Summary
Natural Killer (NK) cells were first identified for their capacity to reject bone marrow allografts in lethally irradiated mice without prior sensitization. Subsequently, human NK cells were detected and defined by their non-major histocompatibility complex (MHC)-restricted cytotoxicity towards transformed or virally infected target cells. Karre and colleagues later proposed ‘the missing self hypothesis’ to explain the mechanism by which self-tolerant cells could kill targets that had lost self of MHC class I. Subsequently, the receptors that recognize MHC class I to mediate tolerance in the host were identified on NK cells. These class I-recognizing receptors contribute to the acquisition of function by a dynamic process known as NK cell education or licensing. In the past, NK cells were assumed to be short lived, but more recently NK cells have been shown to mediate immunologic memory to secondary exposures to cytomegalovirus (CMV) infection. Because of their ability to lyse tumors with aberrant MHC class I expression and to produce cytokines and chemokines upon activation, NK cells may be primed by many stimuli, including viruses and inflammation, to contribute to a graft-versus-tumor effect. In addition interactions with other immune cells support the therapeutic potential of NK cells to eradicate tumor and to enhance outcomes after hematopoietic cell transplantation (HCT).
Keywords: NK cells, innate immunity, killer immunoglobulin-like receptors, transplantation, leukemia, cytomegalovirus
Human NK cell development
The earliest stages of lymphoid cell development take place in the bone marrow where a subpopulation of multi-potent CD34+ hematopoietic progenitor cells gives rise to T, B, natural killer (NK) and dendritic cells (1). During the maturation process, CD34 expression wanes as the expression of lineage-specific markers increases. Irreversible commitment to the NK cell lineage is associated with the loss of CD34 and the acquisition of the natural cytotoxicity receptors NKp44 and NKp46 (2). Further NK cell differentiation is marked by surface expression of CD56. Two phenotypically and functionally distinct populations of peripheral blood NK cells, which comprise ~10-15% of circulating lymphocytes, can be discriminated based on their CD56 surface density levels (3). Of these NK cells, approximately 2-10% have high surface density expression of CD56 and are designated CD56bright. This subset proliferates robustly upon IL-2 stimulation (3), produces high levels of inflammatory cytokines (4), and lacks expression of CD16 (4) and killer immunoglobulin-like receptors (KIRs) (5). While they comprise a minor fraction of total peripheral blood and spleen NK cells, CD56bright cells are significantly enriched in secondary lymphoid tissues where they are presumed to differentiate into CD56dim NK cells (6, 7). Around 90% of the NK cells in peripheral blood are classified as CD56dim. This subset demonstrates limited proliferative potential upon ex vivo interleukin-2 (IL-2) or IL-15 stimulation and expresses CD16, KIR, and the maturation marker CD57, and contains an abundance of cytotoxic granules that arm them for effector function (5, 8, 9). The differentiation process into bright and dim NK cells can be recapitulated in vitro with the use of stromal cells and exogenous cytokines (10-13).
IL-15 is typically regarded as the central cytokine promoting the development of NK cells in vivo, as mice lacking IL-15 (14), IL-15Rα (15), or STAT5 (16) exhibit severe NK cell deficiencies. Although high-dose soluble IL-15 supports NK cell differentiation and proliferation in vitro (17), IL-15 primarily exists in a complex with IL-15Rα in vivo and functions as a membrane-bound ligand on accessory cells that can activate NK cells in trans (18, 19). This trans-presentation of IL-15 stabilizes the cytokine in vivo for physiologic activation of NK cells and CD8+ T-cells (20).
NK cell receptors
NK cells express an array of activating and inhibitory receptors that finely tune their effector function. There are two main types of inhibitory receptors expressed by NK cells that recognize human leukocyte antigen (HLA) molecules: killer immunoglobulin-like receptors (KIR) that recognize HLA-A, HLA-B, or HLA-C allotypes and CD94/NKG2A, a heterodimer that recognizes HLA-E (21). Both NKG2A and inhibitory KIRs have long cytoplasmic tails containing tandem immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which are phosphorylated upon crosslinking, resulting in the recruitment of tyrosine phosphatases that inhibit NK cell activation (22, 23). When NK cells interact with cells that have reduced HLA expression as a consequence of viral infection or transformation they are released from inhibition. This tips the signaling balance toward activation, allowing NK cells to exert their cytotoxic and cytokine production functions.
Activating KIRs have short cytoplasmic tails that associate non-covalently with the DAP12 signaling adapter. DAP12 is recruited as a homodimer and contains an immunoreceptor tyrosine-based activation motif (ITAM). Cross-linking of KIR-DAP12 complexes leads to activation through the recruitment of SYK and ZAP70 protein tyrosine kinases (24). The ligands for activating KIR are also believed to be HLA mimics or allotypes (21). The conditions under which these interactions have physiological relevance remain somewhat enigmatic but appear to be influenced by viral peptides (25) or viral-encoded class I MHC like molecules.
KIR mRNA transcripts were discovered through subtractive hybridization in 1995 (26-28). Since then, fifteen KIR genes and two pseudogenes have been identified within the KIR locus on chromosome 19. However, individuals differ in the number of KIR genes that are contained within their genome, creating haplotypes. Two groups of KIR haplotypes have been distinguished and are found at varying frequencies within different ethnic groups. The Group A haplotype contains mainly inhibitory KIR and only one activating KIR. Group B KIR haplotypes are comprised by other gene content with more activating KIR (29). A remarkable amount of allelic and haplotypic variability has evolved within the KIR locus through extensive deletion/duplication, intergenic sequence exchange and unequal crossing over (30, 31).
In addition to the genetic diversity, KIR expression is stochastic, and individual NK cells express different numbers and types of KIR in a probabilistic manner (32) that is dependent upon promoter DNA methylation (33). Our group has recently shown that KIR expression is regulated at the transcriptional level through the coordinated activities of a bi-directional proximal promoter, a distal promoter element located 1 kb upstream of the transcriptional start site and an additional promoter located within intron 2 (34-39). The expression of KIR is a step towards development of a functional NK cell repertoire, but many receptor families interact to modulate the NK cell response.
Besides KIR family genes, NK cells encode a variety of other activating and inhibitory receptors. The NKG2 family is comprised of several members that generally require heterodimerization with CD94 to bind ligands (40). NKG2A is inhibitory and binds HLA-E, while NKG2C is activating and binds HLA-E but with lower affinity (41). Given that HLA-E contains a conserved sequence of HLA-A, -B, and -C (the leader sequence), these NK receptors likely gauge the level of classical class I MHC expressed on a cell. On the other hand, NKG2D is expressed on the cell surface as a homodimer and recognizes nonclassical MHC molecules (MICA and MICB) as well as other non-MHC molecules (ULBP1-6) typically upregulated in response to stresses such as viral infection or tumor formation (42). Another family of inhibitory receptors, the leukocyte immunoglobulin-like receptors (LILRs), also bind several classical and nonclassical HLA class I molecules as well as UL-18, a CMV-encoded glycoprotein (43, 44). The best-characterized LILR family member is LILRB1 (LIR-1). Natural cytotoxicity receptors (NCRs) are usually activating and, in the case of NKp44 and NKp46, can bind viral hemagglutinins (45, 46). Other NCRs, such as NKp30, can also recognize a variety of other tumor or heat-shock-associated proteins. Recently, a ligand for NKp44 has been described and shown be expressed in transformed cells (47). Other activating receptors on NK cells include: CD96 and DNAM-1, which bind poliovirus receptors and trigger killing and adhesion; CD160, which triggers cytotoxicity through binding of HLA-C; NKp80, thought to be involved in killing of acute myelogenous leukemia (AML) through recognition of AICL on myeloid cells; CD2, which serves a costimulatory molecule through binding of LFA-3; and SLAM family receptors 2B4 (CD244), which yields activation through binding of CD48, NTB-A, and CRACC, which are homophilic and promote NK cell activation (48, 49). Finally, CD16 (Fcγ receptor III) controls antibody-dependent cell-mediated cytotoxicity (ADCC) in NK cells through recognition of the Fc portion of antibodies (50). Although two isoforms exist in humans, CD16A and CD16B, only CD16A (referred to henceforth as CD16) is expressed on NK cells (51). CD16 is primarily present on the more mature CD56dim subset of NK cells (52). Upon engagement, CD16 potently signals through the ITAMs of the associated FcγRIγ and CD3ζ adapters, inducing both cytokine and cytotoxic responses (53-55). NK cell-mediated ADCC plays a role in response to viral infection, autoimmunity, and in protection from some forms of tumors (56-58). Thus, NK cell-mediated ADCC through utilization of monoclonal antibodies (mAbs) specific for tumor antigens is a valuable therapeutic tool (59-63).
NK cell education and tolerance
The development of NK cell function and self tolerance is a complex process involving signaling via inhibitory receptors. Several theories to explain how inhibitory receptor recognition of cognate MHC ligand (HLA in humans) affects this process (64-68). For the purpose of this review, we refer to this process as NK cell education, but the terms licensing, arming, and rheostat tuning have been used interchangeably. The expression of inhibitory receptors during development of human NK cells is critical for acquisition of function. Individual NK cells that do not express inhibitory receptors in particular KIR, and to a lesser extent NKG2A, lack the necessary signaling required to undergo education and remain hypo-responsive (13, 66, 69). For example, NK cells expressing the inhibitory KIR3DL1+ from an individual with its ligand HLA-Bw4 exhibit greater function than do KIR3DL1+ NK cells from an individual homozygous for HLA-Bw6 (lacking HLA-Bw4).
The mechanisms that lead to augmented NK cell function following NK education remain poorly understood. Vivier and colleagues (70) have shown that activating receptors are confined to nanodomains in the plasma membrane of educated NK cells, whereas in uneducated cells activating receptors remain within the actin meshwork. The education process appears to be dynamic, as mature, uneducated NK cells put into an environment with self-ligands will become quickly educated, while the opposite is true if educated NK cells are placed in an environment devoid of self-ligands (71, 72). Of note, a ligand-instructed model of KIR acquisition has been proposed, whereby initial education of an NK cell by a self-ligand through a KIR can influence subsequent acquisition of other KIR specific for the same HLA-ligand (73). A more recent study showed that, although KIR gene dose did not have an effect on education at the single cell level, KIR gene copy number variation affected the frequency of educated NK cells found within peripheral blood (74). In an MHC transgenic in vivo model used to model education in an MHC dose-dependent manner, greater class I engagement led to enhanced NK cell survival and skewing of the NK cell repertoire to favor circulating cells educated through Ly49 engagement of MHC (75). Unpublished data from our group confirms these findings in humans. Given the functional and survival advantages associated with NK cell education, it is unclear whether uneducated cells have a distinct biological role or if they are just developmental intermediates waiting to become educated or to be deleted if unstimulated. One study seems to indicate that uneducated NK cells are important during viral responses (at least to CMV) in mice (76). Yet, we and others have shown that CMV infection in humans results in expansion of educated subsets, indicating that NK cells with specificity for self-HLA ligand also play a role during human CMV infection (77-79). Although major biologic paradigms are the same between mouse and man, this example highlights the extent to which individual differences may define important interspecies differences
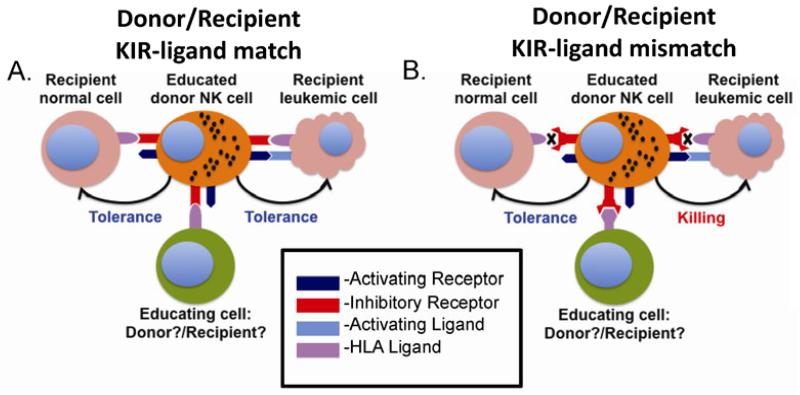
In hematopoietic cell transplantation (HCT), NK cell education and the resulting acquisition of function are necessary to render cells alloreactive against tumor targets missing self-HLA ligand. Uneducated or hyporesponsive NK cells do not contribute to the clinical benefits of alloreactive NK cells (80). It is clear that after HCT the education and function of engrafting alloreactive cells is affected by interactions with Class I HLA. However some details about the mechanisms underlying this education are not fully understood. For example, HLA expression on either donor or recipient hematopoietic cells can influence the function of engrafting NK cells (Fig. 1). The ability of HLA ligands presented on recipient non-hematopoietic tissues to subtly tune NK cell education underscores the complexity of this process (72). In addition, after transplantation donor NK cells may be educated by hematopoietic cells transferred with the donor graft itself (81-84). Furthermore, our understanding of the rules governing the maintenance of self tolerance is incomplete. Substantial evidence supports the ligand instructed model of inhibitory KIR (73) and Ly49 (75, 85-87) expression, which proposes that MHC can modulate the inhibitory repertoire formation on NK cells. In murine models of hybrid resistance this process has been shown to be mediated by the donor bone marrow indicating that donor MHC is responsible for control of inhibitory receptor expression. Yet some studies indicate that residual recipient NK cells, if present in adequate quantities, may eliminate allogeneic donor stem cells, a situation in which the rules of the marrow instruction model do not apply (88-91). One interesting study has demonstrated that while infusion of a low marrow cell dose is not adequate to prevent host elimination of donor NK cells, a high marrow cell dose can induce recipient tolerance to the donor NK cells. It is postulated that the high exposure to donor MHC results in reprogramming of NK cell tolerance from the recipient to the donor. Additional studies have demonstrated that donor instruction by donor MHC can direct the recipient NK cells to tolerate the allogeneic stem cells (92-94). Differences in these studies might stem from a disparity in the number of cells transplanted and the associated treatment of recipients or donor cells, which might provoke increased stress ligands, resulting in decreased induction of NK cell tolerance.
Fig. 1. Role of KIR ligand mismatch in GVL.
This figure illustrates the role of reconstituting NK cells after transplantation. (A) In the setting of KIR-ligand (e.g. HLA/MHC) match, reconstituting NK cells can be educated by class I MHC presentation by donor hematopoietic cells, recipient non-hematopoietic cells or both. This leads to a gain of function. In the absence of KIR ligand mismatch or a missing self setting, donor NK cells would be tolerant against recipient residual leukemia despite expression of activating ligands. Normal recipient tissue is also tolerant based on both inhibition by MHC class I and lack of activating ligands. (B) In contrast, a KIR ligand mismatch in the graft versus host direction results in missing self combined with NK cell activation of stress ligands (e.g. MICA/B) leads to recipient leukemia killing and protection from relapse. Normal tissue is still tolerant based on absence of activating ligands despite absence of inhibition through class I MHC receptors. If educating cells are missing or contain mismatched ligands for inhibitory receptors, donor NK cells will be hypo-responsive and will not be capable of killing leukemic targets, even if inhibitory signaling is disrupted and activating signals are triggered.
After HCT, donor NK cell tolerance to recipient leukemic cells can be broken by increased expression of activating ligands to NKG2D, DNAM-1, and NCRs on the leukemic cells (95-98). Alternatively, loss of inhibitory signaling in the setting of donor KIR recipient KIR ligand (HLA) mismatch can also break tolerance. Residual recipient leukemic cells which express activating ligands can be targeted by educated NK cells from an alloreactive donor to reduce relapse. Engrafting NK cells are tolerant to healthy non-hematopoietic tissues in the recipient, which do not express activating ligands. Furthermore, alloreactive NK cells may eliminate host dendritic cells that activate allogeneic T cells (99, 100) to reduce graft vs. host disease. The role of NK cells in mediating tolerance or rejection after solid organ transplantation is not well defined (101).
NK cell reconstitution after HCT
For NK cells to mediate an effect, they must develop in vivo and acquire function. Their mere presence is not enough to impact on clinical outcomes. It is well accepted that NK cells are the first lymphocyte to reconstitute following HCT. The maturation and functional properties of these reconstituting NK cells may depend on donor source, different functional capabilities of the NK cells in the graft itself and NK cells that develop from graft stem cells, which is difficult to distinguish in vivo. Compared to normal donor NK cells, early engrafting NK cells are developmentally immature. This relative immaturity is characterized by altered receptor profiles including higher NKG2A expression, lower KIR and NCR expression, and lower than normal overall function (102, 103).
Our group (104) compared NK cell function and receptor repertoires following HCT with three different graft preparations: unmanipulated (T-cell-replete) adult grafts, CD34+ selected (T-cell-deplete) adult grafts, and umbilical cord blood (UCB). All groups were treated with standard graft-versus-host disease immunosuppressive prophylaxis except the CD34+ selected grafts that were already T-cell-depleted. Across all groups, K562 target cell-mediated IFNγ production was severely impaired compared to healthy donors, while CD107a degranulation was only slightly impaired post-HCT. By 6 months post-HCT, target cell-mediated IFNγ production by NK cells from recipients of unmanipulated or CD34+ selected grafts approached the level observed in NK cells from the normal volunteer donors. In contrast, the IFNγ response of NK cells from recipients of UCB grafts remained low. This defect was specific to target cell-induced responses, as IFNγ production by NK cells was potent after transplantation with all graft sources following IL-12 and IL-18 stimulation. This is not surprising, as these NK cells have a developmentally immature phenotype similar to CD56bright NK cells in healthy donors that are high producers of IFNγ when stimulated with IL-12 and IL-18 (105). In all groups, NKG2A expression was increased and KIR expression was low. Surprisingly, the group with the highest level of KIR expression was observed in the unmanipulated graft group, where KIR expression correlated with better function. This confirmed earlier observations by Nguyen and colleagues (106) that recipients who received partial T-cell-depleted haploidentical grafts had lower NKG2A expression and higher cytotoxicity against AML blasts than recipients of fully T-cell-depleted grafts. This may be due to help from T cells, which are known to produce IL-2 and stimulate CD56bright NK cells in the lymph nodes to produce IFNγ (6). Alternatively, donor NK cells in unmanipulated grafts may persist for much longer than previously thought and may be responsible for the increased function. In summary, the recovery of normal function and receptor repertoires may take at least 1 year after HCT. Enhancing NK cell function post-HCT has the potential to enhance the graft-versus-leukemia (GvL) effect and viral immune responses as well as to prevent graft-versus-host disease (GvHD) by deleting host antigen-presenting cells.
NK cell alloreactivity and donor KIR affects outcome after HCT
Although NK cells have been known to play a role in HCT, it was not until the characterization of NK cell inhibitory receptors and their recognition of self class I HLA that researchers could elucidate and exploit the natural properties of NK cells to mediate GvL effects to improve allogeneic transplant outcomes. Donor NK cells expressing inhibitory KIR for which the recipient does not express the class I HLA ligand (a situation of missing self) will not be inhibited and therefore, could lyse residual leukemic cells in the recipient (Fig. 1). This would be particularly common in haploidentical transplants where the donor and recipient are only matched for one HLA haplotype. The therapeutic utility of NK cell alloreactivity was first demonstrated in a 2002 study by the Perugia group (82). Transplants with predicted donor NK cell alloreactivity were associated with decreased graft rejection, enhanced engraftment, decreased relapse and increases in overall survival in the absence of GvHD. Of the 92 patients with AML, 34 patients were predicted to have NK cell alloreactivity in the GvH direction. Thus, their NK cells were considered mismatched with the recipient. The probability of overall survival at 5 years was 65% in these patients compared to 5% for those patients who were not predicted to have NK cell alloreactivity. No beneficial effect of NK cell mismatching was seen in patients with acute lymphoblastic leukemia (ALL), perhaps due to the lack of the cell adhesion molecule LFA-1 on the surface of ALL blasts (81) or the fact that ALL blasts express higher levels of class I HLA then AML blasts. In a follow up study published in 2007 (83), KIR-ligand mismatching still resulted in decreased relapse and increased overall survival, but other effects were not sustained suggesting that the biology of this system is complex.
Several subsequent studies evaluated the KIR-ligand incompatibility model. However, not all studies detected a beneficial effect of NK cell alloreactivity, perhaps due to differences in the transplant platforms studied. Reports from Davies et al. (107) using adult unrelated donors found no effect of KIR ligand mismatching, whereas Giebel and colleagues (108), using a similar cohort but with T-cell depletion in vivo using anti-thymocyte globulin, reported improved overall survival with KIR ligand mismatching. In a large study of over 1500 unrelated transplants for CML, AML, and MDS, predicted NK cell alloreactivity was not associated with protection from relapse (109). Yet in another large cohort of 1770 patients receiving fully ablative T-cell-replete transplants for hematologic disease, a beneficial effect was observed when HLA-B and C mismatches between donor and recipient were present and resulted in KIR ligand absence in the recipient, suggesting better NK cell activity against residual tumor (110). Interestingly, recipient HLA-C2 homozygosity correlated with increased relapse and low overall survival (111), a setting discussed more in subsequent sections below. In a third large cohort of over 2000 T-cell-replete unrelated transplant recipients, the absence of 1 or more KIR ligands in the recipient was associated with less relapse in AML patients in first complete remission (CR1) and in CML patients in the first chronic phase (112). However, there was no effect in advanced disease, and KIR ligand absence was associated with an increase in chronic GvHD in patients with CML. Despite differences between these studies, one conclusion is firm: NK cells play a role in transplantation outcome. The individual differences between studies may merely point out the complexity of the transplant platform and the underlying NK cell biology.
KIR ligand incompatibility has been tested beyond donor transplants with adult cells. In recipients of UCB grafts, where most individuals received T-cell depletion in vivo with anti-thymocyte globulin, KIR ligand mismatching was reported by Willemze et al. (113) to be associated with reduced risk of leukemic relapse and improved overall survival, whereas Brunstein et al. (114) who used less anti-thymocyte globulin reported different results. After myeloablative conditioning, KIR ligand mismatch had no effect on GvHD, transplantation-related mortality, relapse, or survival. In contrast, after reduced intensity conditioning, KIR ligand mismatch between the engrafted unit and the recipient resulted in significantly higher rates of GvHD [42% (CI, 27-59) vs 13% (CI, 5-21), P < .01] with inferior survival [32% (CI, 15%-59%) vs 52% (CI, 47%-67%), P = .03]. Multivariate analysis identified KIR ligand mismatch as the only predictive factor associated with the development of GvHD and a significant association between KIR ligand mismatch and increased risk of death. The varying reports of beneficial and detrimental effects of NK cell alloreactivity may be explained by several key differences in the cohorts. For example, all grafts in the Perugia study were extensively T-cell-depleted, whereas T-cell depletion methods (or functionally naive T cells in the setting of UCB grafts) and degree of depletion differed amongst the other studies. One could predict that higher T-cell content in the graft may lead to competition for cytokines, and these T cells may initiate GvHD before the alloreactive NK cells are able to mediate their beneficial effects. Secondly, the preparative regimens (fully ablative vs. reduced intensity conditioning) and types of GvHD prophylaxis could also influence NK cell recovery and function post-HCT. Another major difference between published studies has been the degree of HLA disparity with the donor. In the Perugia study, NK cells from donors were all haploidentical. Unrelated and UCB grafts were heterogeneous in their HLA-matching. This is particularly important as donor KIR are predicted to interact with recipient HLA to determine NK cell function, a topic that will be discussed in more detail in subsequent sections.
As an alternative to NK cell predictive models based on HLA-typing alone, we have been exploring the immunogenetics of donor and recipient KIR genes. As described earlier, KIR genes can be divided into two main haplotypes based on gene content: haplotype A and haplotype B. A haplotypes have a fixed gene content of the main inhibitory KIR and only one activating KIR, whereas B haplotypes have invariable gene content comprising of up to 5 activating KIR. The genes on each KIR haplotype can be divided into either centromeric (2DS2, 2DS3/5) or telomeric (2DS1, 3DS1) regions or those that can be on either or both (2DL5 and 2DS3/5). The haplotype gene content is denoted as Tel-A or Cen-A for the A haplotype and Tel-B and Cen-B for the B haplotype. The impact of these haplotypes and the individual KIR within them has been described in several studies.
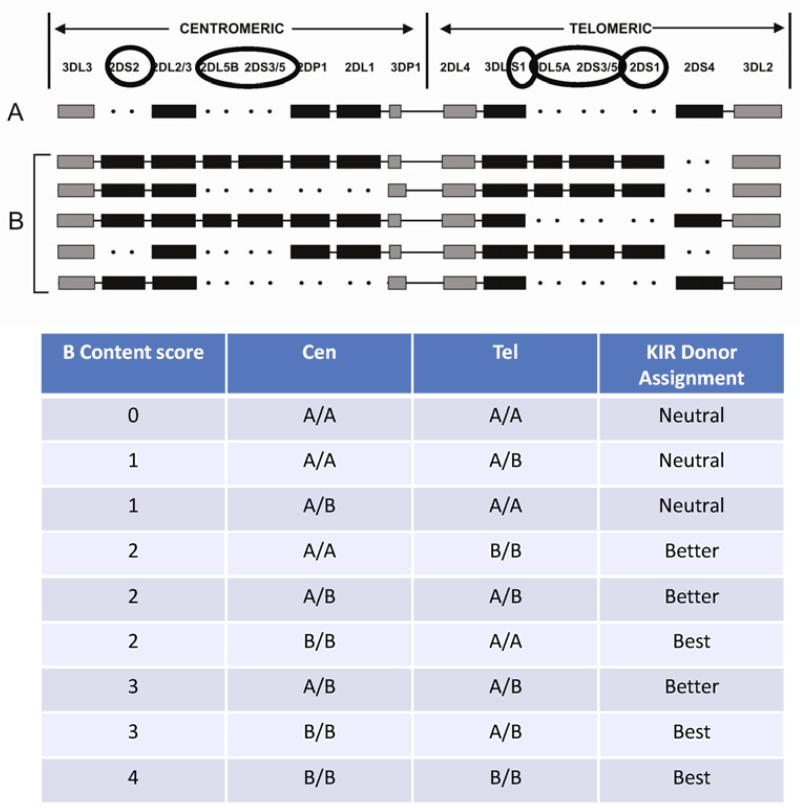
McQueen and colleagues (115) evaluated a small cohort of HLA-matched T-cell-replete sibling transplants and found that transplants from KIR A/A genotype donors into KIR B/x genotype recipients were associated with poorer survival. In another cohort of HLA identical siblings who received T-cell-depleted grafts, Stringaris and colleagues (116) reported a beneficial effect of donor KIR2DS1, KIR3DS1 and KIR2DL5A (all present on the KIR B haplotype) in reducing relapse and overall survival in AML patients. In another study examining KIR B gene and outcomes, donor KIR3DS1 was shown to be protective against acute GvHD, an effect lost when B KIR haplotypes were considered as a group (117). In the largest study at that time, Cooley et al. (118) reported in 2009 on the beneficial effect of unrelated donors with at least one KIR B haplotype on relapse protection and disease-free survival (DFS) for 448 patients with AML. There was no effect of recipient KIR genes. A subsequent analysis in 2010 of 1086 AML and 323 ALL transplant pairs evaluated the relative contributions of centromeric and telomeric KIR B genes (119). Donors with ≥2 centromeric or telomeric KIR B motifs were associated with better outcome. There was no effect when the same strategy was applied to patients with ALL. The best protection from relapse and improved DFS was seen when donors were also homozygous for Cen-B. Thus KIR gene content can be used to define donors as ‘neutral’ (0 or 1 KIR B motifs), ‘better’ (≥2 KIR B motifs but not Cen-B/B), or ‘best’ (≥2 KIR B motifs with Cen-B/B) (Fig. 2). A multicenter prospective trial incorporating KIR genotyping into donor selection coordinated through the National Marrow Donor Program is ongoing.
Fig. 2. The KIR gene cluster is comprised of activating and inhibitory KIR genes on the centromeric or telomeric sides of chromosome 19.
This upper figure depicts the KIR locus on chromosome 19. The individual genes circled depict those defined as KIR B on the centromeric (Cen) or telomeric (Tel) side of the chromosome. The KIR A haplotype has absence of KIR B defining genes. The KIR B haplotype contains at least one of these KIR B motifs. The presence of 1 or 2 KIR B motifs on the Cen or Tel portion determines the B Content score and correlation with relapse protection leads to a KIR Donor Assignment designation (lower table).
Most of the effects of NK cells in transplant relate to relapse and infection protection, but they may influence GvHD. NK cells are assumed not to initiate GvHD directly, but they are found in GvHD lesions and may contribute to the pathology once GvHD is established. In mouse models, alloreactive NK cells were shown to lyse APCs and prevent the initiation of GvHD (81) and were also found to lyse allogeneic DCs in lymph nodes (120). They may also produce cytokines such as TGFβ that help suppress GvHD (121). NK cells may also play a role in regulating T-cell function post-HCT. NK cells were able to control the antigen-driven proliferation of donor CD4+ T cells and lower the incidence of chronic GvHD in mice (122). NK cells may also regulate CD8+ T-cell proliferation by competing for IL-15 (123). Therefore, while NK cells are able to influence GvHD in a more direct manner through various receptors and production of cytokines, they may also modify post-HCT T-cell activation and proliferation and eliminate host DCs before they can initiate GvHD.
KIR interactions with HLA
Inhibitory KIR and activating KIR2DS1 recognize HLA, and it is presumed that differing affinities of binding influence NK cell education. The class I HLA molecules are highly polymorphic and can be divided based on a few differences in the specific amino acids. The HLA-B alleles with the Bw4 epitope can be divided into two groups: those with an isoleucine at position 80 (80I) and those with a threonine (80T). Certain HLA-B alleles with an 80I were found to have a stronger interaction with KIR3DL1 than those with an 80T (124, 125). Presence of 80I has also been implicated in AIDS progression where HIV patients expressing 80I had a slower progression to AIDS than those with an 80T (126). This could suggest that a stronger interaction with KIR3DL1 may promote the development of NK cells with higher function and increased capabilities to eliminate aberrant cells. Certain HLA-A alleles also express the Bw4 epitope and have been found to interact with KIR3DL1 (127-129). Furthermore, donors who have an HLA-A Bw4-expressing allele as their only Bw4 allele have educated KIR3DL1+ NK cells (128). HLA-B*1301 and HLA-B*1302 both express the Bw4 epitope and, therefore, would be predicted to interact with KIR3DL1. However, these HLA haplotypes did not inhibit NK cell function in vitro (128). In support of this finding, donors who expressed either haplotype as their only Bw4 allele did not have any NK cells that were educated through KIR3DL1.
Not all HLA-C alleles behave as predicted either. Yawata and colleagues (130) reported a hierarchical effect on NK cell function dependent on different HLA-C1 alleles. No differences were observed for HLA-C2 alleles. Donors who were homozygous for HLA-C*07 (HLA-C1) were found to be high producers of IFNγ compared to those donors expressing either HLA-C*01, *03, *08 or 1403. Donors who were homozygous for HLA-C*1402 were very poor producers of IFNγ. As described earlier, NK cell education is dependent on the strength of the interactions between KIR and their ligands. Strong interactions result in increased functional potential, whereas weak interactions result in low functional potential. Therefore, certain HLA combinations may result in better NK cell function that could enhance the GvL effect in vivo. Certain HLA-C2 alleles have also been shown to bind to KIR2DL2 and KIR2DL3 (131), and in one study KIR2DL2/3+ NK clones were unable to lyse HLA-C2 homozygote leukemic blasts due to this inhibition (132). Interestingly, the impact of the interaction between KIR2DL2 and HLA-C2 can be observed in vivo. Schonberg and colleagues (73) reported that if a donor was KIR2DL3+ (KIR A haplotype) and HLA-C1 homozygous, a higher proportion of their NK cells would express KIR2DL3. Similar findings were observed for HLA-C2 and KIR2DL1. However, in donors expressing both KIR2DL2 and KIR2DL1 (KIR B haplotypes), NK cells predominately expressed KIR2DL2 with only a low proportion of cells expressing KIR2DL1 irrespective of HLA-C1 or HLA-C2. Since KIR2DL2 is acquired before KIR2DL1 during development, KIR2DL2 interactions with HLA-C2 prior to KIR2DL1 expression may explain the skewing towards KIR2DL2 in KIR B haplotype individuals.
KIR allelic differences may also result in differing KIR/HLA interactions and differing levels of function. The KIR gene locus is highly polymorphic: not only are the KIR genes divided into A and B haplotypes, but several alleles exist for each of the genes, and all alleles do not necessarily behave alike. One of the most polymorphic genes is KIR3DL1 with over 60 alleles described. Different alleles of KIR3DL1 have different surface levels of expression (high and low), with one common allele, KIR3DL1*004, not even being expressed on the surface (133). NK cells with high- or low-expressing KIR3DL1 alleles have been shown to have varying interactions with their ligand Bw4. KIR3DL1*01052 has been shown to be a stronger allele for Bw4 than KIR3DL1*007 (130). Differences in alleles with the similar surface expression have also been reported. KIR3DL1*002 was shown to be a stronger inhibitory receptor than KIR3DL1*007 (134). The difference in the strength of the interaction between these two alleles and Bw4 was attributed to two amino acids: one in the D2 domain and one in the transmembrane region. Strength of binding is also different amongst the KIR-recognizing HLA-C alleles. The interaction between KIR2DL1 and its ligand HLA-C2 results in the strongest inhibitory combination followed by KIR2DL2 then KIR2DL3 (131). Differences in binding strengths between KIR2DL2 and KIR2DL3 may explain the protective effect observed in recipients transplanted with donors homozygous for the centromeric portion of the B haplotype. These donors are homozygous for KIR2DL2 and lack KIR2DL3. If during NK cell education, KIR2DL2 confers a stronger signal, this may result in NK cells with enhanced function that may be able to exert their effects post-transplant. Allelic differences also confer different strengths in binding. All KIR2DL1 alleles have either an arginine or cysteine at position 245. The alleles with arginine have stronger signaling capacity (135). Individuals homozygous for KIR2DL1*004 were found to be low producers of IFNγ (130), suggesting that this allele has a weak interaction with HLA-C2. A recent study compared different alleles of KIR2DL2 and KIR2DL3 and found that KIR2DL3*005 behaved more similarly to KIR2DL2*001 than KIR2DL3*001 and KIR2DL3*002 (136). KIR2DL3*005 had stronger interactions with HLA-C1 and higher IFNγ production.
KIR interactions with HLA affects outcome after HCT
As described earlier, the presence of activating KIR on KIR B haplotypes have been implicated in transplant outcomes. Understanding how each of the activating KIR may contribute to reducing relapse and improving overall disease-free survival is difficult because many of their ligands still are unknown. KIR2DS1, however, has been shown to bind to HLA-C group 2 alleles (25, 137-140) and eliminate leukemic blasts from HLA-C2 homozygous patients (132). In vitro, KIR2DS1 binding to HLA-C2 was found to override NKG2A mediated inhibition (132, 139), a particularly interesting find due to the high expression of NKG2A post transplant. In a recent study, Venstrom and colleagues (141) looked at the presence or absence of KIR genes in 1277 patients undergoing unrelated donor transplantation and found that the presence of KIR2DS1 in the donor (and in most recipients as well) conferred protection from relapse. Protection was abrogated if the donor was HLA-C2 homozygous, as any NK cells expressing KIR2DS1 in those donors would be uneducated and therefore hyporesponsive as predicted by Fauriat et al. (142). In the small proportion of patients who were mismatched at HLA-C, where the recipient was HLA-C2 homozygous and the donor KIR2DS1 positive and the donor had at least one copy of HLA-C1, relapse was lower. Whether this is a KIR2DS1-dependent effect or a gene in linkage disequilibrium with other KIR genes is currently unknown. KIR3DS1, which is in strong linkage disequilibrium with KIR2DS1, is also associated with a reduction in relapse (116, 117) and in non-transplant settings, a role in slowing HIV progression to AIDS (126). Venstrom also found that donor KIR3DS1 positivity was associated with lower relapse. However, this was not sustained when donors were stratified based on KIR2DS1, but KIR3DS1 was still associated with decreased overall mortality in transplant recipients. In any analysis that involves a protection or deleterious effect dependent upon the presence of an activating KIR gene, it is difficult to draw firm conclusions due to the lack of known ligands for the activating KIR and the strong linkage between the KIR B haplotype genes.
As described above, we associated relapse protection in our cohort (119) to the presence of Cen B and Tel B genes in the donor. KIR2DS2 is the activating KIR present in the centromeric portion of the B haplotype. How, if at all, KIR2DS2 or any of the other activating KIR with unknown ligands protect against relapse is unknown. They may recognize a ligand present on AML blasts. Or perhaps another receptor in linkage with these activating KIR may be responsible. Alternatively, absence of certain inhibitory KIR in donors with high activating KIR content may result in NK cells with less inhibition in the recipient.
In a recent analysis, we evaluated 1007 myeloablative, T-cell-replete URD transplants for AML. We found that leukemia free survival (LFS) was associated with ‘Better’ and ‘Best’ (KIR-Better/Best) donors, and this effect is amplified if recipients had 1 or 2 HLA-C allotypes carrying the HLA-C1 epitope (HLA-C1/x). The survival advantage and relapse protection in HLA-C1/x recipients compared to HLA-C2/C2 recipients was similar irrespective of their particular KIR B genes, but different clinical outcomes correlated with the HLA-matched status of the transplant. In the HLA-mismatched transplants (n=513) relapse protection was enhanced with KIR-Better/Best donors and recipients that expressed HLA-C1 (HLA-C1/x vs. HLA-C2/C2) (RR of relapse 0.35 (0.16-.76); P <0.01). Conversely, in the HLA-matched transplants (n=494), reduced treatment related mortality (TRM) was observed with KIR-Better/Best donors and recipient HLA-C1 [RR of TRM 0.40 (0.20-.0.81); P=0.01]. Relapse protection is not explained by increased acute GvHD, as the larger group of B/x donors had less acute GvHD in HLA-C1/C1 recipients vs. HLA-C2/C2. In agreement with our study and the Venstrom study, outcomes of unrelated HCT for AML were associated with KIR B haplotype donors, which were improved by interactions with HLA-C1.
Other receptors and ligands that affect outcome after HCT
NK cell receptor repertoires are far more complex than just KIR, and any number of other receptors (both activating and inhibitory) may play a role in mediating the GvL effect post-HCT. The activating receptor NKG2D, whose ligands include stress-induced molecules upregulated on many tumor cells, has been shown to be important in tumor surveillance in the mouse (95). NKG2D-deficient mice had a higher incidence of spontaneous malignancy and faster progression. Despite lacking NKG2D, these mice had otherwise normal NK cell function. Brenner and colleagues (143) suggested a role for NKG2D in controlling B-cell lymphoma in the early stages. DNAM-1, an adhesion molecule, expressed by a wide variety of cells, may also play a role in tumor surveillance. Similar to NKG2D deficient mice, mice lacking DNAM-1 had accelerated tumor growth (144), and DNAM-1 may be particularly important when tumors lack NKG2D ligands (145). Pende and colleagues (96) reported an association between the level of NK cell killing of leukemic blasts and the expression of the ligands for DNAM-1. PVR and Nectin-2 were expressed by most AML blasts, and only lymphoblasts expressing these ligands were killed. DNAM-1 blocking inhibited NK cell lysis. Interestingly, the ligands for NKG2D were expressed at low levels on AML blasts suggesting a larger role for DNAM-1 in eliminating AML blasts. In patients with MDS, blast cells expressed the ligands for DNAM-1. However, their NK cells had reduced expression of DNAM-1, which correlated with an inability to lyse MDS blasts (146). Members of the natural cytotoxicity receptor family may also be involved in eliminating tumors. One of the ligands for NKp30, B7-H6 is exclusively expressed on human tumor cells including myeloid leukemias, and NK cells can be activated through binding of NKp30 to B7-H6 (97). Additionally, release of the NKp30 ligand, nuclear factor HLA-B associated transcript 3 (BAT3), by tumor cells was also able to activate NK cells to produce IFNγ and TNF (147). As discussed earlier, a ligand for NKp44 was recently described to be present on tumor cells and blocking it yields less NK-mediated tumor cytotoxicity (47). Collectively, these data suggest that various NK cell activating receptor/ligand interactions are important for enabling NK cells to eliminate tumors and survey tumor progression. In the post-HCT setting, sufficient NK cell activating interactions with tumor ligands is essential for the elimination of residual leukemic cells, and deciphering these interactions is important in understanding how NK cells eliminate these cells.
NK cell memory
NK cells have traditionally been viewed as a short-lived lymphocyte population with limited proliferative capacity and a lack of antigen specificity. These observations, combined with the fact that NK cells do not undergo somatic DNA rearrangement, have led to their classification as innate immune cells. Recent studies have challenged this notion by demonstrating unexpected flexibility in responding to stress, providing evidence that NK cells possess at least some adaptive immune traits. The first evidence for NK cell memory came from von Andrian and colleagues who demonstrated that a subpopulation of liver-resident NK cells is capable of mediating hapten-induced contact hypersensitivity responses in mice lacking T and B cells. In this model, NK cells were able to discriminate between haptens during secondary challenge and persist for at least 4 weeks (148). Interestingly, this population of hepatic NK cells, which express the chemokine receptor CXCR6, also mounted specific hypersensitivity responses against influenza and vesicular stomatitis virus-like particles (149).
NK cell memory responses have also been observed in mice infected with mouse cytomegalovirus (MCMV). NK cells expressing Ly49H (a mouse homologue of activating KIR in humans) undergo an m157 antigen-specific expansion phase, followed by contraction and establishment of a long-lived pool of memory cells that persist for at least 70 days post-infection. Upon secondary MCMV challenge, the memory pool expands and displays enhanced effector function ex vivo relative to naive NK cells. Importantly, these memory NK cells are protective against lethal MCMV infections in newborn mice that lack mature NK cells (150).
Similar to the response of Ly49H+ NK cells during MCMV infection in mice, human NK cells expressing the DAP12-coupled CD94-NKG2C receptor complex expand during acute human CMV infection (151). Human NK cells that expand in response to CMV express self-specific inhibitory KIR, suggesting a role for education in the formation of memory NK cell populations (79). How CMV drives the expansion of human NK cells remains unclear, as a human CMV ligand-NK receptor pair has not been identified. It also remains to be determined whether other pathogens can elicit memory NK cell responses. Individuals with rare NK cell-specific deficiencies experience uncontrolled herpes simplex virus, varicella zoster virus, Epstein-Barr virus, and papillomavirus infections in addition to cytomegalovirus infections (152). Therefore, it is tempting to hypothesize that antigen-specific, adaptive NK cell responses are critical for the control of a range of viral infections.
Human NK cell memory induced by CMV infection
CMV can be a serious and potentially life threatening complication after HCT. NK cells have long been implicated in the control of CMV infection, particularly in murine models. As discussed above, CMV infection may result in the generation of memory NK cells in these mice. In humans, CMV has been shown to play a unique role in shaping the NK cell receptor repertoire (79). This is partly due to the clonal-like expansion of a population of NK cells that express NKG2C and an inhibitory KIR that recognizes self-HLA. These expanding NK cells express the maturation marker CD57 (151) and exhibit increased capacity to produce cytokines and mediate ADCC. In recipients of UCB transplants at the University of Minnesota CMV reactivation significantly altered the function and receptor repertoires of reconstituting NK cells (78). During the acute response to CMV, NK cells expressing NKG2C significantly increased following viral diagnosis, peaking at 4 weeks post anti-viral therapy before declining by 8 weeks. Not only were the percentage of NKG2C+ NK cells increased, the absolute number of these cells were also increased. Capacity to produce IFNγ when stimulated with the class I negative cell line K562 was increased in NK cells expressing NKG2C. Interestingly when expression of NKG2C was measured over the first year of transplant, NK cells expressing NKG2C continued to increase so that by 1-year post transplant NKG2C+ NK cells accounted for on average half of the reconstituting NK cell receptor repertoire.
NK cells from UCB transplants are considered to be developmentally immature and take longer to recover target cell mediated cytokine production than NK cells from a peripheral blood or bone marrow graft. This is characterized by a higher expression of NKG2A and lower KIR expression. However, in our UCB cohort, recipients who reactivated CMV had a more rapid decline in NKG2A expression and rise in KIR expression, accompanied by an increase in their ability to produce IFNγ when stimulated with K562 cells. These findings were associated with the expansion of NKG2C+ NK cells, as these expanding NK cells lacked NKG2A and expressed KIR. All expanding NK cells expressed an inhibitory KIR that recognized self-HLA, and this was predominately found to be CD158b (KIR2DL2, KIR2DL3 and KIR2DS2). When we further dissected KIR expression at the mRNA level, the dominant KIR transcript expressed was KIR2DL3. These expanding NKG2C+ NK cells were found to preferentially acquire CD57 during the first year after transplant, suggesting that these NK cells were highly differentiated. Furthermore, recipients who reactivated CMV had higher mRNA transcripts for the transcription factor T-bet and IFNγ, suggesting they have an increased ability to respond rapidly with IFNγ.
In a follow up study (153), we investigated the effect of CMV reactivation in recipients of adult HLA matched allografts were the donor cells were either from the bone marrow or peripheral blood. Unlike our UCB cohort were all grafts are considered CMV naive, the CMV serostatus of the donor in this cohort may influence NK cell reconstitution in the recipient. Similar to the UCB cohort, NKG2C+ NK cells expanded in the presence of CMV reactivation. However, these NKG2C+ NK cells did not continue to expand 1-year post-transplant, although they still persisted at higher frequencies compared to donor/recipient pairs who were both seronegative. One explanation for this discrepancy may be the different graft sources. After UCB transplantation, NK cells are the predominate lymphocyte, and NKG2C+ NK cells have more ‘space’ to expand compared with peripheral blood or bone marrow grafts that contain higher numbers of graft T cells competing with NK cells. Unlike the UCB cohort, NK cells expressing NKG2C also expanded in the absence of CMV viremia. In CMV seropositive donor/recipient pairs, NKG2C+ NK cells from the donor continued to persist in the recipient and continued to expand 1-year post-transplant. This would suggest that NKG2C+ NK cells from seropositive donors have an increased capacity to survive post-transplant. Strikingly, this increased survival appeared to be dependent on the CMV status of the recipient and presumed presence of latent CMV antigen, as NKG2C+ NK cells from seropositive donors transplanted into CMV seronegative recipient failed to increase in numbers and declined to levels comparable with CMV seronegative donor/recipient pairs. While a modest increase in the percentage of NK cells expressing NKG2C was noted by 1 year in CMV seronegative donors transplanted into CMV seropositive recipients, it appeared that detectable CMV viremia is required for high expansion of these cells. Additionally, CMV reactivation or the presence of latent CMV antigen was associated with lower NKG2A expression, increased KIR expression (predominately self KIR), acquisition of CD57 and an increased capacity to produce IFNγ. The capacity to produce IFNγ was increased in patients who reactivated CMV where the donor was CMV seropositive.
Collectively, the results from both studies demonstrate an effect of CMV in shaping NK cell receptor repertoires and function post HCT, resulting in the expansion of a population of NK cells that are potent producers of IFNγ and have the ability to be long-lived. Additionally, the presence of latent CMV antigen in the recipient is required to maintain expanded population of NKG2C+ NK cells. The increase in IFNγ production following re-exposure in the recipient coupled with the expansion and persistence of these cells suggest that NKG2C+ NK cells may represent memory-like cells in humans akin to the Ly49H+ response to MCMV in mice (150).
Recently, Kim and colleagues (154) reported that a population of NK cells do not express the intracellular signaling adapter molecule FcγRIγ, and downregulation of this molecule correlated with CMV seropositivity (155). CD16, NKp30, and NKp46 use the adapter molecules FcγRIγ to signal. CD16 also utilizes CD3ζ to transduce activating signals. Loss of both FcγRIγ and CD3ζ, which has been reported to occur in HIV-infected individuals (156), results in diminished CD16 and NCR signaling. However, in healthy CMV seropositive individuals, down-regulation of FcγRIγ alone results in enhanced CD16 signaling (155). NK cells lacking FcγRIγ had poor functional responses against CMV infected fibroblasts. However, when CMV specific antibodies were added to the culture, NK cell activity markedly increased and was characterized by an increase in both CD107a expression and cytokine production (IFNγ and TNFα). Interestingly, while only found in CMV seropositive individuals, these NK cells also had enhanced antibody-dependent responses to HSV-1. Additionally, NK cells lacking FcγRIγ expressed significantly more CD57, tended to co-express NKG2C and had higher Bcl-2 expression than conventional NK cells. In our UCB cohort, we have observed that downregulation of FcγRIγ also occurs following CMV reactivation (unpublished data) and that these NK cells have enhanced cytokine production when triggered through CD16.
While many transplant studies have shown a complicated interaction between CMV reactivation and transplant outcomes, recent studies suggest that, in some cases, CMV reactivation may be associated with a lower incidence of relapse. Considering the above data on the effect of CMV on NK cell acquisition of function, these findings are intriguing. For example, it is possible that the reported lower risk of relapse in recipients of UCB grafts may be associated with the higher incidence of CMV reactivation (157). In direct support of this notion, Elmaagacli and colleagues (158) reported in their cohort of 266 AML patients receiving grafts from either a sibling or unrelated donor that early CMV reactivation was associated with a reduced risk of leukemic relapse. The risk of leukemic relapse at ten years post-transplant in the presence of CMV reactivation was 9% compared to 42% in its absence. In multivariate analysis accounting for GvHD and other factors, early CMV reactivation resulted in an independent antileukemic effect. Ito and colleagues (159) also reported a significant reduction in relapse, this time in CML patients who reactivated CMV prior to Day 100. Recently, a large study from the Seattle group was conducted to evaluate the effect of early CMV reactivation on leukemic relapse (160) in patients with AML (n=761), ALL (n=322), CML (n=646), lymphoma (n=254) and myelodysplastic syndrome (MDS) (n=371). They reported that CMV pp65 antigenemia prior to Day 100 was associated with a decrease in leukemic relapse, but only for AML patients, This effect was not as striking as to what was observed for Elmaagacli et al. Interestingly, in all cohorts there was a trend towards reduced relapse following CMV reactivation in recipients who were CMV seropositive compared with CMV seronegative recipients with primary CMV infection. Reductions in leukemic relapse in AML patients who reactivate CMV early after transplant could be attributed to the expansion of functionally competent NKG2C+ NK cells after transplant. HLA class I expression is known to be down-regulated on AML blasts, but HLA-E expression is maintained on the surface (102), which could activate NK cells through NKG2C. Alternatively, there may exist a virus-versus-leukemia effect where the virus itself infects AML blasts, and these AML cells may present viral antigens to cytotoxic NK and T cells.
As our understanding of the effect of CMV in shaping NK cell receptor repertoires and altering NK cell functional properties increases, it could be hypothesized that carefully monitored CMV reactivation may be beneficial rather than detrimental to the recipient. Alternatively, ways to mimic the effects of CMV reactivation in the absence of CMV viremia are certainly warranted.
Adoptive transfer of adult NK cells to further enhance efficacy
The first trials in humans to harness the anti-tumor properties of NK cells focused on the use of IL-2 to activate autologous NK cells. Ex vivo IL-2-stimulated cell infusions enhanced recovery of NK cell cytotoxicity in vivo compared to IL-2 administration alone, but efficacy was probably limited by: (i) competition with the recipient’s lymphocytes for cytokines and ‘space’, (ii) autologous NK cell inhibition by self-MHC, (iii) chronic immunosuppression induced by the tumor on host immunity, and (iv) the realization that low dose IL-2 stimulated T-regulatory subpopulations. As inhibitory KIR and their ligands were further characterized, the next approach to utilizing NK cells as immunotherapy focused on allogeneic NK cells from healthy related donors. In this setting, allogeneic NK cells avoid tumor-induced suppression and have the advantage of being educated and fully functional. The first trial of this approach was published in 2005 from the University of Minnesota (161). Forty-three patients with metastatic melanoma, metastatic renal cell carcinoma or poor prognosis AML were enrolled in the trial. Peripheral blood was collected by apheresis from haploidentical related donors and CD3 depleted before being incubated overnight in high dose IL-2. Prior to NK cell infusion, patients underwent a regimen that involved three different chemotherapy preparative regimens: high cyclophosphamide and fludarabine (Hi-Cy/Flu), low cyclophosphamide and methylprednisone, or fludarabine alone. Following infusion, patients received IL-2 daily (1.75 million units (MU)/m2) for 14 days (subsequently modified to 6 higher doses (10 MU without m2 correction) over 2 weeks). NK cell expansion was only observed for patients receiving the preparatory regimen of Hi-Cy/Flu. In subsequent trials, we prospectively defined successful NK cell expansion as a measurement of greater than 100 NK cells/μL of blood 12-16 days after infusion. On this initial protocol, 30% of poor prognosis AML patients achieved a complete remission. However, this remission was not durable, and patients ultimately relapsed. Since the lack of NK cell expansion may be the result of host-mediated rejection of adoptively transferred cells, the addition of 400cGy of total body irradiation (TBI) was added to Hi-Cy/Flu to further deplete host immune cells and create space for donor NK cells to expand. On this modified protocol NK cell expansion was much more successful, and 50% of patients achieved measurable NK cell expansion based on our current definition. Furthermore, leukemia clearance was observed in 66% of patients, which was higher than patients who did not expand NK cells in vivo, suggesting that the NK cells themselves played a role in the anti-leukemia response over and above the activity of the high dose chemotherapy preparative regimen. It should be highlighted that the absolute level of in vivo NK cell expansion needed to induce a clinical response is unknown. It is possible that lower donor NK cell levels or donor chimerism for shorter time intervals (day 7 but not day 14 for example) may be sufficient for clinical efficacy. These parameters need to be measured and correlated with clinical response in all donor NK cell trials to address this question.
Our current strategy at the University of Minnesota is to use NK cells, cytokines, and lymphodepleting chemotherapy (Hi-Cy/Flu) as therapy to achieve remission in patients with refractory AML, a cohort that is generally not eligible for allogeneic transplantation. These trials use donor NK cell persistence and in vivo expansion as a surrogate to improve clinical efficacy given the correlation between leukemia clearance and donor-derived NK cells 7 and 14 days after adoptive transfer. Several modifications to our initial platform are expected to improve results. Building on preclinical data in the mouse where T-regulatory cell depletion with IL-2 diphtheria toxin (denileukin diftitox) induced enhanced responses to AML (162), we piloted this approach as part of our NK cell adoptive transfer strategy. Although Treg precursor depletion was incomplete, NK cell expansion was found in 30% of patients, and leukemia clearance was higher than we have seen previously, allowing 50% of patients to move on to ‘best’ donor allogeneic transplantation.
The use of adoptive transfer of NK cells to treat various malignancies has resulted in mixed results. Shi and colleagues (163) infused haploidentical KIR mismatched NK cells into 10 patients with relapsed multiple myeloma followed 14 days later with an autologous stem cell graft. Five patients achieved near complete remission. Bachanova and colleagues (164) treated 6 patients with non-Hodgkin’s lymphoma with infusion of haploidentical NK cells and found that NK cells poorly expanded in vivo, and host Tregs were significantly increased after NK cell infusion and IL-2 administration. Similarly, adoptively transferred NK cells failed to expand in patients with breast and ovarian cancers, and a similar increase in host Tregs was also observed (165).
Monoclonal and bispecific antibody therapy
Because NK cell infusions are costly, cumbersome and difficult to export to all centers, strategies to enhance NK cell function in vivo are warranted. One such strategy is the administration of monoclonal antibodies to either increase an NK cells’ ability to lyse leukemic blasts or to block inhibitory signals such as inhibitory KIR:MHC interactions. An antibody directed against KIR2DL1, KIR2DL2, and KIR2DL3, (Lirilumab IPH2101) (166) is currently in phase 1 clinical trials and may enhance NK cell function by blocking inhibitory signals (167, 168). Another antibody-based approach is to exploit the ability of NK cells to recognize antibody-coated targets through CD16 [antibody-dependent cellular cytotoxicity (ADCC)] by adding antibodies in the peritransplant setting. Several monoclonal antibodies such as rituximab (169) are effective due to NK cell-mediated ADCC. However, antibody-based NK cell mediated ADCC anti-tumor therapy is limited by CD16 expression on NK cells. Additionally, activation of CD16 leads to rapid cleavage from the surface by matrix metalloproteinases (MMPs) (170-173), likely decreasing mAb therapy efficacy. Also of relevance, particularly in cases where NK cells are expanded in vitro for therapeutic use, cytokines can induce expression of MMPs (174). To address these issues, several groups have recently experimented with metalloproteinase inhibitors already tested in the clinic for other indications (175) to enhance NK cell-mediated ADCC. We have shown that treatment of NK cells with a highly selective ADAM17 (A disintegrin and metalloprotease-17) inhibitor, BMS566394, leads to increased IFN-γ production after CD16 crosslinking (176). Furthermore, ADAM17 inhibition on NK cells combined with rituximab treatment of the human Burkitt’s lymphoma cell line Raji led to increased IFN-γ, TNF, and CD107a production by NK cells. Another group tested a broader spectrum MMP inhibitor, GM6001, combined with trastuzumab, rituximab, and cetuximab on cell lines expressing corresponding antigens and showed similar phenotypic and functional results (177). Finally, utilizing siRNA knockdown to interfere with membrane-type 6 matrix metalloproteinase (MT6 or MMP25), one group showed that this MMP is also involved in cleavage of CD16 on human NK cells, and interference with MT6/MMP25 function leads to enhanced ADCC (178). While the physiologic role of MMP-mediated CD16 cleavage on healthy donor settings remains in question, these studies convey a compelling argument for use of MMP inhibitors in the clinic to enhance NK mediated ADCC tumor therapy.
Another way to exploit NK cells for ADCC-mediated tumor therapy is through use of engineered antibodies with multiple specificities (179). These bispecific and trispecific killer cell engagers (BiKEs and TriKEs) contain two or three different cloned variable fragments from the light and heavy chains from antibodies respectively: one specific for CD16 on the NK cell and one or two specific for tumor antigens. The obvious advantage offered by BiKEs and TriKEs is that they can bring NK cells in contact with tumors through specified antigen(s) while triggering potent activation through CD16. Although the methodology for producing bispecific and trispecific antibodies has changed over time, these multivalent antibodies have been used for over 20 years to target CD16 on the killer cell and CD30 on the tumor cell for Hodgkin’s disease (180-187), CD20 for B-cell Non-Hodgkin’s lymphomas (188), CD19 (189-192) and/or CD33 (193, 194) for B-cell Non-Hodgkin’s lymphomas and leukemias, HER-2/neu for metastatic breast cancer as well as other types of cancer (195-198) including EGF-R for EGF-R+ tumors (199) and MOV19 for ovarian carcinoma (200). Recently, our group has used BiKEs and TriKEs to target tumor cells and further understand the NK cell biology involved in utilizing these reagents.
BiKEs and TriKEs specific for CD16 and CD19/CD22 not only targeted Raji tumor cells efficiently for killing, but they also resulted in increased secretion of IFN-γ, TNF, MIP-1α, GM-CSF, RANTES, and IL-8, indicating that these reagents elicit both direct killing and NK cell-mediated inflammatory responses (201). This study also showed that the CD16/CD19/CD22 TriKE could elicit better CD107a responses to primary ALL and CLL targets than rituximab. Another study from our group utilizing CD16/EpCAM BiKE showed in vitro that prostate, breast, colon, head, and neck carcinomas, usually resistant to NK-mediated killing, could be targeted with this methodology (202). Finally, our most recent study showed that CD16/CD33 BiKEs triggered NK cell degranulation and cytokine secretion against relapse/refractory AML targets expressing CD33 (203). Furthermore, bringing both metalloproteinase inhibitor and BiKE concepts together, combinational therapy with ADAM17 inhibitor and the BiKE augmented killing further. These findings illustrate the future potential of using these combinational therapies in order to overcome the threshold necessary to kill tumor cells in the clinic.
Additionally, we investigated the ability of the CD16x33 BiKE to increase post-HCT NK cell activity against AML blasts. As discussed above, NK cells from UCB recipients who reactivate CMV have a more mature phenotype and increased capacity for cytokine production. Given these findings, we divided recipients based on CMV reactivation. There was no difference in CD16 expression between the two groups, but expression was decreased compared to healthy donors as expected. However, by 1-year CD16 expression was nearing normal levels. NK cells from recipients who reactivated CMV were far more responsive to the CD16x33 BiKE at both 3 months and 1-year, with increases in both CD107a expression and cytokine production compared to CMV seronegative recipients. Interestingly, while CMV seronegative recipients still had poor functional responses at 1-year post-HCT, NK cell function was comparable at 1 year post-HCT to healthy donors for recipients who had reactivated CMV. It would be interesting to determine if the increase in CD16 signaling in recipients who had reactivated CMV was due to loss of FcγRIγ, as NK cells that had downregulated the signaling adapter molecule have been reported to have increased ADCC responses (155). Collectively, these results suggest that administration of this CD16x33 BiKE post-HCT may increase the GvL effect mediated by NK cells, and this is enhanced if the recipient reactivates CMV.
Concluding remarks
NK cells play an important role in cancer therapy, and this is best documented after HCT. As we continue to improve our understanding of how NK cells acquire function, the interactions of NK cell activating and inhibitory receptors with their ligands, their immunoregulatory roles and their potential to mediate immune memory will only increase our ability to exploit these properties to enhance NK cell function post-HCT. Despite many positive and negative studies, it is clear that NK cells play a role in AML relapse protection. Higher resolution analysis considering interactions between KIR and HLA at the antigen and allele levels are likely to further strengthen correlations between NK cells and transplant outcomes, as these variables definitively control NK cell function. It may also better define the role of NK cells in other diseases. Lastly, NK cells have adaptive immune properties endowed by CMV infection. How best to translate these findings into the clinic require further study. Although it is tantalizing to propose deliberate CMV reactivation to prime adaptive immunity, this needs to be balanced with the morbidity of CMV disease risk and its therapy. Understanding mechanisms to induce memory NK cells with CMV infection would be of great interest. Optimal activation of NK cells by IL-15 and IL-15/IL-15Ra complexes combined with targeting through CD16 that overcomes inhibitory signaling offers great promise for the future of NK cell therapeutics.
Acknowledgements
This work has been funded in part with federal funds from the National Cancer Institute (NCI) CA65493 and CA111412.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Galy A, et al. Human T,B. natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3(4):459–73. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 2.Freud AG, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203(4):1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baume DM, et al. Differential responses to interleukin 2 define functionally distinct subsets of human natural killer cells. Eur J Immunol. 1992;22(1):1–6. doi: 10.1002/eji.1830220102. [DOI] [PubMed] [Google Scholar]
- 4.Nagler A, et al. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143(10):3183–91. [PubMed] [Google Scholar]
- 5.Jacobs R, et al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31(10):3121–7. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Fehniger TA, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101(8):3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 7.Ferlazzo G, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172(3):1455–62. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 8.Robertson MJ, et al. Costimulatory signals are required for optimal proliferation of human natural killer cells. J Immunol. 1993;150(5):1705–14. [PubMed] [Google Scholar]
- 9.Lanier LL, et al. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136(12):4480–6. [PubMed] [Google Scholar]
- 10.Grzywacz B, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood. 2006;108(12):3824–33. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JS, Verfaillie C, McGlave P. The generation of human natural killer cells from CD34+/DR− primitive progenitors in long-term bone marrow culture. Blood. 1992;80(9):2182–7. [PubMed] [Google Scholar]
- 12.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83(9):2594–601. [PubMed] [Google Scholar]
- 13.Cooley S, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110(2):578–86. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 16.Imada K, et al. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188(11):2067–74. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87(7):2632–40. [PubMed] [Google Scholar]
- 18.Dubois S, et al. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17(5):537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 19.Burkett PR, et al. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200(7):825–34. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freud AG, Caligiuri MA. Human natural killer cell development. Immunological reviews. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 21.Thielens A, Vivier E, Romagne F. NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Current opinion in immunology. 2012;24(2):239–45. doi: 10.1016/j.coi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Binstadt BA, et al. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5(6):629–38. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 23.Le Drean E, et al. Inhibition of antigen-induced T cell response and antibody-induced NK cell cytotoxicity by NKG2A: association of NKG2A with SHP-1 and SHP-2 protein-tyrosine phosphatases. Eur J Immunol. 1998;28(1):264–76. doi: 10.1002/(SICI)1521-4141(199801)28:01<264::AID-IMMU264>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24.Lanier LL, et al. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391(6668):703–7. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 25.Stewart CA, et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(37):13224–9. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268(5209):405–8. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 27.Wagtmann N, et al. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995;2(5):439–49. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 28.D’Andrea A, et al. Molecular cloning of NKB1. A natural killer cell receptor for HLA-B allotypes. Journal of immunology. 1995;155(5):2306–10. [PubMed] [Google Scholar]
- 29.Uhrberg M, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7(6):753–63. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 30.Wilson MJ, et al. Plasticity in the organization and sequences of human KIR/ILT gene families. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4778–83. doi: 10.1073/pnas.080588597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin MP, et al. Cutting edge: expansion of the KIR locus by unequal crossing over. Journal of immunology. 2003;171(5):2192–5. doi: 10.4049/jimmunol.171.5.2192. [DOI] [PubMed] [Google Scholar]
- 32.Valiante NM, et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7(6):739–51. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 33.Chan HW, et al. DNA methylation maintains allele-specific KIR gene expression in human natural killer cells. J Exp Med. 2003;197(2):245–55. doi: 10.1084/jem.20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies GE, et al. Identification of bidirectional promoters in the human KIR genes. Genes and immunity. 2007;8(3):245–53. doi: 10.1038/sj.gene.6364381. [DOI] [PubMed] [Google Scholar]
- 35.Stulberg MJ, et al. Identification of distal KIR promoters and transcripts. Genes and immunity. 2007;8(2):124–30. doi: 10.1038/sj.gene.6364363. [DOI] [PubMed] [Google Scholar]
- 36.Li H, et al. Genetic control of variegated KIR gene expression: polymorphisms of the bi-directional KIR3DL1 promoter are associated with distinct frequencies of gene expression. PLoS genetics. 2008;4(11):e1000254. doi: 10.1371/journal.pgen.1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cichocki F, et al. The transcription factor c-Myc enhances KIR gene transcription through direct binding to an upstream distal promoter element. Blood. 2009;113(14):3245–53. doi: 10.1182/blood-2008-07-166389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cichocki F, et al. Cutting edge: KIR antisense transcripts are processed into a 28-base PIWI-like RNA in human NK cells. Journal of immunology. 2010;185(4):2009–12. doi: 10.4049/jimmunol.1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright PW, et al. Identification of a KIR antisense lncRNA expressed by progenitor cells. Genes and immunity. 2013 doi: 10.1038/gene.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazetic S, et al. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J Immunol. 1996;157(11):4741–5. [PubMed] [Google Scholar]
- 41.Vales-Gomez M, et al. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 1999;18(15):4250–60. doi: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 43.Meyaard L, et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7(2):283–90. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 44.Cosman D, et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7(2):273–82. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 45.Hershkovitz O, et al. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol. 2009;183(4):2610–21. doi: 10.4049/jimmunol.0802806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–60. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 47.Baychelier F, et al. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood. 122(17):2935–42. doi: 10.1182/blood-2013-03-489054. [DOI] [PubMed] [Google Scholar]
- 48.Bottino C, et al. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26(4):221–6. doi: 10.1016/j.it.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Claus M, et al. Regulation of NK cell activity by 2B4, NTB-A and CRACC. Front Biosci. 2008;13:956–65. doi: 10.2741/2735. [DOI] [PubMed] [Google Scholar]
- 50.Anegon I, et al. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J Exp Med. 1988;167(2):452–72. doi: 10.1084/jem.167.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 52.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 53.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 54.Vivier E, et al. Tyrosine phosphorylation of the Fc gamma RIII(CD16): zeta complex in human natural killer cells. Induction by antibody-dependent cytotoxicity but not by natural killing. J Immunol. 1991;146(1):206–10. [PubMed] [Google Scholar]
- 55.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 56.Shore SL, et al. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974;251(5473):350–2. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- 57.Laszlo A, Petri I, Ilyes M. Antibody dependent cellular cytotoxicity (ADCC)-reaction and an in vitro steroid sensitivity test of peripheral lymphocytes in children with malignant haematological and autoimmune diseases. Acta Paediatr Hung. 1986;27(1):23–9. [PubMed] [Google Scholar]
- 58.Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- 59.Albertini MR, Hank JA, Sondel PM. Native and genetically engineered anti-disialoganglioside monoclonal antibody treatment of melanoma. Cancer Chemother Biol Response Modif. 2005;22:789–97. doi: 10.1016/s0921-4410(04)22037-3. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Foncillas J, Diaz-Rubio E. Progress in metastatic colorectal cancer: growing role of cetuximab to optimize clinical outcome. Clin Transl Oncol. 2010;12(8):533–42. doi: 10.1007/s12094-010-0551-3. [DOI] [PubMed] [Google Scholar]
- 61.Garnock-Jones KP, Keating GM, Scott LJ. Trastuzumab: A review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs. 2010;70(2):215–39. doi: 10.2165/11203700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 62.Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr Cancer Drug Targets. 2010;10(2):200–9. doi: 10.2174/156800910791054167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winter MC, Hancock BW. Ten years of rituximab in NHL. Expert Opin Drug Saf. 2009;8(2):223–35. doi: 10.1517/14740330902750114. [DOI] [PubMed] [Google Scholar]
- 64.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 65.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6(7):520–31. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 66.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 67.Brodin P, et al. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113(11):2434–41. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 68.Joncker NT, et al. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182(8):4572–80. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–23. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guia S, et al. Confinement of activating receptors at the plasma membrane controls natural killer cell tolerance. Sci Signal. 2011;4(167):ra21. doi: 10.1126/scisignal.2001608. [DOI] [PubMed] [Google Scholar]
- 71.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207(10):2073–9. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joncker NT, et al. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207(10):2065–72. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schonberg K, et al. Analyses of HLA-C-specific KIR repertoires in donors with group A and B haplotypes suggest a ligand-instructed model of NK cell receptor acquisition. Blood. 2011;117(1):98–107. doi: 10.1182/blood-2010-03-273656. [DOI] [PubMed] [Google Scholar]
- 74.Beziat V, et al. Influence of KIR gene copy number on natural killer cell education. Blood. 2013;121(23):4703–7. doi: 10.1182/blood-2012-10-461442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brodin P, et al. Skewing of the NK cell repertoire by MHC class I via quantitatively controlled enrichment and contraction of specific Ly49 subsets. J Immunol. 2012;188(5):2218–26. doi: 10.4049/jimmunol.1102801. [DOI] [PubMed] [Google Scholar]
- 76.Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11(4):321–7. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foley B, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189(10):5082–8. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foley B, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–74. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beziat V, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–88. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bjorklund AT, et al. NK cells expressing inhibitory KIR for non-self-ligands remain tolerant in HLA-matched sibling stem cell transplantation. Blood. 2010;115(13):2686–94. doi: 10.1182/blood-2009-07-229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruggeri L, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–9. [PubMed] [Google Scholar]
- 82.Ruggeri L, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 83.Ruggeri L, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–40. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haas P, et al. NK-cell education is shaped by donor HLA genotype after unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2011;117(3):1021–9. doi: 10.1182/blood-2010-02-269381. [DOI] [PubMed] [Google Scholar]
- 85.Sykes M, et al. Hematopoietic cells and radioresistant host elements influence natural killer cell differentiation. J Exp Med. 1993;178(1):223–9. doi: 10.1084/jem.178.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manilay JO, Waneck GL, Sykes M. Altered expression of Ly-49 receptors on NK cells developing in mixed allogeneic bone marrow chimeras. Int Immunol. 1998;10(12):1943–55. doi: 10.1093/intimm/10.12.1943. [DOI] [PubMed] [Google Scholar]
- 87.Manilay JO, Waneck GL, Sykes M. Levels of Ly-49 receptor expression are determined by the frequency of interactions with MHC ligands: evidence against receptor calibration to a “useful” level. J Immunol. 1999;163(5):2628–33. [PubMed] [Google Scholar]
- 88.Chargui J, et al. Inhibition of NK cell activity induces improvement and stable chimerism after allogeneic transplantation. Transplant Proc. 2000;32(7):2462–3. doi: 10.1016/s0041-1345(00)01744-9. [DOI] [PubMed] [Google Scholar]
- 89.Cho SG, et al. Anti-NK cell treatment induces stable mixed chimerism in MHC-mismatched, T cell-depleted, nonmyeloablative bone marrow transplantation. Exp Hematol. 2004;32(12):1246–54. doi: 10.1016/j.exphem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 90.Tiberghien P, et al. Anti-asialo GM1 antiserum treatment of lethally irradiated recipients before bone marrow transplantation: evidence that recipient natural killer depletion enhances survival, engraftment, and hematopoietic recovery. Blood. 1990;76(7):1419–30. [PubMed] [Google Scholar]
- 91.Westerhuis G, et al. Long-term mixed chimerism after immunologic conditioning and MHC-mismatched stem-cell transplantation is dependent on NK-cell tolerance. Blood. 2005;106(6):2215–20. doi: 10.1182/blood-2005-04-1391. [DOI] [PubMed] [Google Scholar]
- 92.Aguila HL, Weissman IL. Hematopoietic stem cells are not direct cytotoxic targets of natural killer cells. Blood. 1996;87(4):1225–31. [PubMed] [Google Scholar]
- 93.Lee LA, Sergio JJ, Sykes M. Natural killer cells weakly resist engraftment of allogeneic, long-term, multilineage-repopulating hematopoietic stem cells. Transplantation. 1996;61(1):125–32. doi: 10.1097/00007890-199601150-00024. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, et al. NK cell tolerance in mixed allogeneic chimeras. J Immunol. 2003;170(11):5398–405. doi: 10.4049/jimmunol.170.11.5398. [DOI] [PubMed] [Google Scholar]
- 95.Guerra N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pende D, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105(5):2066–73. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 97.Brandt CS, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baychelier F, et al. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood. 2013;122(17):2935–42. doi: 10.1182/blood-2013-03-489054. [DOI] [PubMed] [Google Scholar]
- 99.Yu G, et al. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203(8):1851–8. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garrod KR, et al. NK cell patrolling and elimination of donor-derived dendritic cells favor indirect alloreactivity. J Immunol. 2010;184(5):2329–36. doi: 10.4049/jimmunol.0902748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreau A, et al. Effector mechanisms of rejection. Cold Spring Harb Perspect Med. 2013;3(11) doi: 10.1101/cshperspect.a015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen S, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105(10):4135–42. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 103.Vago L, et al. Temporal, quantitative, and functional characteristics of single-KIR-positive alloreactive natural killer cell recovery account for impaired graft-versus-leukemia activity after haploidentical hematopoietic stem cell transplantation. Blood. 2008;112(8):3488–99. doi: 10.1182/blood-2007-07-103325. [DOI] [PubMed] [Google Scholar]
- 104.Foley B, et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118(10):2784–92. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fehniger TA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162(8):4511–20. [PubMed] [Google Scholar]
- 106.Nguyen S, et al. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia. 2008;22(2):344–52. doi: 10.1038/sj.leu.2405041. [DOI] [PubMed] [Google Scholar]
- 107.Davies SM, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100(10):3825–7. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- 108.Giebel S, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102(3):814–9. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 109.Farag SS, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876–84. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 110.Hsu KC, et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2006;12(8):828–36. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 111.Giebel S, et al. Homozygosity for human leucocyte antigen-C ligands of KIR2DL1 is associated with increased risk of relapse after human leucocyte antigen-C-matched unrelated donor haematopoietic stem cell transplantation. British journal of haematology. 2005;131(4):483–6. doi: 10.1111/j.1365-2141.2005.05797.x. [DOI] [PubMed] [Google Scholar]
- 112.Miller JS, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–61. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Willemze R, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23(3):492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brunstein CG, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113(22):5628–34. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McQueen KL, et al. Donor-recipient combinations of group A and B KIR haplotypes and HLA class I ligand affect the outcome of HLA-matched, sibling donor hematopoietic cell transplantation. Hum Immunol. 2007;68(5):309–23. doi: 10.1016/j.humimm.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stringaris K, et al. Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2010;16(9):1257–64. doi: 10.1016/j.bbmt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Venstrom JM, et al. Donor activating KIR3DS1 is associated with decreased acute GVHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2010;115(15):3162–5. doi: 10.1182/blood-2009-08-236943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cooley S, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–32. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cooley S, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–9. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laffont S, et al. Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood. 2008;112(3):661–71. doi: 10.1182/blood-2007-10-120089. [DOI] [PubMed] [Google Scholar]
- 121.Asai O, et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101(9):1835–42. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Noval Rivas M, et al. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. Journal of immunology. 2010;184(12):6790–8. doi: 10.4049/jimmunol.0902598. [DOI] [PubMed] [Google Scholar]
- 123.Zecher D, et al. NK cells delay allograft rejection in lymphopenic hosts by downregulating the homeostatic proliferation of CD8+ T cells. Journal of immunology. 2010;184(12):6649–57. doi: 10.4049/jimmunol.0903729. [DOI] [PubMed] [Google Scholar]
- 124.Cella M, et al. NK3-specific natural killer cells are selectively inhibited by Bw4-positive HLA alleles with isoleucine 80. J Exp Med. 1994;180(4):1235–42. doi: 10.1084/jem.180.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Draghi M, et al. Single-cell analysis of the human NK cell response to missing self and its inhibition by HLA class I. Blood. 2005;105(5):2028–35. doi: 10.1182/blood-2004-08-3174. [DOI] [PubMed] [Google Scholar]
- 126.Martin MP, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31(4):429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 127.Gumperz JE, et al. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med. 1995;181(3):1133–44. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Foley BA, et al. The reactivity of Bw4+ HLA-B and HLA-A alleles with KIR3DL1: implications for patient and donor suitability for haploidentical stem cell transplantations. Blood. 2008;112(2):435–43. doi: 10.1182/blood-2008-01-132902. [DOI] [PubMed] [Google Scholar]
- 129.Stern M, et al. Human leukocyte antigens A23, A24, and A32 but not A25 are ligands for KIR3DL1. Blood. 2008;112(3):708–10. doi: 10.1182/blood-2008-02-137521. [DOI] [PubMed] [Google Scholar]
- 130.Yawata M, et al. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008;112(6):2369–80. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moesta AK, et al. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008;180(6):3969–79. doi: 10.4049/jimmunol.180.6.3969. [DOI] [PubMed] [Google Scholar]
- 132.Pende D, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113(13):3119–29. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- 133.Pando MJ, et al. The protein made from a common allele of KIR3DL1 (3DL1*004) is poorly expressed at cell surfaces due to substitution at positions 86 in Ig domain 0 and 182 in Ig domain 1. J Immunol. 2003;171(12):6640–9. doi: 10.4049/jimmunol.171.12.6640. [DOI] [PubMed] [Google Scholar]
- 134.Carr WH, Pando MJ, Parham P. KIR3DL1 polymorphisms that affect NK cell inhibition by HLA-Bw4 ligand. Journal of immunology. 2005;175(8):5222–9. doi: 10.4049/jimmunol.175.8.5222. [DOI] [PubMed] [Google Scholar]
- 135.Bari R, et al. Significant functional heterogeneity among KIR2DL1 alleles and a pivotal role of arginine 245. Blood. 2009;114(25):5182–90. doi: 10.1182/blood-2009-07-231977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frazier WR, et al. Allelic variation in KIR2DL3 generates a KIR2DL2-like receptor with increased binding to its HLA-C ligand. Journal of immunology. 2013;190(12):6198–208. doi: 10.4049/jimmunol.1300464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moretta A, et al. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182(3):875–84. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chewning JH, et al. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol. 2007;179(2):854–68. doi: 10.4049/jimmunol.179.2.854. [DOI] [PubMed] [Google Scholar]
- 139.Foley B, et al. KIR2DS1-mediated activation overrides NKG2A-mediated inhibition in HLA-C C2-negative individuals. Int Immunol. 2008;20(4):555–63. doi: 10.1093/intimm/dxn013. [DOI] [PubMed] [Google Scholar]
- 140.Fauriat C, et al. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 2010;115(6):1166–74. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 141.Venstrom JM, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. The New England journal of medicine. 2012;367(9):805–16. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fauriat C, et al. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. 115(6):1166–74. doi: 10.1182/blood-2009-09-245746. [DOI] [PubMed] [Google Scholar]
- 143.Brenner CD, et al. Requirements for control of B-cell lymphoma by NK cells. Eur J Immunol. 2010;40(2):494–504. doi: 10.1002/eji.200939937. [DOI] [PubMed] [Google Scholar]
- 144.Iguchi-Manaka A, et al. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med. 2008;205(13):2959–64. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gilfillan S, et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205(13):2965–73. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carlsten M, et al. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia. 2010;24(9):1607–16. doi: 10.1038/leu.2010.149. [DOI] [PubMed] [Google Scholar]
- 147.Pogge von Strandmann E, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27(6):965–74. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 148.O’Leary JG, et al. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nature immunology. 2006;7(5):507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 149.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nature immunology. 2010;11(12):1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lopez-Verges S, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(36):14725–32. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes and infection / Institut Pasteur. 2002;4(15):1545–58. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 153.Foley B, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. Journal of immunology. 2012;189(10):5082–8. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hwang I, et al. Identification of human NK cells that are deficient for signaling adapter FcRgamma and specialized for antibody-dependent immune functions. International immunology. 2012;24(12):793–802. doi: 10.1093/intimm/dxs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang T, et al. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. Journal of immunology. 2013;190(4):1402–6. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lichtfuss GF, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. Journal of immunology. 2012;189(3):1491–9. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- 157.Brunstein CG, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Elmaagacli AH, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–12. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 159.Ito S, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone marrow transplantation. 2013 doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Green ML, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–24. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Miller JS, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 162.Zhou Q, et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114(18):3793–802. doi: 10.1182/blood-2009-03-208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi J, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol. 2008;143(5):641–53. doi: 10.1111/j.1365-2141.2008.07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bachanova V, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59(11):1739–44. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Geller MA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Romagne F, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114(13):2667–77. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vey N, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–23. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 168.Benson DM, Jr., et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120(22):4324–33. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reff ME, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–45. [PubMed] [Google Scholar]
- 170.Harrison D, Phillips JH, Lanier LL. Involvement of a metalloprotease in spontaneous and phorbol ester-induced release of natural killer cell-associated Fc gamma RIII (CD16-II) J Immunol. 1991;147(10):3459–65. [PubMed] [Google Scholar]
- 171.Borrego F, et al. Downregulation of Fc gamma receptor IIIA alpha (CD16-II) on natural killer cells induced by anti-CD16 mAb is independent of protein tyrosine kinases and protein kinase C. Cell Immunol. 1994;158(1):208–17. doi: 10.1006/cimm.1994.1268. [DOI] [PubMed] [Google Scholar]
- 172.Grzywacz B, Kataria N, Verneris MR. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia. 2007;21(2):356–9. doi: 10.1038/sj.leu.2404499. author reply 359. [DOI] [PubMed] [Google Scholar]
- 173.Liu Q, et al. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol. 2009;83(17):8705–12. doi: 10.1128/JVI.02666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Edsparr K, et al. Effects of IL-2 on MMP expression in freshly isolated human NK cells and the IL-2-independent NK cell line YT. J Immunother. 2010;33(5):475–81. doi: 10.1097/CJI.0b013e3181d372a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu J, et al. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–98. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 176.Romee R, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121(18):3599–608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou Q, et al. Matrix metalloproteinases inhibition promotes the polyfunctionality of human natural killer cells in therapeutic antibody-based anti-tumour immunotherapy. Clin Exp Immunol. 2013;173(1):131–9. doi: 10.1111/cei.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Peruzzi G, et al. Membrane-Type 6 Matrix Metalloproteinase Regulates the Activation-Induced Downmodulation of CD16 in Human Primary NK Cells. J Immunol. 2013;191(4):1883–94. doi: 10.4049/jimmunol.1300313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ferrini S, et al. Targeting of T or NK lymphocytes against tumor cells by bispecific monoclonal antibodies: role of different triggering molecules. Int J Cancer Suppl. 1992;7:15–8. [PubMed] [Google Scholar]
- 180.Hombach A, et al. A CD16/CD30 bispecific monoclonal antibody induces lysis of Hodgkin’s cells by unstimulated natural killer cells in vitro and in vivo. Int J Cancer. 1993;55(5):830–6. doi: 10.1002/ijc.2910550523. [DOI] [PubMed] [Google Scholar]
- 181.Renner C, Pfreundschuh M. Treatment of heterotransplanted Hodgkin’s tumors in SCID mice by a combination of human NK or T cells and bispecific antibodies. J Hematother. 1995;4(5):447–51. doi: 10.1089/scd.1.1995.4.447. [DOI] [PubMed] [Google Scholar]
- 182.Sahin U, et al. Interleukin-12 increases bispecific-antibody-mediated natural killer cell cytotoxicity against human tumors. Cancer Immunol Immunother. 1996;42(1):9–14. doi: 10.1007/s002620050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Renner C, Hartmann F, Pfreundschuh M. Treatment of refractory Hodgkin’s disease with an anti-CD16/CD30 bispecific antibody. Cancer Immunol Immunother. 1997;45(3-4):184–6. doi: 10.1007/s002620050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hartmann F, et al. Treatment of refractory Hodgkin’s disease with an anti-CD16/CD30 bispecific antibody. Blood. 1997;89(6):2042–7. [PubMed] [Google Scholar]
- 185.Hartmann F, et al. Anti-CD16/CD30 bispecific antibodies as possible treatment for refractory Hodgkin’s disease. Leuk Lymphoma. 1998;31(3-4):385–92. doi: 10.3109/10428199809059232. [DOI] [PubMed] [Google Scholar]
- 186.da Costa L, et al. Immune recruitment by bispecific antibodies for the treatment of Hodgkin disease. Cancer Chemother Pharmacol. 2000;46(Suppl):S33–6. doi: 10.1007/pl00014047. [DOI] [PubMed] [Google Scholar]
- 187.Renner C, et al. Initiation of humoral and cellular immune responses in patients with refractory Hodgkin’s disease by treatment with an anti-CD16/CD30 bispecific antibody. Cancer Immunol Immunother. 2000;49(3):173–80. doi: 10.1007/s002620050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Glorius P, et al. The novel tribody ((CD20)(2)×CD16) efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia. 2013;27(1):190–201. doi: 10.1038/leu.2012.150. [DOI] [PubMed] [Google Scholar]
- 189.Kipriyanov SM, et al. Synergistic antitumor effect of bispecific CD19 × CD3 and CD19 × CD16 diabodies in a preclinical model of non-Hodgkin’s lymphoma. J Immunol. 2002;169(1):137–44. doi: 10.4049/jimmunol.169.1.137. [DOI] [PubMed] [Google Scholar]
- 190.Kellner C, et al. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother. 2008;31(9):871–84. doi: 10.1097/CJI.0b013e318186c8b4. [DOI] [PubMed] [Google Scholar]
- 191.Kellner C, et al. Heterodimeric bispecific antibody-derivatives against CD19 and CD16 induce effective antibody-dependent cellular cytotoxicity against B-lymphoid tumor cells. Cancer Lett. 2011;303(2):128–39. doi: 10.1016/j.canlet.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 192.Portner LM, et al. T and NK cells of B cell NHL patients exert cytotoxicity against lymphoma cells following binding of bispecific tetravalent antibody CD19 × CD3 or CD19 × CD16. Cancer Immunol Immunother. 2012;61(10):1869–75. doi: 10.1007/s00262-012-1339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schubert I, et al. A single-chain triplebody with specificity for CD19 and CD33 mediates effective lysis of mixed lineage leukemia cells by dual targeting. MAbs. 2011;3(1):21–30. doi: 10.4161/mabs.3.1.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Silla LM, et al. Potentiation of lysis of leukaemia cells by a bispecific antibody to CD33 and CD16 (Fc gamma RIII) expressed by human natural killer (NK) cells. Br J Haematol. 1995;89(4):712–8. doi: 10.1111/j.1365-2141.1995.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 195.Weiner LM, et al. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. Cancer Res. 1995;55(20):4586–93. [PubMed] [Google Scholar]
- 196.Weiner LM, et al. Clinical development of 2B1, a bispecific murine monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. J Hematother. 1995;4(5):453–6. doi: 10.1089/scd.1.1995.4.453. [DOI] [PubMed] [Google Scholar]
- 197.Shahied LS, et al. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J Biol Chem. 2004;279(52):53907–14. doi: 10.1074/jbc.M407888200. [DOI] [PubMed] [Google Scholar]
- 198.Xie Z, et al. A trivalent anti-erbB2/anti-CD16 bispecific antibody retargeting NK cells against human breast cancer cells. Biochem Biophys Res Commun. 2003;311(2):307–12. doi: 10.1016/j.bbrc.2003.09.211. [DOI] [PubMed] [Google Scholar]
- 199.Ferrini S, et al. Use of anti-CD3 and anti-CD16 bispecific monoclonal antibodies for the targeting of T and NK cells against tumor cells. Cancer Detect Prev. 1993;17(2):295–300. [PubMed] [Google Scholar]
- 200.Ferrini S, et al. Bispecific monoclonal antibodies directed to CD16 and to a tumor-associated antigen induce target-cell lysis by resting NK cells and by a subset of NK clones. Int J Cancer. 1991;48(2):227–33. doi: 10.1002/ijc.2910480213. [DOI] [PubMed] [Google Scholar]
- 201.Gleason MK, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11(12):2674–84. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vallera DA, et al. Heterodimeric Bispecific Single-Chain Variable-Fragment Antibodies Against EpCAM and CD16 Induce Effective Antibody-Dependent Cellular Cytotoxicity Against Human Carcinoma Cells. Cancer Biother Radiopharm. 2013 doi: 10.1089/cbr.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wiernik A, et al. Targeting Natural Killer Cells to Acute Myeloid Leukemia In Vitro with a CD16 × 33 Bispecific Killer Cell Engager and ADAM17 Inhibition. Clin Cancer Res. 2013;19(14):3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]