Abstract
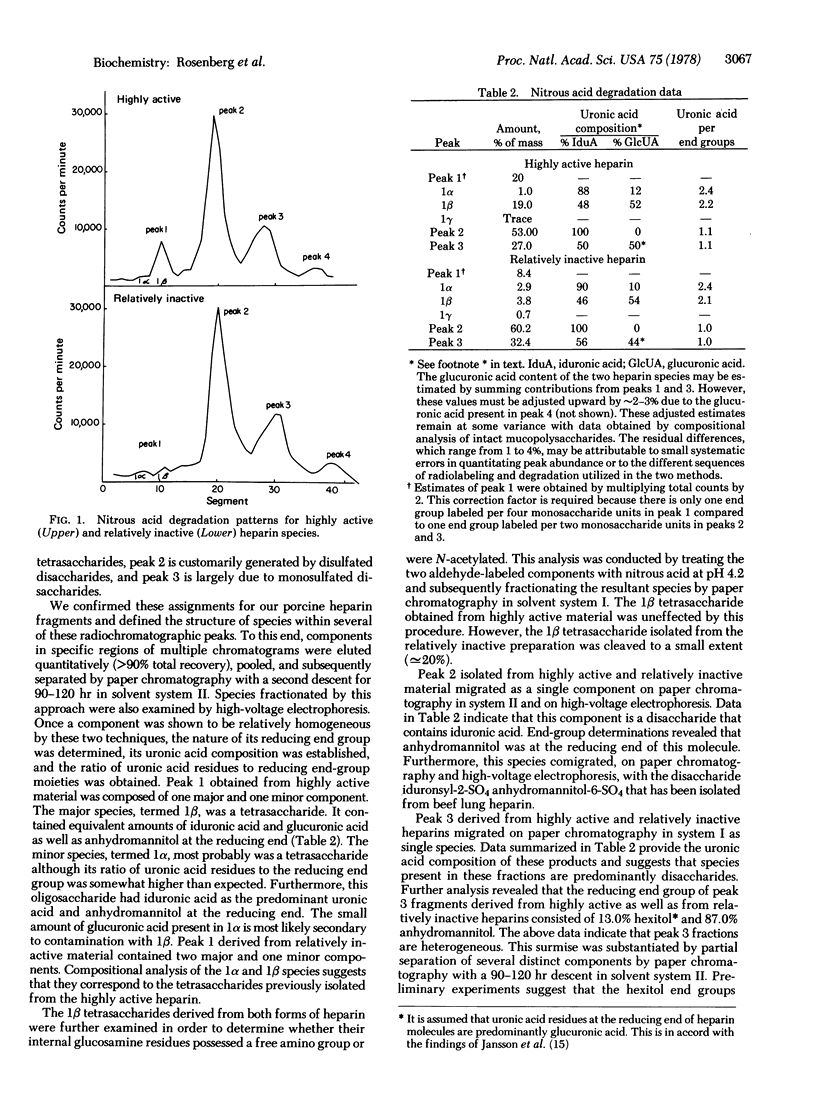
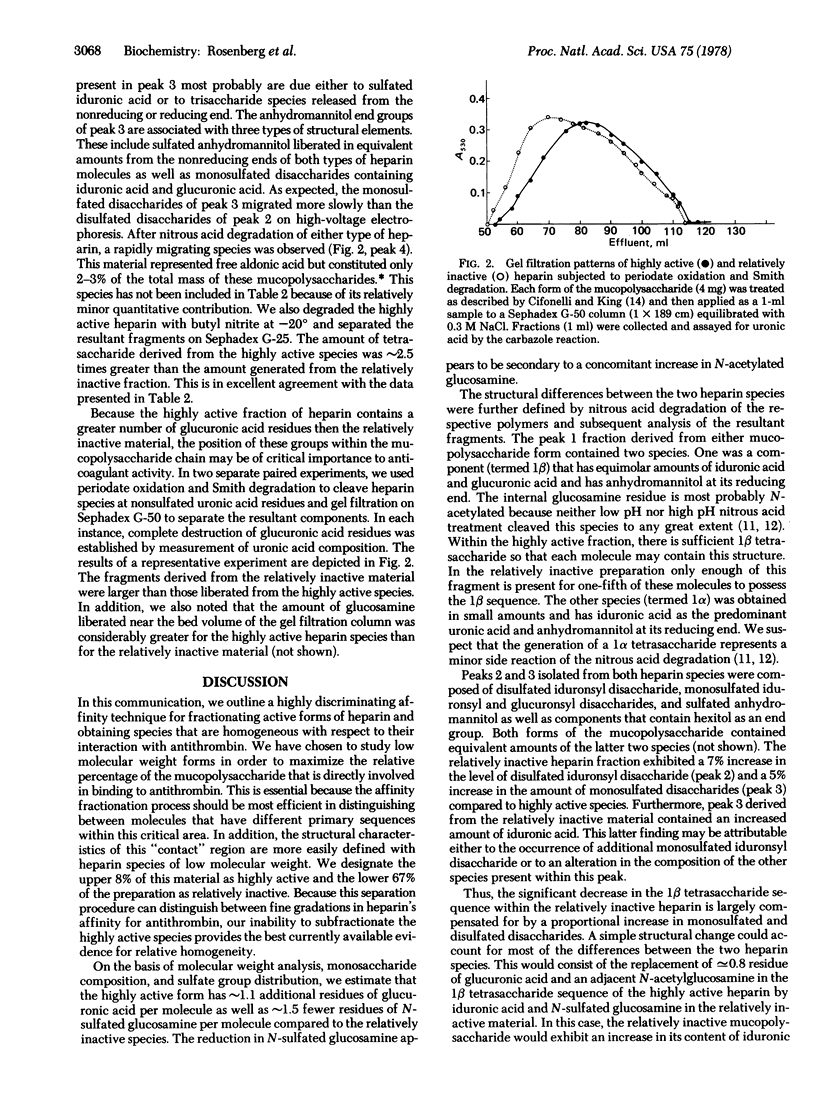
We have fractionated porcine heparin species of low molecular weight, with an average specific anticoagulant activity of 96 units/mg by affinity chromotography. Highly active and relatively inactive preparations of similar size were obtained with specific anticoagulant activities of 360 and 4 units/mg, respectively. The highly active heparin fraction possesses 1.1 additional residues of glucuronic acid and 1.5 fewer residues of N-sulfated glucosamine per molecule compared to the relatively inactive species. This decrease in N-sulfated glucosamine appears to be secondary to a corresponding increase in N-acetylated glucosamine. This form also contains a tetrasaccharide sequence with a N-sulfated glucosamine at its reducing end as well as equivalent amounts of glucuronic acid and iduronic acid. Furthermore, the internal glucosamine residue of this sequence appears to be N-acetylated. Sufficient amounts of this tetrasaccharide sequence are present within the highly active preparation such that each molecule may be endowed with this structure. The relatively inactive product contains a significantly decreased quantity of this tetrasaccharide sequence such that only [unk]20% of these molecules may possess this structure. The mean distance between nonsulfated uronic acid residues of the highly active species is smaller than that separating similar residues of the relatively inactive product. In addition, a larger number of the nonsulfated uronic acid residues of the highly active material appears either to be present in a restricted region of the molecule separated only by glucosamine residues or to be located at penultimate positions within the polysaccharide chain.
Keywords: purification of highly active heparin, partial sequence of heparin, chemical composition of heparin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Balazs E. A., Berntsen K. O., Karossa J., Swann D. A. An automated method for the determination of hexosamines. Anal Biochem. 1965 Sep;12(3):559–564. doi: 10.1016/0003-2697(65)90222-8. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem. 1950 Jun;184(2):517–522. [PubMed] [Google Scholar]
- DODGSON K. S., PRICE R. G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962 Jul;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Jansson L., Ogren S., Lindahl U. Macromolecular properties and end-group analysis of heparin isolated from bovine liver capsule. Biochem J. 1975 Jan;145(1):53–62. doi: 10.1042/bj1450053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D. Biologic actions of heparin. Semin Hematol. 1977 Oct;14(4):427–440. [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of molecular weight dispersion in chondroitin sulphate on a microgram level. Biochim Biophys Acta. 1969 Feb 18;177(1):152–154. doi: 10.1016/0304-4165(69)90076-2. [DOI] [PubMed] [Google Scholar]


