Abstract
Hypertension leads to structural and functional changes at baroreceptor synapses in the medial nucleus tractus solitarius (NTS), but the underlying molecular mechanisms remain unknown. Our previous studies show that brain-derived neurotrophic factor (BDNF) is abundantly expressed by rat nodose ganglion (NG) neurons, including baroreceptor afferents and their central terminals in the medial NTS. We hypothesized that hypertension leads to upregulation of BDNF expression in NG neurons. To test this hypothesis, we used two mechanistically distinct models of hypertension: the spontaneously hypertensive rat (SHR) and the deoxycorticosterone acetate (DOCA)-salt rat. Young adult SHRs, whose blood pressure was significantly elevated compared to age-matched Wistar-Kyoto (WKY) control rats, exhibited dramatic upregulation of BDNF mRNA and protein in the NG. BDNF transcripts from exon 4, known to be regulated by activity, and exon 9 (protein-coding region) showed the largest increases. Electrical stimulation of dispersed NG neurons with patterns that mimic baroreceptor activity during blood pressure elevations led to increases in BDNF mRNA that were also mediated through promoter 4. The increase in BDNF content of the NG in vivo was associated with a significant increase in the percentage of BDNF-immunoreactive NG neurons. Moreover, upregulation of BDNF in cell bodies of NG neurons was accompanied by a significant increase in BDNF in the NTS region, the primary central target of NG afferents. A dramatic increase in BDNF in the NG was also detected in DOCA-salt hypertensive rats. Together, our study identifies BDNF as a candidate molecular mediator of activity-dependent changes at baroafferent synapses during hypertension.
Keywords: Baroreceptor, Blood Pressure, DOCA, Nodose Ganglion, SHR
INTRODUCTION
Blood pressure changes are buffered by the arterial baroreceptor reflex (Dampney and Horiuchi, 2003; Brooks and Sved, 2005; Guyenet, 2006). First-order neurons of this reflex are located in the nodose ganglion (NG), and their central projections terminate in the medial nucleus tractus solitarius (NTS) of the dorsal medulla (Andresen and Kunze, 1994; Guyenet, 2006). It is well established that baroreceptor reflexes remain plastic throughout life. Moreover, hypertension is associated with an increased activity of cardiovascular afferents (Jones and Thoren, 1977) that, in turn, is likely to drive plastic changes at NTS synapses. In fact, previous studies show that hypertension leads to increased density of dendritic spines and GluR1 subunits of AMPA receptors at first-order baroreceptor synapses in the NTS (Aicher et al., 2003; Hermes et al., 2008). These changes are thought to be homeostatic in nature and to strengthen synaptic transmission in baroafferent pathways. However, the underlying molecular mechanisms remain unknown.
Recent findings implicate brain-derived neurotrophic factor (BDNF), a neurotrophin with broad biological actions in both the developing and mature nervous system (Poo, 2001; Huang and Reichardt, 2001), in the plasticity of NTS synapses. BDNF plays a critical role in activity-dependent synaptic plasticity, including sensory pathways (Malcangio and Lessmann, 2003). During embryonic development, BDNF is a target-derived survival factor for a large subset of NG neurons, including arterial baroreceptors (Brady et al., 1999). Postnatally, BDNF is expressed by the NG neurons themselves (Schecterson and Bothwell, 1992; Wetmore and Olson, 1995) and can be released from these neurons by activity (Balkowiec and Katz, 2000). Studies from our laboratory and others indicate that BDNF is a likely mediator of plastic changes specifically at baroreceptor synapses. Namely, BDNF is expressed in arterial baroreceptors and their central terminals in the medial NTS in vivo, and BDNF release from cultured NG neurons is increased by electrical stimulation with patterns that mimic the in vivo activity of baroreceptor afferents (Martin et al., 2009). Moreover, BDNF inhibits AMPA glutamatergic transmission in sensory relay NTS neurons (Balkowiec et al., 2000; Clark et al., 2011) and a microinjection of BDNF to the medial NTS increases mean arterial pressure and heart rate (Clark et al., 2011). Recently, we showed that BDNF also regulates the dendritic morphology of sensory relay neurons from the NTS region (Martin et al., 2012). However, it is unknown whether BDNF contributes to NTS synaptic plasticity during hypertension.
To begin to investigate this question, the current study was undertaken to test the hypothesis that the hypertension-driven increase in activity of NG cardiovascular afferents results in upregulation of BDNF expression in NG neurons. We used two mechanistically distinct rat models of hypertension, spontaneously hypertensive rats (SHRs) and deoxycorticosterone acetate (DOCA)-salt hypertensive rats, and age-matched normotensive controls, to examine BDNF expression in nodose ganglia and the NTS region of the dorsal medulla, the central target of NG afferents. In addition, using an in vitro model, we determined if electrical stimulation of NG neurons with a baroreceptor-like pattern increases BDNF mRNA levels. Portions of this work have previously been published in abstract form (Jenkins et al., 2007).
MATERIALS AND METHODS
Animals
Postnatal day (P) 2–3 Sprague Dawley rat pups of both sexes, Sprague Dawley adult male rats of 250–300 g body weight, and 12- to 18-week-old spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) male rats were obtained from Charles River Laboratories (Wilmington, MA) and used for this study. All procedures were approved by the Institutional Animal Care and Use Committee of the Oregon Health and Science University, and conformed to the Policies on the Use of Animals and Humans in Neuroscience Research approved by the Society for Neuroscience and the NIH Guide for the Health and Use of Laboratory Animals.
DOCA-salt model
Adult male Sprague Dawley rats underwent surgery to remove the right kidney and insert a silicone pellet with or without (sham) 65 mg of deoxycorticosterone acetate (DOCA), as previously described (Brooks et al., 2006; O’Donaughy et al., 2006; O’Donaughy and Brooks, 2006). During 2–3 weeks of recovery from the surgery, and for the remainder of the experiment, all rats drank water with 1% NaCl and 0.2% KCl (O’Donaughy et al., 2006).
Blood pressure measurements
All blood pressure measurements were performed in conscious animals. For each SHR and WKY rat, blood pressure was measured twice, in two consecutive weeks preceding euthanasia and NG harvest, using a non-invasive method based on determining the tail blood volume with a volume-pressure recording sensor and an occlusion tail-cuff (CODA System, Kent Scientific, Torrington, CT (Feng et al., 2008). Blood pressure was measured by multiple readings in individual rats, until an average of 15 stable measurements were obtained. Data were calculated as an average of blood pressure values obtained in the second week of measurements. All Sprague Dawley DOCA-salt and sham-salt animals were instrumented with femoral arterial catheters, and blood pressure was measured one day before euthanasia and tissue collection, using a Statham (Spectramed) pressure transducer, a Grass bridge amplifier [7P1], and a Grass polygraph [7D], as previously described (O’Donaughy et al., 2006). In a subset of animals instrumented for the direct measurements of blood pressure in the femoral artery, the non-invasive tail-cuff method was used for comparison. Blood pressure values obtained with the two techniques were not significantly different from each other (data not shown).
RNA extraction and quantitative RT-PCR
Right and left nodose ganglia were dissected from each euthanized animal (CO2 followed by 0.1 mg/kg of Euthasol® intraperitoneally) and immediately transferred to pre-chilled RNAse-free 1.5-ml microcentrifuge tubes placed on ice. Reverse transcription was performed following the Cells-to-cDNA II kit (Ambion, Foster City, CA) with some modifications. Specifically, nodose ganglia were treated with 2.5 mg/ml collagenase (Sigma, St Louis, MO) for 30 min at 37°C, washed with PBS and resuspended in 75 μl of lysis buffer. Next, samples were heated at 80°C for 10 min and chilled on ice. DNaseI (1.5 μl) was added to each sample and incubated for 15 min at 37°C, followed by heat inactivation (80°C) for 5 min. To make cDNA, 4 μl of the lysate were reverse transcribed using oligo-(dT) primers and the M-MLV RT enzyme following the instructions of the manufacturer. Quantitative PCR was performed in triplicates following the MIQE guidelines (Bustin et al., 2009) using the MX3000P real time PCR system (Stratagene; Cedar Creek, TX), Sensimix Plus SYBR master mix (Quantace, Taunton, MA), 2 μl of cDNA (diluted 1:4), and 3 μM of each forward and reverse primers, in a final volume of 10 μl. All primer sequences (see below) were designed using Primer3 online software and synthesized by Integrated DNA Technologies (Coralville, IA). To check for primer specificity, DNA products were amplified by PCR using Taq polymerase (Invitrogen, Carlsbad, CA). Single PCR products were gel-purified using QIAquick Gel Extraction Kit (Qiagen, Valencia, CA), sequenced (OHSU DNA sequencing facilities) and quantified spectrophotometrically. Quantitative RT-PCR was performed with DNA standard dilutions ranging from 10 pg to 10 ag. The real time amplification data were collected continuously using the software supplied with the MX3000P real time PCR system. Cq values for standard curves were between 10 and 30, with Cq values for samples falling within this range. RT-PCR efficiencies were all above 97%. The forward primers for BDNF were: 5′-aaagccgaacttctcacatgat-3′ (BDNF1), 5′-ctcccccttttaactgaagagaa-3′ (BDNF4), ctttggggcagacgagaaag-3′ (BDNF6), with a common reverse primer, 5′-attcacgctctccagagtcc-3′. Primers for the BDNF protein-coding region (pcr), designed to amplify all BDNF transcripts, were: 5′-ggtcacagcggcagataaaaagac-3′ (forward) and 5′-ttcggcattgcgagttccag-3′ (reverse). The PCR products were 273, 280, 275 and 188 bp, respectively. Data normalization was carried out against the endogenous reference gene transcript glyceraldehyde-3-phosphate dehydrogenase (GAPDH, forward: 5′-ccatcactgccactcagaag-3, reverse: 5′-ggaagaatgggagttgctgt-3′; PCR product of 341 bp in size). Genbank accession numbers are EF125675.1 (BDNF-I), EF125679.1 (BDNF-IV/formerly known as BDNF-III), EF125680.1 (BDNF-VI/formerly known as BDNF-IV), NM012513 (BDNF-pcr), and NM017008.3 (GAPDH).
BDNF ELISA
Nodose ganglia and dorsal medulla regions containing the nucleus tractus solitarius (NTS) were dissected from each animal, as previously described by our laboratory (Martin et al., 2009; Martin et al., 2012), immediately following euthanasia, and transferred to individual siliconized (Sigmacote®; Sigma), pre-weighed and pre-chilled 1.5-ml microcentrifuge tubes. The tissue-containing tubes were weighed (to obtain the weight of tissue), and 100 μl of pre-chilled lysis buffer (20 mM Tris buffer, pH 7.4, 137 mM NaCl, 1% Nonidet-P40, 10% glycerol, 1 mM phenylmethanesulfonyl fluoride (PMSF), 0.5 mM sodium vanadate, 10 μM aprotinin, 10 μM actinonin, and 100 μM leupeptin) was added to each tube, followed by homogenization of the tissue with Kontes® Pellet Pestle® (Kimble-Chase, Vineland, NJ). Next, 400 μl of Block & Sample buffer (BDNF Emax™ ImmunoAssay System, Promega, Madison, WI) was added and samples (the total volume of 500 μl) were sonicated on ice (3 × 3.0 W, 5 sec each), using a microprobe sonicator (Sonicator 3000, Misonix, Inc., Farmingdale, NY). The resulting crude lysate was transferred to an anti-BDNF-coated 96-well ELISA plate (100 μl per well; 3 wells per sample). BDNF ELISA was performed according to the manufacturer’s protocol (BDNF Emax™ ImmunoAssay System, Promega). BDNF levels were calculated from the standard curve prepared for each plate, using SOFTmax PRO® software (v. 4.3; Molecular Devices, Sunnyvale, CA). The standard curves were linear within the range used (0–500 pg/ml) and the quantities of BDNF in experimental samples were always within the linear range of the standard curve.
BDNF immunohistochemistry and analysis
Nodose ganglia were processed for BDNF immunostaining as previously described in detail by our laboratory (Martin et al., 2009). Briefly, following euthanasia, three SHRs and three WKY rats were perfused transcardially with phosphate-buffered saline (PBS), followed by 2% paraformaldehyde in 0.1 M sodium phosphate buffer. Nodose ganglia were dissected from each animal, immersion post-fixed in 2% paraformaldehyde for 1 hour, rinsed in PBS, and cryoprotected in 30% sucrose in PBS at 4°C for at least 24 h. Next, the ganglia were embedded in O.C.T. Tissue-Tek® compound (Sakura Finetek USA, Inc., Torrance, CA), and 10-μm cryostat sections of the right NG were collected onto glass slides in a series of two.
Every other section (i.e. one series) was processed for BDNF immunostaining with chicken polyclonal anti-BDNF (1:50; Promega), followed by goat anti-chicken biotinylated IgG (1:200, Vector Laboratories, Burlingame, CA) secondary antibody, avidin-biotin reagent (ABC), and diaminobenzidine.
NG sections were imaged with an Olympus IX-71 inverted microscope (Olympus America Inc., Center Valley, PA) and images were captured with a Hamamatsu ORCA-ER CCD camera (Hamamatsu, Bridgewater, NJ) controlled by Olympus Microsuite software (v. 5.0, Olympus America Inc). Sections were analyzed both directly from the microscope and through images using ImageJ software (National Institutes of Health, Bethesda, MD).
The entire neuronal population of the right NG was randomly sampled, and BDNF content was assessed in each profile that contained a nucleus. In order to determine the percentage of BDNF-immunoreactive profiles, approximately 200 profiles in at least three randomly chosen sections were selected, and a characteristic punctate staining of BDNF imunoreactivity was assessed. For each animal, the numbers of total and BDNF-immunoreactive profiles in each examined section were pooled and expressed as a fraction of BDNF-immunoreactive profiles in the entire neuronal population.
Preparation of NG cultures
Rat pups were euthanized by intraperitoneal injection of Euthasol® (0.1 mg/kg) and decapitated, followed by dissection of both nodose ganglia, which were immediately transferred to ice-cold Ca2+/Mg2+-free Dulbecco’s salt solution. Neuron-enriched NG cultures were prepared as previously described by our laboratory (Buldyrev et al., 2006; Martin et al., 2009; Tarsa and Balkowiec, 2009), and grown in Neurobasal-A medium (Invitrogen, Carlsbad, CA) supplemented with B-27 (Invitrogen), 0.05 mg/ml penicillin-0.05 mg/ml streptomycin-0.1 mg/ml neomycin (Invitrogen), 0.5 mM L-glutamine (Invitrogen), and 2.5% fetal bovine serum (HyClone, Logan, UT) for 6 days in 48-well tissue culture-treated plates (Nalge Nunc Int., Naperville, IL) pre-coated with poly-D-lysine (Sigma) and laminin (Sigma). The neuronal nature of the NG cultures has been verified in our previous study (Fig. 6a in Martin et al., 2009).
Electrical field stimulation
Following the initial 6-day incubation, NG cultures were stimulated as previously described by our laboratory (Buldyrev et al., 2006; Martin et al., 2009; Hsieh et al., 2010), except that 48-well plates were used and wells were fitted with paired platinum electrodes (0.2 mm wire diameter). The neurons were stimulated with biphasic rectangular pulses of 0.2 ms duration and amplitude of 100 mA per well, delivered at 6 Hz.
RNA extraction from NG cultures
RNA extraction and cDNA synthesis were performed as previously described by our laboratory (Hsieh et al., 2010). Briefly, total RNA was extracted using TRIzol (Invitrogen), and 1 μg of total RNA was reverse transcribed using Tetro cDNA synthesis kit (Bioline, Taunton, MA). Quantitative RT-PCR was performed as described above for the ex vivo NG.
Calculations and statistical analysis
Data are expressed as mean ± standard error, and the sample size (n) represents the number of individual animals or cultures. Samples were compared using analysis of variance (ANOVA) followed by post-hoc analysis with Duncan’s test, or by an independent sample Student’s t-test for two-group comparisons. Proportional data (immunohistochemical analysis of NG sections) were arcsin-transformed before their further analysis by t-test; P<0.05 was considered significant.
RESULTS
BDNF transcripts are upregulated in nodose ganglia of SHRs compared to WKY rats
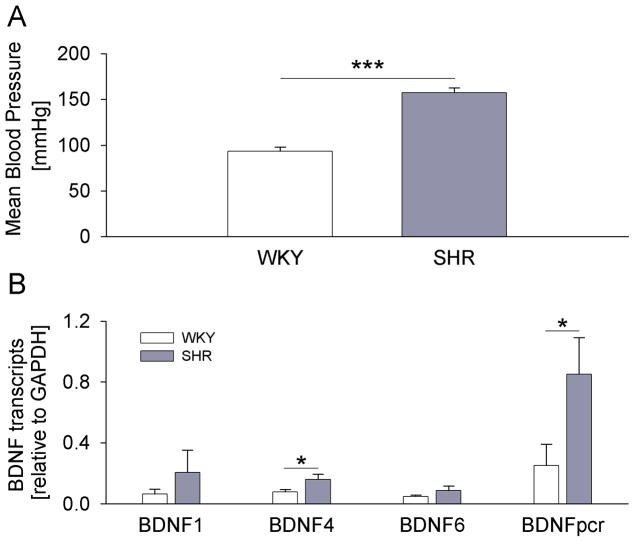
It is well established that neuronal activity upregulates BDNF expression (Isackson et al., 1991; Ernfors et al., 1991; Castren et al., 1992). BDNF mRNA expression is controlled by nine differentially regulated promoters (Aid et al., 2007), and rat BDNF transcripts from exons 1, 4, and 6 are known to be regulated by activity (Tabuchi et al., 2000; Hong et al., 2008). Therefore, we first compared expression of BDNF transcripts 1, 4, 6, and the protein-coding region, between SHRs and age-matched WKY control rats. Mean blood pressure of SHRs was significantly higher compared to WKY rats, as measured 3–7 days before harvesting the ganglia (Fig. 1A). The analysis of individual BDNF transcripts by quantitative RT-PCR revealed a strong trend toward increased BDNF expression in nodose ganglia of SHRs compared to WKY rats for all examined transcripts. Moreover, the increase was statistically significant for BDNF 4 and the protein-coding region (Fig. 1B), consistent with earlier studies pointing to BDNF 4 (formerly known as BDNF 3) as the transcript that is most strongly regulated by activity (Tao et al., 1998; Hong et al., 2008; Tabuchi, 2008). The transcripts derived from the protein-coding region of the BDNF gene showed significantly higher expression compared to the transcript derived from exon 4 (Fig. 1B). The latter indicates that other transcripts, whose individual upregulation did not reach statistical significance, contribute to the increase in total BDNF transcription observed in SHRs relative to WKY rats.
Figure 1. BDNF transcripts are upregulated in nodose ganglia of SHR compared to WKY rats.
(A) Mean arterial pressure of WKY (n=6) and SHR (n=4) rats measured in the week preceding tissue collection; (B) Levels of BDNF transcripts 1, 4, 6 and the protein-coding region (pcr) relative to the reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), measured by quantitative RT-PCR in nodose ganglia of WKY rats (n=6) and SHRs (n=4); * P<0.05, *** P<0.001.
Electrical stimulation of NG neurons in vitro at a pattern that mimics the in vivo activity of baroreceptor afferents leads to BDNF transcription
The upregulation of BDNF transcription in NG afferents of hypertensive animals is likely due to a hypertension-driven increase in afferent activity. To begin testing this hypothesis, we examined the effects of electrical field stimulation of newborn rat NG neurons in vitro on expression of BDNF transcripts. We chose a 6-Hz stimulation frequency, which mimics newborn rat heart rate and, consequently, the basic discharge frequency of baroreceptors and other cardiovascular afferents in these animals. NG cultures (6 days in vitro) were stimulated for 4 hours, the minimal duration of stimulation that results in a significant, consistent, and readily detectable upregulation of BDNF transcripts (A. Vermehren-Schmaedick and A. Balkowiec, unpublished observations). Consistent with our data from hypertensive animals in vivo (Fig. 1B), electrical activation of NG neurons resulted in a trend toward upregulation of all BDNF transcripts examined (data not shown) and a statistically significant upregulation of transcript 4, which is known to be regulated by activity (Fig. 2). These data support our hypothesis that the hypertension-mediated increase in BDNF transcription in nodose ganglia is a result of increased activity of NG afferents.
Figure 2. Electrical stimulation of NG neurons in vitro at the frequency that mimics in vivo activity of baroreceptor afferents leads to BDNF transcription.
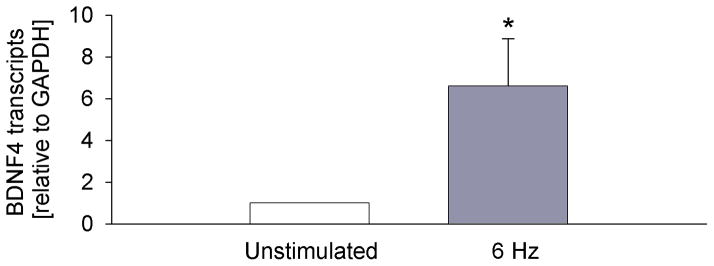
Levels of BDNF transcript 4 (which showed significant upregulation in hypertensive rats in vivo, Fig. 1B) in newborn NG neurons in vitro in control (Unstimulated, n=4) and following a 4-hour electrical field stimulation at 6 Hz with 0.2 ms/100 mA pulses (6 Hz, n=4). The levels of the BDNF transcript were measured by quantitative RT-PCR, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the reference gene, and normalized (the transcript levels in unstimulated cultures are considered 1); * P<0.05.
The increase in BDNF transcripts is accompanied by an upregulation of BDNF protein content in nodose ganglia of SHRs
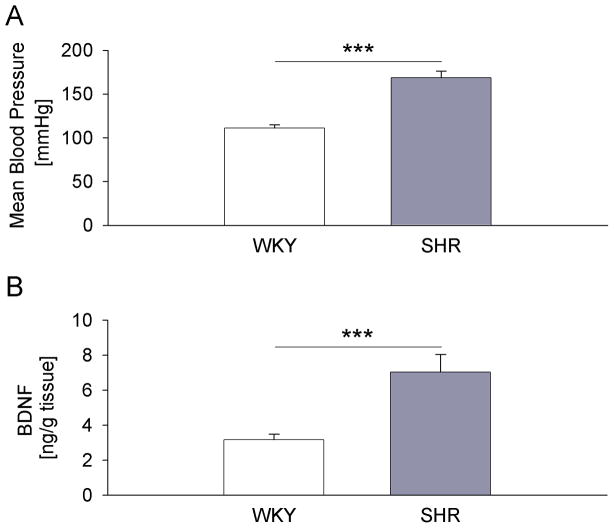
The biological significance of the induction of the BDNF gene is determined by changes in BDNF protein. However, activity-dependent expression of BDNF mRNA and protein can be regulated differently, such that increases in BDNF transcripts are not necessarily accompanied by increases in BDNF protein (Nanda and Mack, 2000; Tropea et al., 2001). Therefore, we next examined whether the increased transcription of BDNF in the NG of SHRs results in higher levels of BDNF protein, by assaying littermates of animals that were used for the analysis of BDNF transcripts. As expected, SHRs showed significantly higher mean arterial blood pressure compared to WKY rats (Fig. 3A). Consistent with the results of the BDNF transcript analysis, BDNF protein was significantly increased, and over twofold greater, in SHRs compared with WKY control rats, as measured by a standard sandwich ELISA (Fig. 3B). This result indicates that the increased transcription of the BDNF gene is followed by increased translation.
Figure 3. BDNF protein is upregulated in nodose ganglia of SHR compared to WKY rats.
(A) Mean arterial pressure of WKY (n=14) and SHR (n=13) rats measured in the week preceding tissue collection; (B) Levels of BDNF protein measured by sandwich ELISA in nodose ganglia of WKY rats (n=14) and SHRs (n=13); *** P<0.001.
Nodose ganglia of SHRs show a significantly increased proportion of BDNF-immunoreactive neurons
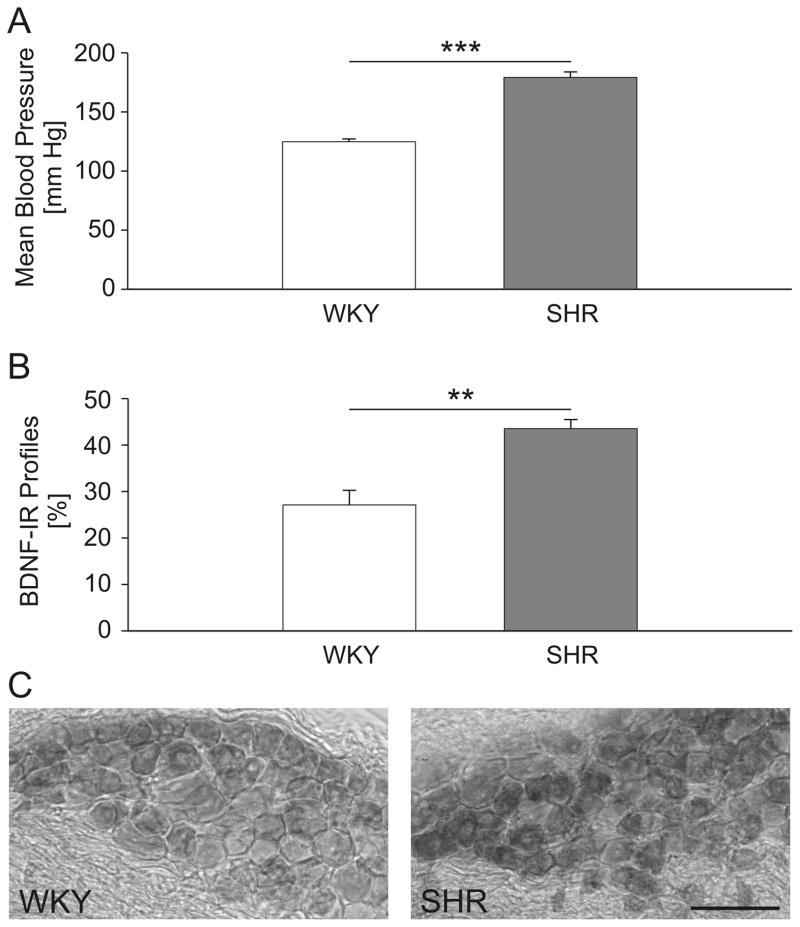
The increase in BDNF mRNA and protein measured in the whole NG of SHRs could potentially be accounted for by an increased expression in neurons that synthesize BDNF in the normotensive state, but could also be a result of recruitment of neurons that normally do not express detectable amounts of this neurotrophin. To distinguish between these two possibilities, we performed histological analysis of BDNF immunoreactivity in sections of the right-side NG from three SHRs and three WKY rats that showed significant differences in mean arterial blood pressure (Fig. 4A). We first identified somata with a clearly delineated plasma membrane and a nuclear profile, and BDNF immunoreactivity was assessed for every profile meeting these criteria. This analysis revealed that the percentage of BDNF-immunoreactive cells is 1.6-fold higher in SHRs (43.5 ± 1.9%) compared to WKY control rats (27.1 ± 3.2%; P<0.01; Fig. 4B). The observed increase in the percentage of BDNF-immunoreactive cells was accompanied by a stronger intensity of the BDNF staining in SHRs (Fig. 4C), suggesting an increased BDNF synthesis in individual neurons. Together, these data are consistent with the notion that the large increase in NG BDNF content of SHRs measured by ELISA (Fig. 3B) is likely a result of both an increase in the number of cells expressing BDNF at detectable levels and an increase in BDNF expression per neuron.
Figure 4. The proportion of BDNF-immunoreactive neurons is increased in nodose ganglia of SHR compared to WKY rats.
(A) Mean arterial pressure of WKY (n=3) and SHR (n=3) rats measured in the week preceding tissue collection; (B) Mean percentage of BDNF-immunoreactive (BDNF-IR) cells among all NG somata with a clearly delineated plasma membrane and a nuclear profile of WKY and SHR rats; WKY (n=3 rats; 703 neurons), SHR (n=3 rats; 591 neurons); ** P<0.01, *** P<0.001; (C) A representative section through the right NG of a WKY (left panel) and SHR (right panel) rat, immunostained for BDNF; scale bar, 100 μm.
The upregulation of BDNF expression in nodose ganglia of SHRs is paralleled by an increase in BDNF protein levels in the nucleus tractus solitarius (NTS) region of the dorsal medulla
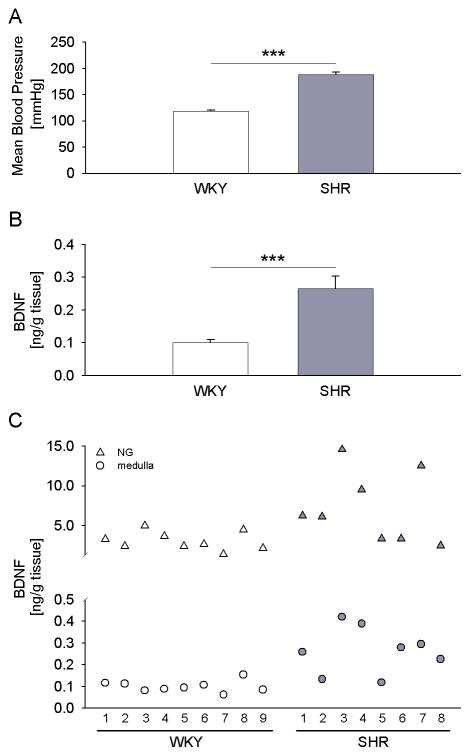
Our previous studies have indicated that BDNF expressed in NG neurons is faithfully transported to their central terminals in the brainstem NTS (Martin et al., 2009). Based upon these data, we hypothesized that the upregulation of BDNF expression in the NG results in increased BDNF protein in the terminal fields of NG afferents. To test this hypothesis, we examined BDNF levels in tissue blocks containing the caudal portion of the NTS, including the medial NTS, which is the primary central target of baroreceptor afferents. For these studies, we used a subset of SHR and WKY rats in which BDNF protein content was also examined in the NG. These animals showed significant differences in mean arterial blood pressure (Fig. 5A). In support of our hypothesis, BDNF protein was significantly increased in SHRs compared with WKY control rats, and the fold increase was similar to that in the NG (2.6-fold in the medulla versus 2.2-fold in the NG; Figs. 3B and 5B). Moreover, the analysis of individual animals revealed that a greater increase in BDNF levels in the NG was generally paralleled by a greater increase in this neurotrophin levels in the medulla (Fig. 5C). Together, these results suggest that the upregulation of BDNF expression in NG neurons of SHRs leads to an increased BDNF release at their synapses in the brainstem.
Figure 5. BDNF protein is upregulated in the dorsal medulla, the primary central target of NG afferents, in SHR compared to WKY rats.
(A) Mean arterial pressure of WKY (n=9) and SHR (n=8) rats measured in the week preceding tissue collection; (B) Levels of BDNF protein measured by sandwich ELISA in the dorsal medulla of WKY rats (n=9) and SHRs (n=8); *** P<0.001; (C) Levels of BDNF protein in the dorsal medulla (circles) and the NG (triangles) of each individual WKY rat and SHR analyzed.
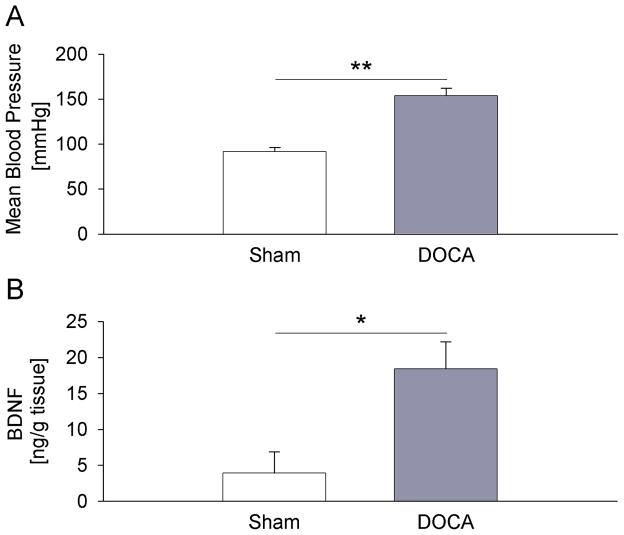
BDNF expression is also increased in nodose ganglia of DOCA-salt rats, a mechanistically distinct model of hypertension
To rule out the possibility that the observed increase in BDNF expression in the NG of SHRs is a unique feature related to the genetic make-up of this rat strain, we examined BDNF protein levels in another model of rat hypertension, DOCA-salt-sensitive hypertension (Brooks et al., 2006; O’Donaughy et al., 2006; O’Donaughy and Brooks, 2006). The mean arterial blood pressure was significantly higher in DOCA-salt rats compared to sham-salt controls (Fig. 6A), and not different from that measured in SHR and WKY rats, respectively (Figs. 1A, 3A, 4A, 5A). Similar to SHRs, DOCA-salt hypertensive animals showed a dramatic, nearly fivefold, increase in BDNF protein in the NG, compared to sham-salt normotensive controls (Fig. 6B). These data are consistent with our hypothesis that hypertension is the cause of upregulation of BDNF expression in NG primary afferents.
Figure 6. BDNF expression is increased in nodose ganglia of the deoxycorticosterone acetate (DOCA)-salt rats, a mechanistically distinct model of hypertension.
(A) Mean arterial pressure of sham-salt (Sham, n=3) and DOCA-salt (DOCA, n=4) rats measured one day before tissue collection; (B) Levels of BDNF protein measured by sandwich ELISA in nodose ganglia of sham-salt (Sham, n=7) and DOCA-salt (DOCA, n=9) rats; * P<0.05, ** P<0.01.
DISCUSSION
The present study provides the first evidence that hypertension upregulates BDNF expression in NG neurons and dorsal medulla, the primary central target of NG afferents, and that this effect is likely a result of the hypertension-induced increase in NG afferent activity. This conclusion is based on the fact that BDNF is upregulated in two mechanistically distinct rat models of hypertension. Moreover, the increase in BDNF transcripts is largely mediated through BDNF promoter 4, which is known to be regulated by neuronal activity. Finally, electrical stimulation of NG neurons in vitro mirrors the results obtained in hypertensive animals, i.e. a significant upregulation of BDNF transcription is also mediated through BDNF promoter 4.
Previous work from our laboratory and others has provided several lines of evidence to support BDNF as a likely mediator of activity-dependent plasticity at first-order baroafferent synapses (Balkowiec et al., 2000; Martin et al., 2009; Clark et al., 2011; Martin et al., 2012). Most significantly, BDNF is present not only in cell bodies of arterial baroreceptor neurons in the NG, but also in their central terminals in the medial NTS. This suggests that BDNF is released at first-order baroafferent synapses in vivo. In support of this possibility, BDNF release from NG neurons in vitro is enhanced by electrical stimulation with phasic patterns mimicking baroreceptor activity (Martin et al., 2009) that are known to evoke plastic changes at baroafferent synapses (Scheuer et al., 1996; Liu et al., 1998; Chen et al., 1999; Liu et al., 2000; Doyle and Andresen, 2001). The current study shows that during hypertension, the levels of BDNF in both NG neurons and their central targets in the dorsal medulla are substantially increased, suggesting increased BDNF availability at first-order synapses in the NTS and, in turn, more pronounced BDNF effects at first-order baroafferent synapses. The increased BDNF content in the brainstem suggests that BDNF trafficking along NG afferents to their central terminals is enhanced in hypertension. However, a possibility exists that the increased brainstem levels of BDNF do not originate in NG afferents, but in the brainstem itself or other brainstem projections.
There are several lines of evidence that hypertension results in increased baroafferent activity. First, direct recordings in rabbits show that baroafferent activity is dramatically upregulated in both myelinated and unmyelinated fibers of hypertensive animals (Jones and Thoren, 1977). Nota bene, baroreceptor afferents in rabbits behave much like those in rats (Thoren et al., 1999). Second, although myelinated baroreceptors reset during chronic hypertension to have higher thresholds and lower sensitivity to pressure, the extent of resetting (i.e., the ratio of change in pressure threshold to change in mean arterial pressure) is substantially less than 1, suggesting that myelinated fibers are more active at prevailing hypertensive levels of arterial pressure (Andresen et al., 1978; Munch et al., 1983). Third, aortic unmyelinated baroreceptors do not reset during the early established phase of hypertension in SHRs (Thoren et al., 1983). Lastly, an increased cardiac output at the initial stages of hypertension development results in an increase in the blood pressure amplitude, likely leading to increased baroreceptor firing rates (Rau and Elbert, 2001). Based on this evidence, it has been hypothesized that an afferent activity-dependent mechanism is responsible for hypertension-mediated plastic changes at NTS synapses (Aicher et al., 2003; Hermes et al., 2008), and previous studies provide evidence for such changes. Namely, Aicher and colleagues (2003) demonstrated increased number of GluR1-containing dendritic spines and increased total number of spines specifically in baroafferent-relay regions of the NTS after the development of hypertension in SHRs, compared to WKY controls. Overall, the synapses in SHRs showed evidence of dynamic synaptic remodeling (Aicher et al., 2003). In the follow-up study, the same group more definitively linked the synaptic changes to hypertension by examining another rat model of hypertension, i.e. DOCA-salt-sensitive rats (Hermes et al., 2008), as in our present study. However, the molecular mechanisms of the hypertension-induced synaptic remodeling remained unknown.
BDNF has a well-established role in increasing AMPA glutamate receptor expression, including the GluR1 subunit, in various parts of the central nervous system (Narisawa-Saito et al., 1999; Narisawa-Saito et al., 2002; Caldeira et al., 2007). Equally well established are BDNF effects promoting dendritic spine formation (Murphy et al., 1998; Shimada et al., 1998; Kumar et al., 2005). Our present findings, taken together with these data, strongly suggest that the hypertension-mediated increases in GluR1 receptor expression and spine formation are mediated by BDNF.
Our earlier studies show that BDNF is a potent inhibitor of glutamatergic AMPA currents in a large subset of second-order sensory neurons from the newborn rat NTS (Balkowiec et al., 2000). More recent studies demonstrate a similar effect of BDNF on AMPA receptor-mediated synaptic transmission in adult rat NTS slices (Clark et al., 2011). Other studies show that inhibition of AMPA receptors leads to their accumulation at synapses by rapid membrane trafficking and by regulating the turnover of postsynaptic AMPA receptors. Within minutes, this leads to pronounced increases in synaptic efficacy (O’Brien et al., 1998; Lissin et al., 1999). These data from other systems suggest that BDNF, by inhibiting AMPA currents in second-order sensory neurons in the NTS, leads to increased AMPA receptor density in these neurons, as seen in hypertension.
It is well established that the operational range of pressures to which the baroreceptors respond resets in the direction of a sustained change in blood pressure (Kunze, 1981; Heesch et al., 1984a; Heesch et al., 1984b; Kunze, 1986; Andresen and Yang, 1989; Xie et al., 1991; Thrasher, 2004; Brooks and Sved, 2005). Acute resetting, which results in a 30–40% shift, begins within seconds and concludes within a few minutes. On the other hand, chronic resetting of baroreceptors takes several weeks and causes further shifts in the curve in the direction of the pressure change, but is likely incomplete (Thrasher, 2004). In multiple forms of hypertension, the baroreceptors clearly reset (Kunze, 1981; Heesch et al., 1984a; Heesch et al., 1984b; Kunze, 1986; Andresen and Yang, 1989; Xie et al., 1991; Brooks and Sved, 2005), albeit incompletely (Lohmeier et al., 2010). Despite this resetting of the baroreceptor afferents, hypertension-induced resetting of baroreflex control of sympathetic nerve activity can be minimal, particularly early in hypertension development (Barrett et al., 2003; Barrett et al., 2005; Armitage et al., 2012). Yet, the molecular mechanisms by which the brain counteracts baroreceptor resetting are unknown. Based on the present results, we speculate that increased arterial pressure could produce sustained increases in BDNF in the NTS, which, in turn, increase AMPA receptor expression and synaptic strength, thereby effectively counteracting baroreceptor resetting (i.e., a relative reduction in baroreceptor afferent activity for a given level of arterial pressure). BDNF is known to acutely inhibit glutamatergic currents in NTS slices. However, the magnitude of the inhibition is approximately 20% (Clark et al., 2011), compared with the almost two-fold increase in the density of puncta labeled for the GluR1 subunit of AMPA receptors in hypertensive animals (Aicher et al., 2003). Even though the increase in GluR1 density does not necessarily equate to an increase in sensitivity to glutamate, the final outcome of BDNF action is likely to be an increase in synaptic strength, which counteracts the reduced afferent baroreceptor input due to resetting. Our ongoing studies using RNA interference in vivo will provide the definitive answer regarding the net effect of BDNF in baroreceptor resetting.
It is also interesting that different components of the reflex show differential sensitivity to the resetting phenomenon. For example, while the cardiac component resets completely, the renal sympathetic activity appears to be resistant to resetting (Head and Burke, 2001; Barrett et al., 2003; Barrett and Malpas, 2005). Our previous study indicates that BDNF is expressed only by a subset of NG afferents in normotensive animals and preferentially in myelinated AΔ-type neurons (Martin et al., 2009), known to reset to blood pressure changes. Thus, the differential expression of BDNF could contribute to the differential sensitivity of individual components of the baroreceptor reflex to resetting. Moreover, it is also possible that hypertension, by recruiting previously silent neurons, increases BDNF expression at new or previously silent synapses. Neuronal connections in the NTS are characterized by an extremely high level of complexity and functional diversity of second-order sensory neurons that include both excitatory and GABA-ergic inhibitory populations (Andresen et al., 2001; Jin et al., 2004; Bailey et al., 2006). Thus, despite the fact that BDNF seems to be an ideal candidate as a factor that counteracts resetting of baroreceptors, the details of the cellular/molecular mechanisms remain to be determined.
In conclusion, our present data identify hypertension as a natural condition that leads to increased BDNF in visceral primary sensory neurons and their central targets in the dorsal medulla. In view of the well-documented role of BDNF in activity-dependent synaptic plasticity in other systems, our data point to BDNF as a likely candidate mediator of the hypertension-induced remodeling of baroafferent pathways that counteracts baroreflex resetting, and/or cardiorespiratory reflexes in general. In addition to a potentially high clinical significance of these findings, our study has identified a new model for analysis of activity-dependent regulation of BDNF expression and synaptic effects of BDNF in vivo in this well-controllable blood pressure regulation system.
Acknowledgments
GRANT INFORMATION: This work was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL076113 to AB, and HL070962 and HL088552 to VLB), the American Heart Association (AB and VLB), and the Medical Research Foundation of Oregon (AB).
The authors thank Dr. Michael C. Andresen for invaluable input on the discussion of the data presented in this paper.
Footnotes
DISCLOSURES: No conflicts of interest, financial or otherwise, are declared by the authors.
References
- Aicher SA, Sharma S, Mitchell JL. Structural changes in AMPA-receptive neurons in the nucleus of the solitary tract of spontaneously hypertensive rats. Hypertension. 2003;41:1246–1252. doi: 10.1161/01.HYP.0000069007.98987.E0. [DOI] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Doyle MW, Jin YH, Bailey TW. Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Ann NY Acad Sci. 2001;940:132–141. doi: 10.1111/j.1749-6632.2001.tb03672.x. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Krauhs JM, Brown AM. Relationship of aortic wall and baroreceptor properties during development in normotensive and spontaneously hypertensive rats. Circ Res. 1978;43:728–738. doi: 10.1161/01.res.43.5.728. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius--gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang M. Arterial baroreceptor resetting: contributions of chronic and acute processes. Clin Exp Pharmacol Physiol Suppl. 1989;15:19–30. doi: 10.1111/j.1440-1681.1989.tb02993.x. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Kunze DL, Katz DM. Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons. J Neurosci. 2000;20:1904–1911. doi: 10.1523/JNEUROSCI.20-05-01904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CJ, Guild SJ, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension. 2005;46:168–172. doi: 10.1161/01.HYP.0000168047.09637.d4. [DOI] [PubMed] [Google Scholar]
- Barrett CJ, Malpas SC. Problems, possibilities, and pitfalls in studying the arterial baroreflexes’ influence over long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R837–R845. doi: 10.1152/ajpregu.00456.2004. [DOI] [PubMed] [Google Scholar]
- Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res. 2003;92:1330–1336. doi: 10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- Brady R, Zaidi SI, Mayer C, Katz DM. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J Neurosci. 1999;19:2131–2142. doi: 10.1523/JNEUROSCI.19-06-02131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks VL, Freeman KL, Qi Y. Time course of synergistic interaction between DOCA and salt on blood pressure: roles of vasopressin and hepatic osmoreceptors. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1825–R1834. doi: 10.1152/ajpregu.00068.2006. [DOI] [PubMed] [Google Scholar]
- Brooks VL, Sved AF. Pressure to change? Re-evaluating the role of baroreceptors in the long-term control of arterial pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R815–R818. doi: 10.1152/ajpregu.00012.2005. [DOI] [PubMed] [Google Scholar]
- Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006;99:1338–1350. doi: 10.1111/j.1471-4159.2006.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, Carvalho AL, Esteban JA, Duarte CB. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2007;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Horowitz JM, Bonham AC. A presynaptic mechanism contributes to depression of autonomic signal transmission in NTS. Am J Physiol Heart Circ Physiol. 1999;277:H1350–H1360. doi: 10.1152/ajpheart.1999.277.4.H1350. [DOI] [PubMed] [Google Scholar]
- Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J Neurosci. 2011;31:12318–12329. doi: 10.1523/JNEUROSCI.0746-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol. 2003;71:359–384. doi: 10.1016/j.pneurobio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol. 2001;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nature Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Abboud FM, Thames MD. Acute resetting of carotid sinus baroreceptors. II. Possible involvement of electrogenic Na+ pump. Am J Physiol Heart Circ Physiol. 1984a;247:H833–H839. doi: 10.1152/ajpheart.1984.247.5.H833. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Thames MD, Abboud FM. Acute resetting of carotid sinus baroreceptors. I. Dissociation between discharge and wall changes. Am J Physiol Heart Circ Physiol. 1984b;247:H824–H832. doi: 10.1152/ajpheart.1984.247.5.H824. [DOI] [PubMed] [Google Scholar]
- Hermes SM, Mitchell JL, Silverman MB, Lynch PJ, McKee BL, Bailey TW, Andresen MC, Aicher SA. Sustained hypertension increases the density of AMPA receptor subunit, GluR1, in baroreceptive regions of the nucleus tractus solitarii of the rat. Brain Res. 2008;1187:125–136. doi: 10.1016/j.brainres.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HY, Robertson CL, Vermehren-Schmaedick A, Balkowiec A. Nitric oxide regulates BDNF release from nodose ganglion neurons in a pattern-dependent and cGMP-independent manner. J Neurosci Res. 2010;88:1285–1297. doi: 10.1002/jnr.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jenkins VK, Pricher M, O’Donaughy T, Hsieh HY, Brooks VL, Balkowiec A. Activity-dependent regulation of BDNF expression in nodose ganglia of DOCA-salt hypertensive rats. Soc Neurosci Abstr. 2007:139.11. [Google Scholar]
- Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JV, Thorén PN. Characteristics of aortic baroreceptors with non-medullated afferents arising from the aortic arch of rabbits with chronic renovascular hypertension. Acta Physiol Scand. 1977;101:286–293. doi: 10.1111/j.1748-1716.1977.tb06010.x. [DOI] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze DL. Rapid resetting of the carotid baroreceptor reflex in the cat. Am J Physiol Heart Circ Physiol. 1981;241:H802–H806. doi: 10.1152/ajpheart.1981.241.6.H802. [DOI] [PubMed] [Google Scholar]
- Kunze DL. Acute resetting of baroreceptor reflex in rabbits: a central component. Am J Physiol Heart Circ Physiol. 1986;250:H866–H870. doi: 10.1152/ajpheart.1986.250.5.H866. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Carroll RC, Nicoll RA, Malenka RC, Von Zastrow M. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen CY, Bonham AC. Metabotropic glutamate receptors depress vagal and aortic baroreceptor signal transmission in the NTS. Am J Physiol Heart Circ Physiol. 1998;275:H1682–H1694. doi: 10.1152/ajpheart.1998.275.5.H1682. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen CY, Bonham AC. Frequency limits on aortic baroreceptor input to nucleus tractus solitarii. Am J Physiol Heart Circ Physiol. 2000;278:H577–H585. doi: 10.1152/ajpheart.2000.278.2.H577. [DOI] [PubMed] [Google Scholar]
- Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol. 2010;299:H402–H409. doi: 10.1152/ajpheart.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M, Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends Pharmacol Sci. 2003;24:116–121. doi: 10.1016/S0165-6147(03)00025-7. [DOI] [PubMed] [Google Scholar]
- Martin JL, Brown AL, Balkowiec A. Glia determine the course of brain-derived neurotrophic factor-mediated dendritogenesis and provide a soluble inhibitory cue to dendritic growth in the brainstem. Neuroscience. 2012;207:333–346. doi: 10.1016/j.neuroscience.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Jenkins VK, Hsieh HY, Balkowiec A. Brain-derived neurotrophic factor in arterial baroreceptor pathways: implications for activity-dependent plasticity at baroafferent synapses. J Neurochem. 2009;108:450–464. doi: 10.1111/j.1471-4159.2008.05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch PA, Andresen MC, Brown AM. Rapid resetting of aortic baroreceptors in vitro. Am J Physiol Heart Circ Physiol. 1983;244:H672–H680. doi: 10.1152/ajpheart.1983.244.5.H672. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda SA, Mack KJ. Seizures and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Brain Res Mol Brain Res. 2000;78:1–14. doi: 10.1016/s0169-328x(00)00054-1. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Brain-derived neurotrophic factor regulates the expression of AMPA receptor proteins in neocortical neurons. Neuroscience. 1999;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Iwakura Y, Kawamura M, Araki K, Kozaki S, Takei N, Nawa H. Brain-derived neurotrophic factor regulates surface expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors by enhancing the N-ethylmaleimide-sensitive factor/GluR2 interaction in developing neocortical neurons. J Biol Chem. 2002;277:40901–40910. doi: 10.1074/jbc.M202158200. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- O’Donaughy TL, Brooks VL. Deoxycorticosterone acetate-salt rats: hypertension and sympathoexcitation driven by increased NaCl levels. Hypertension. 2006;47:680–685. doi: 10.1161/01.HYP.0000214362.18612.6e. [DOI] [PubMed] [Google Scholar]
- O’Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension. 2006;48:658–663. doi: 10.1161/01.HYP.0000238140.06251.7a. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nature Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Rau H, Elbert T. Psychophysiology of arterial baroreceptors and the etiology of hypertension. Biol Psychol. 2001;57:179–201. doi: 10.1016/s0301-0511(01)00094-1. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Zhang J, Toney GM, Mifflin SW. Temporal processing of aortic nerve evoked activity in the nucleus of the solitary tract. J Neurophysiol. 1996;76:3750–3757. doi: 10.1152/jn.1996.76.6.3750. [DOI] [PubMed] [Google Scholar]
- Shimada A, Mason CA, Morrison ME. TrkB signaling modulates spine density and morphology independent of dendrite structure in cultured neonatal Purkinje cells. J Neurosci. 1998;18:8559–8570. doi: 10.1523/JNEUROSCI.18-21-08559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi A. Synaptic plasticity-regulated gene expression: a key event in the long-lasting changes of neuronal function. Biol Pharm Bull. 2008;31:327–335. doi: 10.1248/bpb.31.327. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem. 2000;275:17269–17275. doi: 10.1074/jbc.M909538199. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tarsa L, Balkowiec A. Nerve growth factor regulates synaptophysin expression in developing trigeminal ganglion neurons in vitro. Neuropeptides. 2009;43:47–52. doi: 10.1016/j.npep.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoren P, Andresen MC, Brown AM. Resetting of aortic baroreceptors with non-myelinated afferent fibers in spontaneously hypertensive rats. Acta Physiol Scand. 1983;117:91–97. doi: 10.1111/j.1748-1716.1983.tb07182.x. [DOI] [PubMed] [Google Scholar]
- Thoren P, Munch PA, Brown AM. Mechanisms for activation of aortic baroreceptor C-fibres in rabbits and rats. Acta Physiol Scand. 1999;166:167–174. doi: 10.1046/j.1365-201x.1999.00556.x. [DOI] [PubMed] [Google Scholar]
- Thrasher TN. Baroreceptors and the long-term control of blood pressure. Exp Physiol. 2004;89:331–335. doi: 10.1113/expphysiol.2004.027441. [DOI] [PubMed] [Google Scholar]
- Tropea D, Capsoni S, Tongiorgi E, Giannotta S, Cattaneo A, Domenici L. Mismatch between BDNF mRNA and protein expression in the developing visual cortex: the role of visual experience. Eur J Neurosci. 2001;13:709–721. doi: 10.1046/j.0953-816x.2000.01436.x. [DOI] [PubMed] [Google Scholar]
- Wetmore C, Olson L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J Comp Neurol. 1995;353:143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]
- Xie PL, McDowell TS, Chapleau MW, Hajduczok G, Abboud FM. Rapid baroreceptor resetting in chronic hypertension. Implications for normalization of arterial pressure. Hypertension. 1991;17:72–79. doi: 10.1161/01.hyp.17.1.72. [DOI] [PubMed] [Google Scholar]