Abstract
Background:
An important part of neurosurgical training is the improvement of surgical skills. Acquiring microsurgical skills follows a learning curve, influenced by specific exercises, feedback, and training. Aim of training should be rapid learning success. The study shows the way in which video-based training can influence the learning curve.
Methods:
Over a period of 18 months (2011-2012) 12 residents were evaluated in spinal surgery (12 cases per resident) by a skilled evaluator based on different criteria. The evaluation criteria (exposition of important anatomy, intraoperative bleeding, efficacy of using bipolar cauterization) were weighted and added to a single quality-score. The participating residents were divided into two groups. Only one group (n = 5) received video-based training.
Results:
Residents showed an individually different but explicit increase in microsurgical skills. The quality-score during the first surgery compared with the end point of the study demonstrated a faster improvement of surgical skills in the group with video-based training than in the group without special training. Considering all residents together, the video-training group displayed a steeper gradient of microsurgical success. Comparison of the single resident's microsurgical skills showed individual disparities. Various biases that influence the learning success are under examination.
Conclusion:
Video-based training can improve microsurgical skills, leading to an improved learning curve. An earlier entry of the learning curve plateau in the video-training group promotes a higher acquisition of surgical skills. Because of the positive effect, we plan to apply the video-based training model to other neurosurgical subspecialties, especially neurovascular and skull base surgery.
Keywords: Learning curve, microsurgery, surgical skills, video-based training
INTRODUCTION
The increased financial pressure placed on hospitals and the required short-term hospitalization of patients is the main fact that negatively impacts the surgical skills of residents.[8] In surgery, a detailed intraoperative training is expected, leading to longer operation length and increased expenses for the surgical department.[4,9] For these reasons the rapid development of surgical skills should be an important part of education.[4,9] The learning process of surgery capabilities can be mapped by a learning curve.[1,4,7,9] However, the learning curve is influenced by specific exercises and training methods.[1,4,7,9] Recent studies have shown that surgical procedures – especially microsurgical techniques – consist of a high-leveled motion sequence that requires substantial practice and training.[2,3,4,5,8,9,10,11,12]
Following our assumption, that the most important part of training is the intensive feedback of the supervisor, we have created an appropriate training model, with an additional video support. This training model can be easily integrated into the clinical routine. The aim of our study was to evaluate whether intraoperative video-based training model improves neurosurgical skills compared with a control group.
MATERIALS AND METHODS
Residents of our Neurosurgical Department (n = 12) were randomly selected and divided into two groups. The names of the 14 participants were allotted to the two different groups by a nurse who was not involved in the study. Most residents (n = 9) were between the first and third year of their training. Only three residents were in an advanced stage (fourth and fifth year). Five residents were assigned to the video-based training group (VbG) and seven residents in the group did without video-based training (control group [cG]). This unbalance of the number of residents was the consequence of withdrawal of two residents after starting the study. One resident changes the department of neurosurgery and one resident finished his residency.
The study was limited to lumbar spine surgeries, according to the year of residency of the participants. All surgical procedures were video recorded using the surgical microscope Zeiss Pentero 800 with video recording capability. The operations were assisted and evaluated by a supervising attending neurosurgeon. One week after surgery the supervisor and the resident analyzed the intraoperative video and discussed the steps of the surgery together. In this discussion, the pitfalls and failures were pointed out by the supervisor to enable correct understanding during regarding the video.
The evaluation for the assessments of surgical skills was done using a special score sheet from 1 (poor) to 10 (best marks). The parameters were carefully selected on the basis of a literature study and specialist experience. All criteria are listed in Figure 1. Some parameters such as the intraoperative bleeding or the exposition of important anatomic structures had already been used in several other studies.[1,4,6,7,8,9,10,12]
Figure 1.
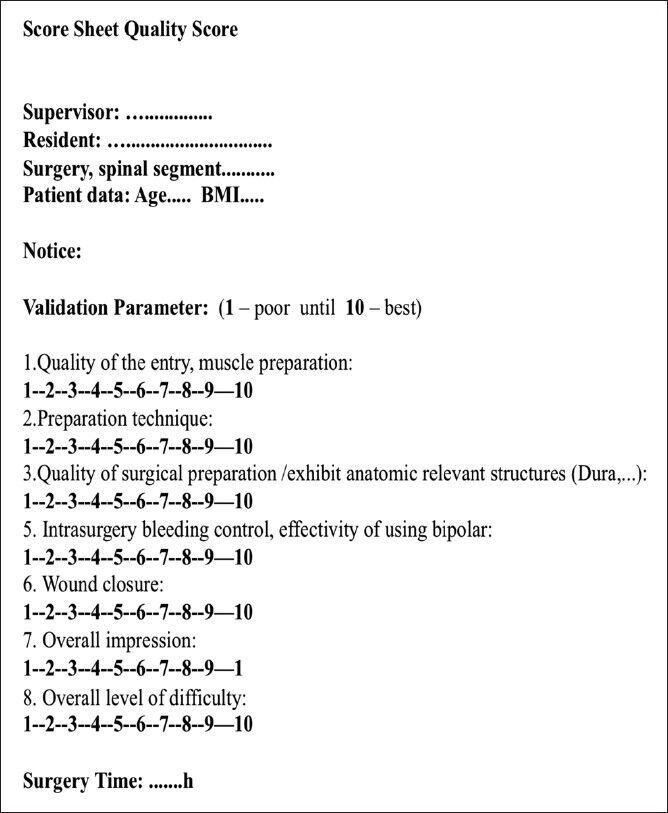
An example of a score sheet, which was used to validate the surgical interventions. The supervisor completed this score sheet directly after the surgery
In addition, we used the time of surgery for further evaluation of each procedure. The time of surgery is a well-known parameter to evaluate surgical experience and could be used to follow up the resident's improvement. The evaluation criteria were weighted and added to a single quality-score (QS). Each parameter was individually ranked based on the importance of the surgical process and clinical outcome. For example, wound closure was less important than the exposure of the dura or the efficacy of using the bipolar. Exposure of anatomic structures and effective hemostasis were weighted higher and the points were multiplied by the factor 2. The end score was calculated by summarizing the points of each parameter. The level of difficulty should bring all surgery to the same level. For the final score, all points were multiplied by 1.5 of the level of difficulty. After the calculation, the acquirable score is between 5.33 and 533.33.
Video-based training
In our video-based training model, the residents had to review the recorded surgery and discuss each step with an experienced neurosurgeon. Figure 4 shows the different surgical steps that were often discussed in the training sessions especially the dura preparation, intraoperative bleeding control, and optimization handling the microscope. The separability of this video-based training model is the intensive response from the supervisor, as well as the visual feedback. The supervisor interacts as a crucial player because of the double feedback in surgery and training [Figure 2c].
Figure 4.
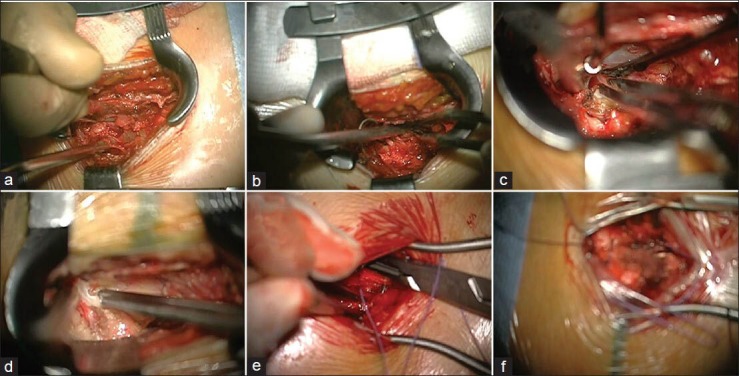
Different pictures of typical situations, which were important to validate the surgery. (a) Different preparation techniques for entry into subcutis and muscles. (b) Effective use of the bipolar (c) Preparation of the dura. (d) Resection of the intervertebral disk tissue. (e) Wound closure with microscopic help. This trains the residents to handle the microscope in general. (f) An example for ineffective use of the microscope due to problems with correct focus
Figure 2.
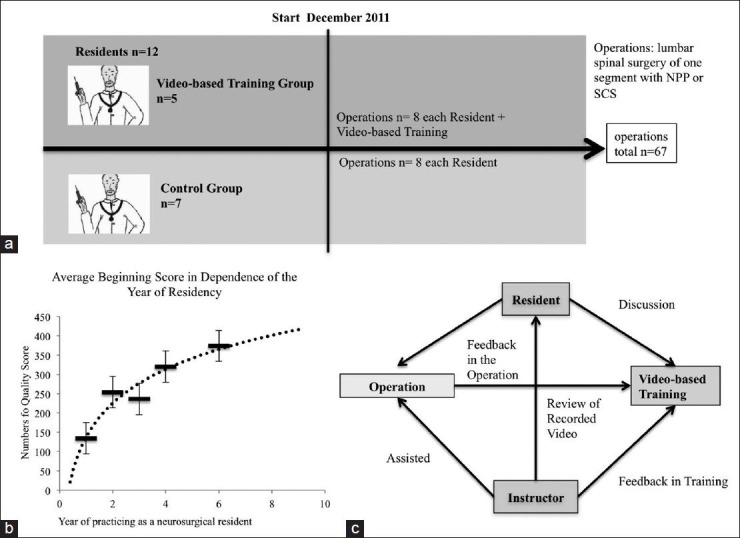
(a) A flowchart of the study design. (b) A diagram of the beginning point, which presents the average quality score depending in the year of neurosurgical residency. A significant difference in the skill level (P= 0.04) was detectable between the first and second and the following years. The values were connected by a logarithm function, which shows a typical learning curve. (c) A flowchart reveals the training session procedure. The video-based training focused on the resident, the supervisor, the surgery and the training. The main effect of the learn successes was presented by this double feedback. On one side the intraoperative feedback, on the other side feedback of the training session
Statistical analysis
The statistical analysis was performed using a student's t-test and regression analysis. A P ≤ 0.05 was considered statistically significant. The QS was calculated by using the parameter of the score sheet and the calculation to adapt the difficulty level. These values were used for the learning curve. To analyze the average beginning score a student's t-test was used. Regression analysis was the best way to analyze the learning curve data and to compare the individual progress. To validate the individual learning success between the two groups, a student's t-test was used.
Ethic permission
The study was approved by the ethical review committee of the Ruhr University Bochum.
RESULTS
In the VbG, 43 surgeries were included in the study. No data were collected in 5 surgeries, leaving 38 cases for analysis. In the cG, 38 operations were performed in which 3 were not included in the statistical analysis due to missing patient consent.
The primary aim was to find out how the surgical skill level changed depending on of the training year of the residents. When compared with the average beginning score [Figure 2b], a clear progress of surgical skills could be measured. Between the first and the second year of education the difference in the skill level changed significantly (P = 0.04). This shows that the very beginning of the neurosurgical residency is the perfect time point to influence the surgical skills with a special training program.
The VbG revealed more improvement of the QS. For the learning curve, we compared the arithmetic mean of each group and surgery to work against individual differences. This value for each group is presented in Figure 3a. Both groups showed clear improvement in their surgical skills [Figure 3a]. Our first aim was to analyze the starting time point of the study. Both groups started with a similar skill level. This acted as a control for the randomized distribution of both groups and revealed an equal initial skill level. After the sixth surgery the groups started to increase their skill level in a different way. The VbG was able to improve their skills faster than the control group. After the eleventh surgery both groups exhibited similar skill levels. Between the first and sixth surgery a regression analysis [Figure 3b and c] revealed a significant difference between the VbG and the cG. The gradient of a linear regression curve between the first and seventh surgery was used for the student's t-test and reveals a significantly difference (P = 0.02) between both groups. This difference could be seen in Figure 3b, which shows the average gradient of both groups. The strongest effect of the video-based training could be seen at the beginning of the learning process but the improvement was truncated after 12-14 surgeries.
Figure 3.
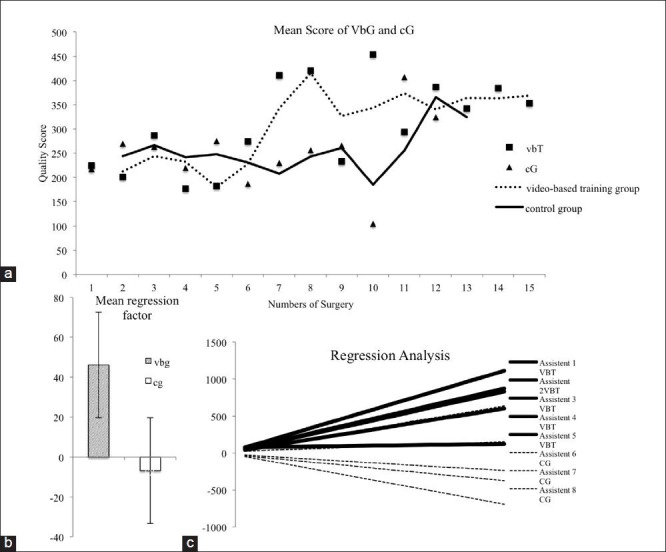
(a) The learning curve of the video-based training group (VbG) and the control group (cG). The values shown were the mean quality score of all members in one surgical intervention of one group. The learning curve of the video-based group revealed a faster increase of the surgical skills after the sixth surgery and an earlier entry of the learning plateau. (b) The diagram presents the different mean gradients of the regression analysis. The video-based training group presents a significantly (P= 0.02) steeper gradient of their learning success between the beginning point and the seventh surgical interventions. (c) All linear regression curves are shown
DISCUSSION
Every learning process has a learning curve.[4] A learning curve shows the learning success within the training time. In the background of surgery, a certain repetition rate of surgical operations had been done to reach a learn plateau. Further recurrence of operations would not increase the surgical skill as much as at the beginning of the learning process. The aim of the training should be to decrease the numbers of repetitions until the resident reaches his learning plateau. We introduced video-based training as a method to improve skills without increasing duration or animal models. Additionally, the training model can easily be integrated in the clinical daily routine. In the beginning, both groups were on the same level but the VbG was able to improve its surgical skills with statistically significant results (P < 0.05). This result reveals that the average skill level was virtually the same in both groups and the random group splitting was successful. When analyzing the individual scores of each group member some differences could be seen. After five to six surgeries, the VbG started to differentiate itself from the control group. In the period of time between the sixth and twelfth surgical interventions, the VbG was able to improve their skills much faster than the cG due to the influence of the video-based training. In addition, the VbG was under special observation. This could influence the results and worked as a bias. After the twelfth surgery, both groups reached the same learning level, from which further improvement could be expected. The highest differences were measured in the speed of improvement of surgical skills.
These results indicate that the video-based training is an easy and effective way to analyze intraoperative mistakes. For example, imprecise focus handling was a common mistake, which could easily be optimized by video-based training.
We observed a statistically significant increase in surgical skills, especially in the first two years of training in comparison to the residents in the later years of residency [Figure 2b]. This suggests that effective training should be established in this period of time. The following parameters may also play a decisive role in the learning process and should be considered as possible biases of the study:
The special support for residents in group with video-based training.
The awareness of being under supervision by video recordings.
Individual differences in learning behavior.
Based on the positive results, we recommend the use and expansion of the video-based learning model, especially in more demanding areas such as tumor or vascular neurosurgery, which would require more repetitions to improve surgical skills. These subspecialties are suitable areas in where to use video-based training more effectively. Because of the positive effect, we plan to apply the video-based training model to other neurosurgical subspecialties, especially neurovascular and skull base surgery.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/1/124973
Contributor Information
D. H. Heiland, Email: dieter.henrik.heiland@uniklinik-freiburg.de.
A. K. Petridis, Email: opticdisc@aol.com.
H. Maslehaty, Email: h.maslehaty@gmx.de.
J. Thissen, Email: thissenj@sana.de.
A. Kinzel, Email: kinzela@sana.de.
M. Scholz, Email: martin.scholz-kdu@sana.de.
L. Schreiber, Email: lutzschreiber1@aol.com.
REFERENCES
- 1.Buchmann P, Dinçler S. Learning curve-calculation and value in laparoscopic surgery. Ther Umsch. 2005;62:69–75. doi: 10.1024/0040-5930.62.2.69. [DOI] [PubMed] [Google Scholar]
- 2.Chan WY, Figus A, Ekwobi C, Srinivasan JR, Ramakrishnan VV. The ‘round-the-clock’ training model for assessment and warm up of microsurgical skills: A validation study. J Plast Reconstr Aesthet Surg. 2010;63:1323–8. doi: 10.1016/j.bjps.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Hsu HT, Chang SJ, Yang SS, Chai CL. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J. 2013;22:727–33. doi: 10.1007/s00586-012-2540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lascar I, Totir D, Cinca A, Cortan S, Stefanescu A, Bratianu R, et al. Training program and learning curve in experimental microsurgery during the residency in plastic surgery. Microsurgery. 2007;27:263–7. doi: 10.1002/micr.20352. [DOI] [PubMed] [Google Scholar]
- 5.Neudert M, Kluge A, Beleites T, Kemper M, Zahnert T. Microsurgical skills training with a new tympanoplasty model: Learning curve and motivational impact. Otol Neurotol. 2012;33:364–70. doi: 10.1097/MAO.0b013e318238081c. [DOI] [PubMed] [Google Scholar]
- 6.Pape-Kohler C, Chmelik C, Heiss MM, Jauch KW. E-learning in surgical procedure manuals and blogs. Chirurg. 2010;81:14–8. doi: 10.1007/s00104-009-1759-z. [DOI] [PubMed] [Google Scholar]
- 7.Parikh K, Tomasino A, Knopman J, Boockvar J, Härtl R. Operative results and learning curve: Microscope-assisted tubular microsurgery for 1- and 2-level discectomies and laminectomies. Neurosurg Focus. 2008;25:E14. doi: 10.3171/FOC/2008/25/8/E14. [DOI] [PubMed] [Google Scholar]
- 8.Regelsberger J, Heese O, Horn P, Kirsch M, Eicker S, Sabel M, et al. Training microneurosurgery-four years experiences with an in vivo model. Cent Eur Neurosurg. 2011;72:192–5. doi: 10.1055/s-0030-1261906. [DOI] [PubMed] [Google Scholar]
- 9.Selber JC, Chang EI, Liu J, Suami H, Adelman DM, Garvey P, et al. Tracking the learning curve in microsurgical skill acquisition. Plast Reconstr Surg. 2012;130:550–7e. doi: 10.1097/PRS.0b013e318262f14a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyderman CH, Fernandez-Miranda J, Gardner PA. Training in neurorhinology: The impact of case volume on the learning curve. Otolaryngol Clin North Am. 2011;44:1223–8. doi: 10.1016/j.otc.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Urso-Baiarda F, Shurey S, Grobbelaar AO. Effect of caffeine on microsurgical technical performance. Microsurgery. 2007;27:84–7. doi: 10.1002/micr.20311. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Lü G, Patel AA, Ren P, Cheng I. An evaluation of the learning curve for a complex surgical technique: The full endoscopic interlaminar approach for lumbar disc herniations. Spine J. 2011;11:122–30. doi: 10.1016/j.spinee.2010.12.006. [DOI] [PubMed] [Google Scholar]


