analyses have furthered key theories regarding the primary mechanism. Histologic analysis of Marfan syndrome models in mice have shown significant attenuation of dural tissue and pronounced fibro-connective disorganization, suggesting that inherent structural weakness in the dura itself is responsible for the high rate of tearing.[9]
Marfan syndrome is an autosomal dominant disease affecting the connective tissue protein fibrillin-1, with an estimated prevalence of 1 in 5000-10,000.[22] Disease expressivity is highly variable, yielding a broad range of clinical findings.[4] This is due, in part, to the widespread expression of fibrillin-1 (FBN1) throughout the extracellular matrix in ophthalmologic, pulmonary, integumentary, and dural systems, where the mutant form disrupts normal tissue architecture.[35] Correspondingly, Marfan syndrome is diagnosed using a complex clinical schema called the Ghent Nosology, whose major criteria include skeletal, cardiovascular, ocular, and dural abnormalities, as well as family history.[11] Components of the characteristic habitus observed in Marfan syndrome and related HCTD include dolichostenomelia, which describes disproportionately elongated limbs, and arachnodactyly, which describes long, slender fingers, relative to the palm.
On a molecular level, abnormalities in transforming growth factor beta (TGFβ) are implicated in the relationship between HCTD and weak connective tissue. Loeys–Dietz syndromes types 2A and 2B arise from mutations in the TGFβ receptor gene TGFBR1 and TGFBR2, leading to fibroblast signaling abnormalities, while TGFβ dysregulation and overexpression have been observed in Marfan syndrome pathogenesis.[1,12] Marfan and Loeys–Dietz syndromes are associated with high rates of dural ectasia (DE) and SIH.[33] However, the specificity of abnormalities in TGFβ signaling to DE and SIH is unclear, as other studies have demonstrated comparable rates of DE in HCTD patients with and without mutations in FBN1, TGFBR1, and TGFBR2.[31]
DE is qualitatively defined as widening of the spinal canal, posterior scalloping of the vertebral body, thinning of the cortex of the pedicles and laminae, widening of the neural foramina, or the presence of a meningocele.[2,4,17,20,32] The finding is relatively specific for HCTD, and demonstrable in 60-92% of patients with a diagnosed HCTD.[2,6,7,11] As DEs are a major criteria in the Ghent Nosology, at least five sets of guidelines have been proposed for defining DE by computed tomography (CT) or magnetic resonance (MR) imaging.[8,13,33,37]
Oosterhof's method, which found DE in 88-94% of Marfan patients and 44-47% of controls, measures the ratio of the anteroposterior dural sac diameter to the vertebral body diameter against specified cut-offs.[17] Ahn's method, which found DE in 72-76% or Marfan patients and 29-44% of controls, defines DE when the midsaggital diameter of the spinal canal is greater at S1 than L4.[2] Söylen's method multiplies the transverse and sagittal width of the dural sac at three levels per vertebral body (superior endplate, midcorpus, inferior endplate) and compares the average of the three measurements against another set of cut-offs.[32] Several analyses have compared these criteria and found significant discrepencies between them, with Ahn's and Söylen's methods showing significantly more specificity than Oosterhof's.[32,37] In this report, we omit commentary on the systems outlined by Fattori and Villeirs, as they depend on qualitative and CT criteria, respectively.[5,34]
As these radiographic metrics provide an important tool for evaluating the possibility of underlying HCTD and, therefore, susceptibility to SIH, they provide important diagnostic information that may shape management decisions in patients with postural headaches. Correspondingly, this report incorporates a validation of the three methodologies described earlier in a patient with known HCTD.
Other studies have approached the relationship between HCTD and DE from a clinical perspective, evaluating connections between characteristic disease features and incidence of DE. Although many HCTD features were tested, the only significant association found was between the skeletal manifestations of HCTD and DE, and the authors correspondingly concluded that the SIH mechanism is likely driven by reduced resistance in the osseous structures and structural disturbances at the bone–dura interface.[31] This theory is supported by another study that observed Marfanoid skeletal features in 21% of all patients with SIH.[29] Finally, a case-control analysis showed that the only HCTD feature significantly elevated among patients with diagnosed SIH was dolichostenomelia (Marfanoid habitus).[10]
In spite of the different mechanisms each supports, all of these studies link structurally weak connective tissue to DE and dural structural abnormalities in general and, consequently, elevated risk of SIH. Although a definitive relationship between FBN1 or TGFβ genes and DE has not been proven, these mutations remain areas of active research. Further, the emerging understanding of pathology-driven skeletal and dural abnormalities occuring independently of syndrome-defining mutations suggests a higher-than-estimated prevalence of dural abnormalities in the population at large. Finally, in this report we not only revisit the significant relationship between HCTD and SIH, but also emphasize the central role for noninvasive diagnostic and therapeutic modalities - in particular, MR myelography and conservative mangement. Awareness of these techniques and the powerful clinical insight they offer in SIH is essential, as many patients with Marfan syndrome and other HCTD are anticoagulated for co-morbid valvular disease and are not candidates for more common and invasive interventions, including LP and conventional CT myelography with intrathecal contrast agents.
CASE REPORT
A 58-year-old, right-handed female with a history of Marfan syndrome, mechanical aortic valve replacement on warfarin anticoagulation, and hypertension presented to NYU Medical Center complaining of a severe, sudden-onset headache after straining at stool. She described her headache as diffuse, with radiation to the neck, dizziness, and light-headedness. She had experienced a single episode of severe nausea with vomiting. The headache worsened in the upright position.
On neurologic examination, the patient was awake, alert, and fully oriented. Her cranial nerves were grossly intact. She had no focal motor, sensory, or cerebellar deficits. Fundoscopic examination revealed no papilledema. Physical exam findings consistent with Marfan syndrome included dolichostenomelia and arachnodactyly, and her cardiovascular examination confirmed a mechanical aortic valve. The remainder of her exam was noncontributory. The patient's INR was 4.2, consistent with warfarin anticoagulation. All other laboratory tests were within normal limits.
Imaging of the brain demonstrated mild dilatation of the lateral ventricles with crowding of the gyri, and inferior displacement of the posterior fossa structures, with the cerebellar tonsils extended below the level of the foramen magnum [Figure 1a]. An atrial diverticulum was noted as a medial out-pouching of the trigone of the left lateral ventricle [Figure 1a].
Figure 1.
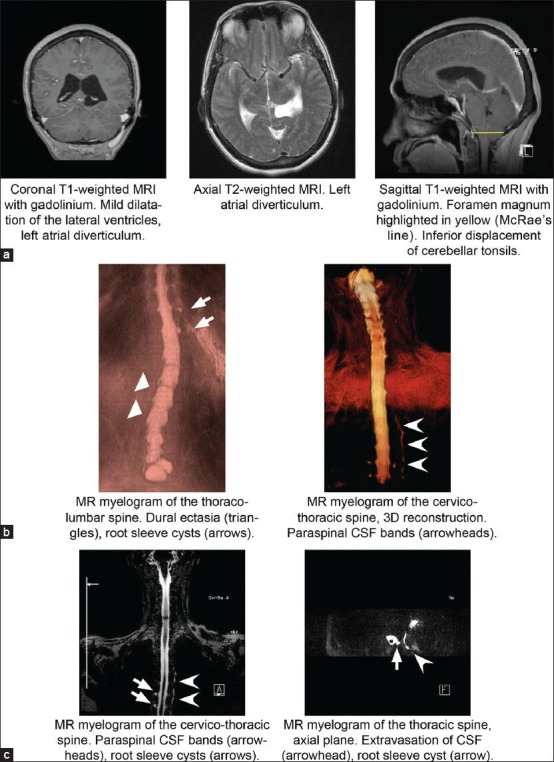
Diagnostic imaging. (a) Coronal and sagittal T1 with gadolinium and axial T2 MRI demonstrating mild dilatation of the lateral ventricles, left atrial diverticulum, and inferior displacement of the cerebellar tonsils (McRae's line in yellow). (b) MR myelography of the thoraco-lumbar and cervico-thoracic spine with 3D reconstruction demonstrating lumbar DE (triangles), root sleeve cysts (arrows), and paraspinal CSF bands (arrowheads). (c) MR myelography of the cervico-thoracic spine demonstrating root sleeve cysts (arrows) and extravasation of CSF with paraspinal CSF band (arrowheads)
Neurosurgery was consulted to evaluate the patient. She was admitted to the neurosurgical intensive care unit for monitoring and repeat imaging to rule out worsening ventriculomegaly. The suspicion of SIH due to tonsillar herniation in the setting of diagnosed Marfan syndrome prompted empiric treatment with intravenous hydration and keeping the patient flat for 48 hours. Warfarin anticoagulation was stopped to prepare for the possibility of an urgent intervention and an intravenous heparin drip was started the following day. Repeat CT was stable, the patient's symptoms declined steadily, and gradual elevation of the head of the bed was tolerated.
As the patient was anticoagulated for a mechanical aortic valve, she was considered ineligible for LP to evaluate opening pressure or administer contrast for conventional myelography. Correspondingly, definitive evaluation was completed via noninvasive MR myelography and spinal MRI [Figure 1b and c]. Multiple sacral and thoracic root sleeve cysts and paraspinal thin bands of CSF were observed in the left thoracic region, consistent with CSF extravasation [Figure 1b and c]. DE was noted at multiple levels from L3-S2, with positive findings by Oosterhof's, Ahn's, and Söylen's criteria [Figure 2]. Of note, the patient also had a significant scoliotic deformity, which may have introduced a degree of error into applying these metrics.
Figure 2.
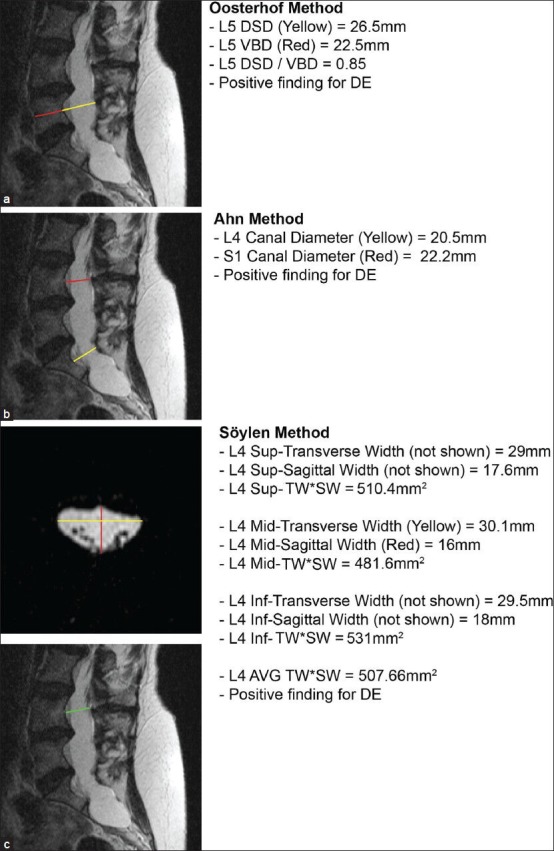
Validation of radiographic metrics for DE. (a) Validation of Oosterho's method, with positive findings for DE. (b) Validation of Ahn's method, with positive findings for DE. (c) Validation of Söylen's method, with positive findings for DE. DSD: Disk space diameter; VBD: Vertebral body diameter; AVG: Average; TW*SW: Transverse width *sagittal width
Given the resolution of the patient's symptoms under conservative management, no further interventions were required. During her hospital course the patient suffered a silent myocardial infarction, but she was ultimately discharged home without additional complications. She has remained free of orthostatic headaches for 15 months since this event.
DISCUSSION
Management of suspected SIH begins with conservative treatment before proceeding to more invasive modalities. Initially, patients are hydrated and maintained in a flat position to reduce hydrostatic pressure and promote dural scaring. Patients who do not improve or who deteriorate during observation may be considered for an epidural blood patch (EBP), a minimally invasive therapy in which a small volume of autologous blood is injected into the epidural space to seal the underlying CSF leak. Although its use as first-line therapy is controversial, EBP has been shown to have 56-77% success rates in prospective trials.[14,16,25,30] If patients continue to deteriorate or fail to improve following EBP, surgical repair of the dura—which has been demonstrated to be a safe and effective treatment for SIH—is a final option for definitive therapy.[27,28]
Definitively diagnosing SIH is a clinical priority, and awareness of a co-morbid HCTD and the limitations it may place on available measures is essential. Conventional radiographic myelography often plays a central role in diagnosis—in terms of measuring CSF opening pressure, assessing DE and evaluating for a site of leak. Sometimes, as our case illustrates, patients are not good candidates for an invasive procedure. In such instances, alternative modalities must be chosen. One such technique is the noninvasive MR myelogram, which utilizes a single shot fast spin echo T2-weighted sequence with long echo time. This results in a heavily T2-weighted sequence allowing for exquisite information of the thecal sac and CSF-intensity-based structures.[23] Advantages of MR myelography over conventional radiographic/CT myelogram include its noninvasive nature, lack of the need to administer intrathecal contrast, and lack of ionizing radiation. A relative limitation of MR myelogram is its inability to obtain a CSF opening pressure. In fact, MR myelogram has been demonstrated to be just as accurate as a CT myelogram in localizing CSF leak in patients with SIH.[19,36] This is well illustrated by the present case, in which DEs were identified in the lumbar spine by MRI, while MR myelography ultimately demonstrated CSF extravasation in the thoracic spine.
The major issue complicating use of MRI and MR myelography in HCTD patients is the lack of standardized radiographic criteria for DE. As described earlier, among the current methodologies for defining DE, several are nonquantitative, while others vary widely in their findings: A head-to-head comparison of the techniques descibed by Villiers, Oosterhof, and Ahn found that all three systems agreed on only 2 of 53 patients, while Ahn's and Oosterhof's methodologies agreed on 13.[37] Although we positively identified DE using all three criteria in the present case, we also note that her DE were severely advanced and some confounding may have occurred due to her underlying scoliosis. As DE and HCTD are significant risk factors for SIH, confirming the diagnosis may guide management decisions in patients with postural headaches, and a more reliable and standardized set of criteria that will accurately capture all patients—in particular, those with more subtle DE—should be developed.
CONCLUSION
Our case also illustrates how an understanding of the association between HCTD and SIH will help mitigate concern for other, less likely diagnoses in comparable clinical circumstances. Based on initial imaging, concern was raised by radiology for acute hydrocephalus or a spontaneous atrial diverticulum as the primary process underlying the patient's symptoms. However, after her history of Marfan syndrome was taken into account, SIH was identified as the most likely primary pathologic event, which subsequently precipitated low CSF pressure originating from the spine and downward herniation of the cerebellar tonsils.
We believe our case illustrates a range of important considerations surrounding common complications of SIH, especially in patients with underlying HCTD. In particular, we stress the value of conservative management and the utility of noninvasive diagnostic techniques in patients on long-term anticoagulation. Our case also adds to mounting evidence that the radiographic criteria defining DE are inadequate and require further reevaluation and standardization to assure accurate diagnosis. We highlight the need to advance our understanding of the underlying mechanisms of disease, both in hope of providing more effective treatment options to patients with HCTD and to enhance our understanding of risk factors that may be predisposing patients both with and without syndromic HCTD to develop SIH. Accordingly, we recommend that neurosurgeons consider SIH in the differential for all cases of acute and chronic postural headaches, with particular attention to patients with history or physical exam findings suggestive of HCTD.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/8/125629
Contributor Information
Luigi Bassani, Email: Luigi.Bassani@gmail.com.
Christopher S. Graffeo, Email: Graffeo@gmail.com.
Navid Behrooz, Email: nab9080@nyp.org.
Vineet Tyagi, Email: Vineet.Tyagi@med.nyu.edu.
Taylor Wilson, Email: Taylor.Wilson@nyumc.org.
Saul Penaranda, Email: Spenaran@hunter.cuny.edu.
David Zagzag, Email: David.Zagzag@nyumc.org.
Daniel B Rifkin, Email: Daniel.Rifkin@nyumc.org.
Mary Helen Barcellos-Hoff, Email: MHBarcellos-Hoff@nyumc.org.
Girish Fatterpekar, Email: Girish.Fatterpekar@nyumc.org.
Dimitris Placantonakis, Email: Dimitris.Placantonakis@nyumc.org.
REFERENCES
- 1.Ades LC, Sullivan K, Biggin A, Haan EA, Brett M, Holman KJ, et al. FBN1, TGFBR1, and the Marfan-craniosynostosis/mental retardation disorders revisited. Am J Med Genet A. 2006;140:1047–58. doi: 10.1002/ajmg.a.31202. [DOI] [PubMed] [Google Scholar]
- 2.Ahn NU, Sponseller PD, Ahn UM, Nallamshetty L, Rose PS, Buchowski JM, et al. Dural ectasia in the Marfan syndrome: MR and CT findings and criteria. Genet Med. 2000;2:173–9. doi: 10.1097/00125817-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 3.De Paepe A. Dural ectasia and the diagnosis of Marfan's syndrome. Lancet. 1999;354:878–9. doi: 10.1016/S0140-6736(99)00168-3. [DOI] [PubMed] [Google Scholar]
- 4.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–26. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Fattori R, Nienaber CA, Descovich B, Ambrosetto P, Reggiani LB, Pepe G, et al. Importance of dural ectasia in phenotypic assessment of Marfan's syndrome. Lancet. 1999;354:910–3. doi: 10.1016/s0140-6736(98)12448-0. [DOI] [PubMed] [Google Scholar]
- 6.Habermann CR, Weiss F, Schoder V, Cramer MC, Kemper J, Wittkugel O, et al. MR evaluation of dural ectasia in Marfan syndrome: Reassessment of the established criteria in children, adolescents, and young adults. Radiology. 2005;234:535–41. doi: 10.1148/radiol.2342031497. [DOI] [PubMed] [Google Scholar]
- 7.Ho NC, Hadley DW, Jain PK, Francomano CA. Case 47: Dural ectasia associated with Marfan syndrome. Radiology. 2002;223:767–71. doi: 10.1148/radiol.2233000971. [DOI] [PubMed] [Google Scholar]
- 8.Iacono MI, Passera K, Magrassi L, Dore R, Lago P, Arbustini E, et al. A method for morphological characterization of dural ectasia in Marfan syndrome. Conf Proc IEEE Eng Med Biol Soc 2009. 2009:5764–7. doi: 10.1109/IEMBS.2009.5332525. [DOI] [PubMed] [Google Scholar]
- 9.Jones KB, Myers L, Judge DP, Kirby PA, Dietz HC, Sponseller PD. Toward an understanding of dural ectasia: A light microscopy study in a murine model of Marfan syndrome. Spine (Phila Pa 1976) 2005;30:291–3. doi: 10.1097/01.brs.0000152166.88174.1c. [DOI] [PubMed] [Google Scholar]
- 10.Liu FC, Fuh JL, Wang YF, Wang SJ. Connective tissue disorders in patients with spontaneous intracranial hypotension. Cephalalgia. 2011;31:691–5. doi: 10.1177/0333102410394676. [DOI] [PubMed] [Google Scholar]
- 11.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 12.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 13.Lundby R, Rand-Hendriksen S, Hald JK, Lilleas FG, Pripp AH, Skaar S, et al. Dural ectasia in Marfan syndrome: A case control study. AJNR Am J Neuroradiol. 2009;30:1534–40. doi: 10.3174/ajnr.A1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokri B. Spontaneous cerebrospinal fluid leaks: From intracranial hypotension to cerebrospinal fluid hypovolemia--evolution of a concept. Mayo Clin Proc. 1999;74:1113–23. doi: 10.4065/74.11.1113. [DOI] [PubMed] [Google Scholar]
- 15.Mokri B, Maher CO, Sencakova D. Spontaneous CSF leaks: Underlying disorder of connective tissue. Neurology. 2002;58:814–6. doi: 10.1212/wnl.58.5.814. [DOI] [PubMed] [Google Scholar]
- 16.Mokri B, Posner JB. Spontaneous intracranial hypotension: The broadening clinical and imaging spectrum of CSF leaks. Neurology. 2000;55:1771–2. doi: 10.1212/wnl.55.12.1771. [DOI] [PubMed] [Google Scholar]
- 17.Oosterhof T, Groenink M, Hulsmans FJ, Mulder BJ, van der Wall EE, Smit R, et al. Quantitative assessment of dural ectasia as a marker for Marfan syndrome. Radiology. 2001;220:514–8. doi: 10.1148/radiology.220.2.r01au08514. [DOI] [PubMed] [Google Scholar]
- 18.Puget S, Kondageski C, Wray A, Boddaert N, Roujeau T, Di Rocco F, et al. Chiari-like tonsillar herniation associated with intracranial hypotension in Marfan syndrome. Case report. J Neurosurg. 2007;106(1 Suppl):48–52. doi: 10.3171/ped.2007.106.1.48. [DOI] [PubMed] [Google Scholar]
- 19.Pui MH, Husen YA. Value of magnetic resonance myelography in the diagnosis of disc herniation and spinal stenosis. Australas Radiol. 2000;44:281–4. doi: 10.1046/j.1440-1673.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- 20.Pyeritz RE, Fishman EK, Bernhardt BA, Siegelman SS. Dural ectasia is a common feature of the Marfan syndrome. Am J Hum Genet. 1988;43:726–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Reinstein E, Pariani M, Bannykh S, Rimoin DL, Schievink WI. Connective tissue spectrum abnormalities associated with spontaneous cerebrospinal fluid leaks: A prospective study. Eur J Hum Genet. 2013;21:386–90. doi: 10.1038/ejhg.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimoin DL, Emery AE. 5th ed. Philadelphia: Churchill Livingstone; 2007. Emery and Rimoin's principles and practice of medical genetics. [Google Scholar]
- 23.Schick U, Musahl C, Papke K. Diagnostics and treatment of spontaneous intracranial hypotension. Minim Invasive Neurosurg. 2010;53:15–20. doi: 10.1055/s-0030-1247552. [DOI] [PubMed] [Google Scholar]
- 24.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks: A review. Neurosurg Focus. 2000;9:e8. doi: 10.3171/foc.2000.9.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Schievink WI, Gordon OK, Tourje J. Connective tissue disorders with spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension: A prospective study. Neurosurgery. 2004;54:65–70. doi: 10.1227/01.neu.0000097200.18478.7b. [DOI] [PubMed] [Google Scholar]
- 26.Schievink WI, Meyer FB, Atkinson JL, Mokri B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg. 1996;84:598–605. doi: 10.3171/jns.1996.84.4.0598. [DOI] [PubMed] [Google Scholar]
- 27.Schievink WI, Morreale VM, Atkinson JL, Meyer FB, Piepgras DG, Ebersold MJ. Surgical treatment of spontaneous spinal cerebrospinal fluid leaks. J Neurosurg. 1998;88:243–6. doi: 10.3171/jns.1998.88.2.0243. [DOI] [PubMed] [Google Scholar]
- 28.Schievink WI, Reimer R, Folger WN. Surgical treatment of spontaneous intracranial hypotension associated with a spinal arachnoid diverticulum. Case report. J Neurosurg. 1994;80:736–9. doi: 10.3171/jns.1994.80.4.0736. [DOI] [PubMed] [Google Scholar]
- 29.Schrijver I, Schievink WI, Godfrey M, Meyer FB, Francke U. Spontaneous spinal cerebrospinal fluid leaks and minor skeletal features of Marfan syndrome: A microfibrillopathy. J Neurosurg. 2002;96:483–9. doi: 10.3171/jns.2002.96.3.0483. [DOI] [PubMed] [Google Scholar]
- 30.Sencakova D, Mokri B, McClelland RL. The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology. 2001;57:1921–3. doi: 10.1212/wnl.57.10.1921. [DOI] [PubMed] [Google Scholar]
- 31.Sheikhzadeh S, Rybczynski M, Habermann CR, Bernhardt AM, Arslan-Kirchner M, Keyser B, et al. Dural ectasia in individuals with Marfan-like features but exclusion of mutations in the genes FBN1, TGFBR1 and TGFBR2. Clin Genet. 2011;79:568–74. doi: 10.1111/j.1399-0004.2010.01494.x. [DOI] [PubMed] [Google Scholar]
- 32.Soylen B, Hinz K, Prokein J, Becker H, Schmidtke J, Arslan-Kirchner M. Performance of a new quantitative method for assessing dural ectasia in patients with FBN1 mutations and clinical features of Marfan syndrome. Neuroradiology. 2009;51:397–400. doi: 10.1007/s00234-009-0508-9. [DOI] [PubMed] [Google Scholar]
- 33.Soylen B, Singh KK, Abuzainin A, Rommel K, Becker H, Arslan-Kirchner M, et al. Prevalence of dural ectasia in 63 gene-mutation-positive patients with features of Marfan syndrome type 1 and Loeys-Dietz syndrome and report of 22 novel FBN1 mutations. Clin Genet. 2009;75:265–70. doi: 10.1111/j.1399-0004.2008.01126.x. [DOI] [PubMed] [Google Scholar]
- 34.Villeirs GM, Van Tongerloo AJ, Verstraete KL, Kunnen MF, De Paepe AM. Widening of the spinal canal and dural ectasia in Marfan's syndrome: Assessment by CT. Neuroradiology. 1999;41:850–4. doi: 10.1007/s002340050856. [DOI] [PubMed] [Google Scholar]
- 35.von Kodolitsch Y, Robinson PN. Marfan syndrome: An update of genetics, medical and surgical management. Heart. 2007;93:755–60. doi: 10.1136/hrt.2006.098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YF, Lirng JF, Fuh JL, Hseu SS, Wang SJ. Heavily T2-weighted MR myelography vs CT myelography in spontaneous intracranial hypotension. Neurology. 2009;73:1892–8. doi: 10.1212/WNL.0b013e3181c3fd99. [DOI] [PubMed] [Google Scholar]
- 37.Weigang E, Ghanem N, Chang XC, Richter H, Frydrychowicz A, Szabo G, et al. Evaluation of three different measurement methods for dural ectasia in Marfan syndrome. Clin Radiol. 2006;61:971–8. doi: 10.1016/j.crad.2006.05.015. [DOI] [PubMed] [Google Scholar]


