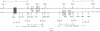Abstract
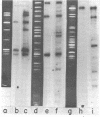
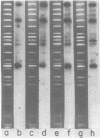
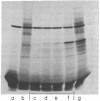
RNAs larger than about 6 S prepared from etioplasts of dark-grown maize seedlings, and from plastids at later stages of light-induced development, were labeled in vitro and hybridized to separated fragments of maize chloroplast DNA digested with endonucleases. The major nonribosomal RNA present in developing plastids, but virtually undetectable in etioplasts, hybridizes to chloroplast DNA Bam fragment 8 and has been mapped on the maize plastid chromosome. Other aliquots of RNA from plastids were translated in a rabbit reticulocyte-derived system. Developing plastids, and mature chloroplasts, but not etioplasts, contain mRNA for an approximately 34,500 dalton polypeptide. The simultaneous appearance, during light-induced maize plastid development, of RNA which hybridizes to Bam 8 and is translated into a 34,500 dalton protein indicates that photoregulated expression of a single gene is being observed.
Keywords: plastid DNA, etioplast, photogene, gene mapping
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Bogorad L. Light-induced increase in the activity of maize plastid DNA-dependent RNA polymerase. Eur J Biochem. 1976 Aug 16;67(2):615–620. doi: 10.1111/j.1432-1033.1976.tb10727.x. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Bogorad L. Endonuclease recognition sites mapped on Zea mays chloroplast DNA. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4309–4313. doi: 10.1073/pnas.73.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Kolodner R., Bogorad L. Zea mays chloroplast ribosomal RNA genes are part of a 22,000 base pair inverted repeat. Cell. 1977 Aug;11(4):739–749. doi: 10.1016/0092-8674(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Bottomley W., Smith H. J., Bogorad L. RNA polymerases of maize: partial purification and properties of the chloroplast enzyme. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2412–2416. doi: 10.1073/pnas.68.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. H., Wildman S. G. Chloroplast DNA codes for the primary structure of the large subunit of fraction I protein. Biochim Biophys Acta. 1972 Sep 14;277(3):677–680. doi: 10.1016/0005-2787(72)90115-3. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Bedbrook J. R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haff L. A., Bogorad L. Hybridization of maize chloroplast DNA with transfer ribonucleic acids. Biochemistry. 1976 Sep 7;15(18):4105–4109. doi: 10.1021/bi00663a029. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Thomas C. A., Jr Molecular cloning of DNA fragments produced by restriction endonucleases Sa1I and BamI. Proc Natl Acad Sci U S A. 1976 May;73(5):1537–1541. doi: 10.1073/pnas.73.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. R., Davidson J. N., Mets L. J., Bogorad L. Characterization of chloroplast and cytoplasmic ribosomal proteins of Chlamydomonas reinhardi by two-dimensional gel electrophoresis. Mol Gen Genet. 1974;132(2):105–118. doi: 10.1007/BF00272176. [DOI] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. The molecular size and conformation of the chloroplast DNA from higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):372–390. doi: 10.1016/0005-2787(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K., Warner R. C. Physical studies on the size and structure of the covalently closed circular chloroplast DNA from higher plants. Biochim Biophys Acta. 1976 Oct 4;447(2):144–155. doi: 10.1016/0005-2787(76)90338-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maizels N. Dictyostelium 17S, 25S, and 5S rDNAs lie within a 38,000 base pair repeated unit. Cell. 1976 Nov;9(3):431–438. doi: 10.1016/0092-8674(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Mendelian and uniparental alterations in erythromycin binding by plastid ribosomes. Science. 1971 Nov 12;174(4010):707–709. doi: 10.1126/science.174.4010.707. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Sagher D., Grosfeld H., Edelman M. Large subunit ribulosebisphosphate carboxylase messenger RNA from Euglena chloroplasts. Proc Natl Acad Sci U S A. 1976 Mar;73(3):722–726. doi: 10.1073/pnas.73.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., Tewari K. K. Conservation of 70S ribosomal RNA genes in the chloroplast DNAs of higher plants. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3147–3151. doi: 10.1073/pnas.71.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]