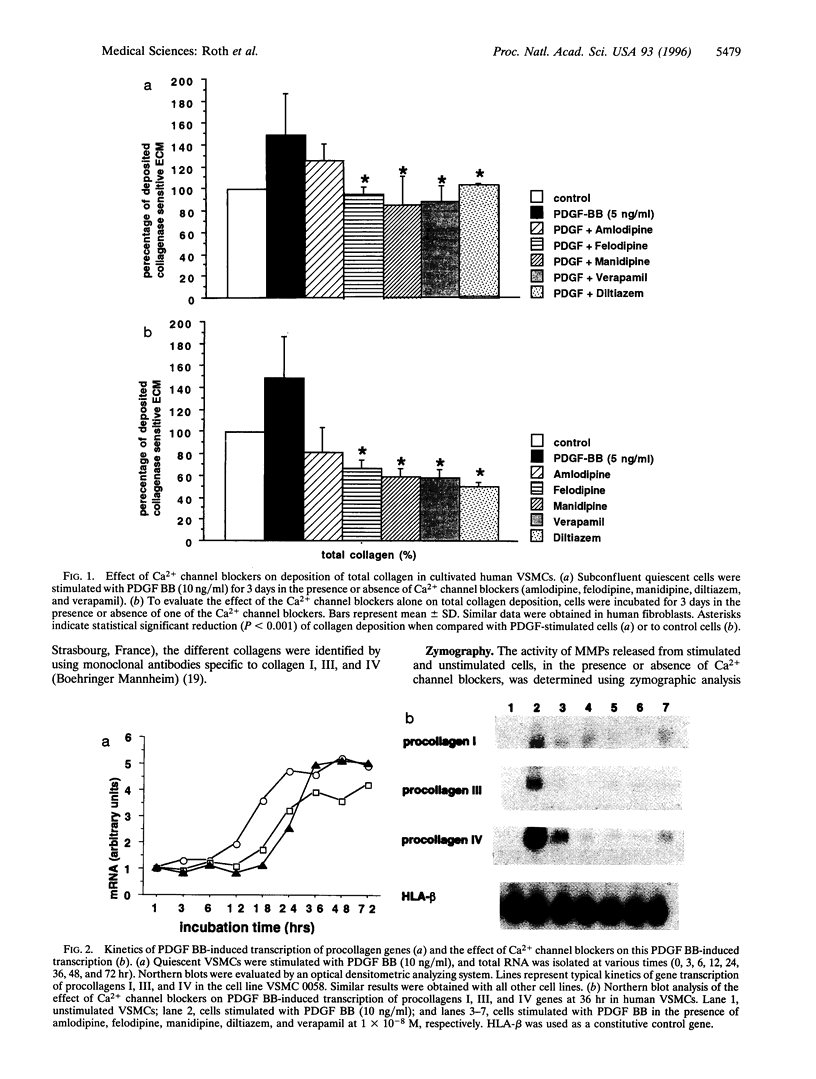
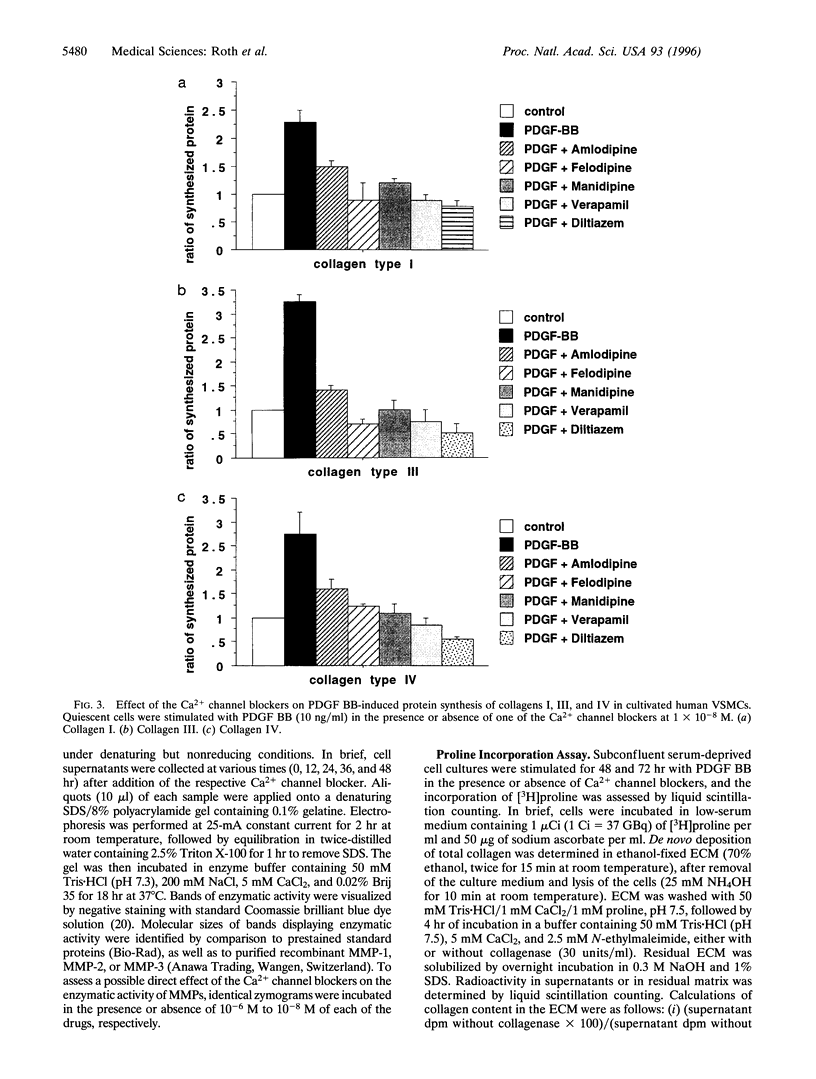
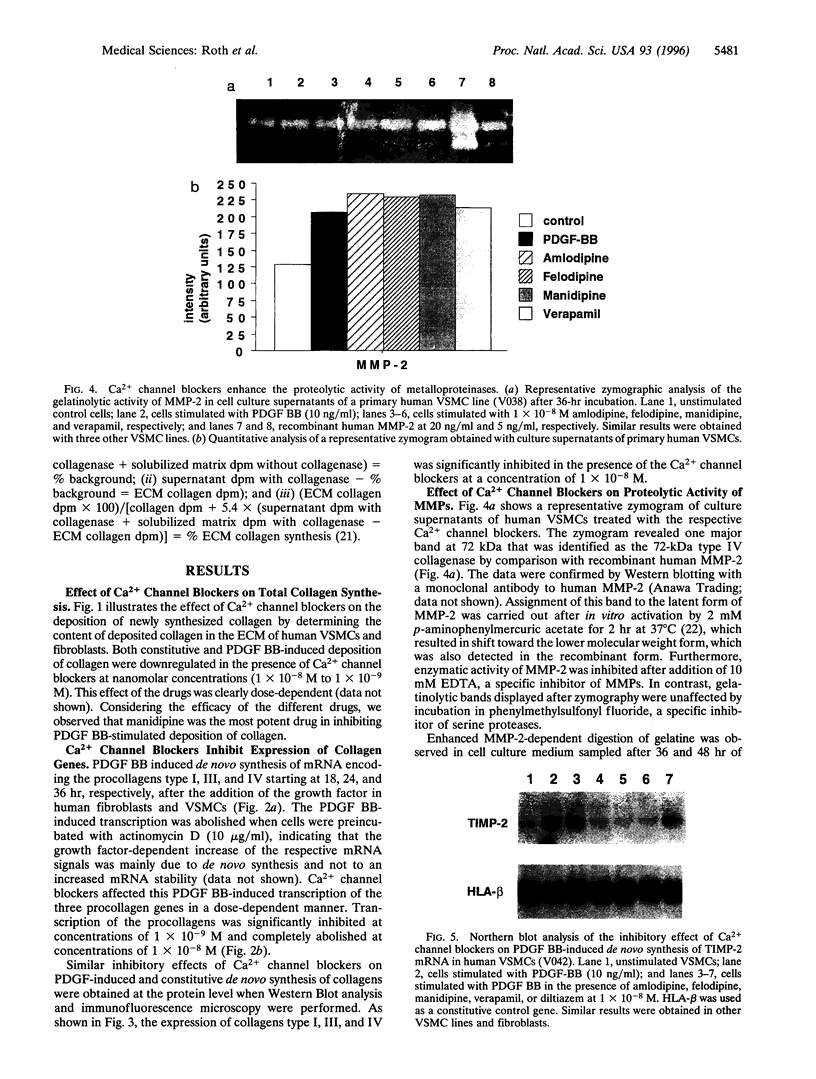
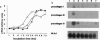Abstract
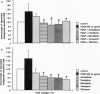
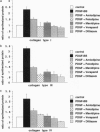
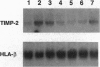
The extracellular matrix (ECM) is an intricate network composed of an array of macromolecules capable of regulating the functional responsiveness of cells. Its composition greatly varies among different types of tissue, and dysregulation of its metabolism may contribute to vascular remodeling during the pathogenesis of various diseases, including atherosclerosis. In view of their antiatherosclerotic effects, the role of Ca2+ channel blockers in the metabolism of ECM was examined. Nanomolar concentrations of the five Ca2+ channel blockers amlodipine, felodipine, manidipine, verapamil, or diltiazem significantly decreased both the constitutive and platelet-derived growth factor BB-dependent collagen deposition in the ECM formed by human vascular smooth muscle cells and fibroblasts. The drugs inhibited the expression of fibrillar collagens type I and III and of basement membrane type IV collagen. Furthermore, Ca2+ channel blockers specifically increased the proteolytic activity of the 72-kDa type IV collagenase as shown by gelatin zymography and inhibited the transcription of tissue inhibitor of metalloproteinases-2.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baragi V. M., Sweet A. M., Thompson M. A., Hawkins K. L., Toy K. A., Rosebury W. S. Inhibition of interleukin 1-induced biosynthesis of stromelysin by the calcium antagonist TMB-8 (8-(N, N-diethylamino)octyl-3,4,5-trimethoxybenzoate HCl). Connect Tissue Res. 1995;31(2):153–160. doi: 10.3109/03008209509028403. [DOI] [PubMed] [Google Scholar]
- Bashey R. I., Donnelly M., Insinga F., Jimenez S. A. Growth properties and biochemical characterization of collagens synthesized by adult rat heart fibroblasts in culture. J Mol Cell Cardiol. 1992 Jul;24(7):691–700. doi: 10.1016/0022-2828(92)93383-u. [DOI] [PubMed] [Google Scholar]
- Block L. H., Matthys H., Emmons L. R., Perruchoud A., Erne P., Roth M. Ca(2+)-channel blockers modulate expression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and low density lipoprotein receptor genes stimulated by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9041–9045. doi: 10.1073/pnas.88.20.9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabos M., Roth M., Hahn A. W., Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J Clin Invest. 1994 Jun;93(6):2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Lark M. W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994 Dec;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardi C., Calzoni P., Marcolongo P., Cavarra E., Vanni L., Lungarella G. Collagen breakdown products and lung collagen metabolism: an in vitro study on fibroblast cultures. Thorax. 1994 Apr;49(4):312–318. doi: 10.1136/thx.49.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaud J. A., Lortat-Jacob H. Matrix receptors to cytokines: from concept to control of tissue fibrosis dynamics. Pathol Res Pract. 1994 Oct;190(9-10):883–890. doi: 10.1016/S0344-0338(11)80991-X. [DOI] [PubMed] [Google Scholar]
- Hatamochi A., Mori K., Ueki H. Role of cytokines in controlling connective tissue gene expression. Arch Dermatol Res. 1994;287(1):115–121. doi: 10.1007/BF00370729. [DOI] [PubMed] [Google Scholar]
- Katsuda S., Okada Y., Minamoto T., Oda Y., Matsui Y., Nakanishi I. Collagens in human atherosclerosis. Immunohistochemical analysis using collagen type-specific antibodies. Arterioscler Thromb. 1992 Apr;12(4):494–502. doi: 10.1161/01.atv.12.4.494. [DOI] [PubMed] [Google Scholar]
- Kleiner D. E., Stetler-Stevenson W. G. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem. 1994 May 1;218(2):325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Alessandro R., Spoonster J., Wersto R. P., Liotta L. A. Angiogenesis: role of calcium-mediated signal transduction. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1307–1311. doi: 10.1073/pnas.92.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn E. C., Jacobs W., Kim Y. S., Alessandro R., Stetler-Stevenson W. G., Liotta L. A. Calcium influx modulates expression of matrix metalloproteinase-2 (72-kDa type IV collagenase, gelatinase A). J Biol Chem. 1994 Aug 26;269(34):21505–21511. [PubMed] [Google Scholar]
- Kunjara-Na-Ayudhya R., Thomson A. B., Kappagoda C. T. Effect of isradipine on the formation and regression of fatty streaks in cholesterol fed rabbits. Cardiovasc Res. 1994 Jul;28(7):1089–1095. doi: 10.1093/cvr/28.7.1089. [DOI] [PubMed] [Google Scholar]
- Laurent G. J. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol. 1987 Jan;252(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- Lin C. Q., Bissell M. J. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993 Jun;7(9):737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Lohi J., Keski-Oja J. Calcium ionophores decrease pericellular gelatinolytic activity via inhibition of 92-kDa gelatinase expression and decrease of 72-kDa gelatinase activation. J Biol Chem. 1995 Jul 21;270(29):17602–17609. doi: 10.1074/jbc.270.29.17602. [DOI] [PubMed] [Google Scholar]
- Nagase H. Matrix metalloproteinases. A mini-review. Contrib Nephrol. 1994;107:85–93. [PubMed] [Google Scholar]
- Nayler W. G., Panagiotopoulos S. The antiatherosclerotic effect of the calcium antagonists and their implications in hypertension. Am Heart J. 1993 Feb;125(2 Pt 2):626–629. doi: 10.1016/0002-8703(93)90213-s. [DOI] [PubMed] [Google Scholar]
- Rekhter M. D., Zhang K., Narayanan A. S., Phan S., Schork M. A., Gordon D. Type I collagen gene expression in human atherosclerosis. Localization to specific plaque regions. Am J Pathol. 1993 Dec;143(6):1634–1648. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Roth M., Keul R., Emmons L. R., Hörl W. H., Block L. H. Manidipine regulates the transcription of cytokine genes. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4071–4075. doi: 10.1073/pnas.89.9.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata N., Kawamura K., Takebayashi S. Effects of collagen matrix on proliferation and differentiation of vascular smooth muscle cells in vitro. Exp Mol Pathol. 1990 Apr;52(2):179–191. doi: 10.1016/0014-4800(90)90003-v. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., deBlois D., O'Brien E. R. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995 Sep;77(3):445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- Stary H. C. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990 Aug;11 (Suppl E):3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Wacher M. P., Margulies I. M., Liotta L. A. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989 Jan 25;264(3):1353–1356. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- van der Rest M., Garrone R. Collagen family of proteins. FASEB J. 1991 Oct;5(13):2814–2823. [PubMed] [Google Scholar]