Abstract
Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) and classical Hodgkin lymphoma (CHL) are classified separately because of their distinct clinical and pathological features. While EBV is detected in the neoplastic cells of 25-70% of CHL, NLPHL is generally considered to be EBV-negative. We assessed EBV status in 302 pediatric and adult cases of NLPHL. A total of 145 pediatric (age ≤18) and 157 adult cases of NLPHL were retrieved from 3 North American centers and tested for EBV by in situ hybridization (EBER). Clinical and pathological features were analyzed. Five (3.4%) pediatric and 7 (4.5%) adult NLPHL cases contained EBV-positive LP cells. While all 12 cases met criteria for the diagnosis of NLPHL, atypical features were present, including capsular fibrosis, atrophic germinal centers and pleomorphic or atypical lymphocyte predominant (LP) cells. Both CD20 and OCT-2 expression were strong and diffuse in all except one case. However, PAX5 and CD79a were weak and/or variable in 7/8 and 6/6 cases tested, respectively. EBV-positive cases were more likely to be CD30-positive (75%) than EBV-negative cases (25%) (p=0.0007); CD15 was negative in all cases. Our results show that EBV-positive LP cells may occur in NLPHL. Distinguishing EBV+ NLPHL from CHL can be challenging, since EBV+ NLPHL can have partial expression of CD30 and weak PAX5 staining as well as pleomorphic-appearing LP cells. However, the overall appearance and maintenance of B-cell phenotype, with strong and diffuse CD20 and OCT-2 expression, support the diagnosis of NLPHL in these cases.
Keywords: Nodular lymphocyte predominant Hodgkin lymphoma, Epstein-Barr virus, epidemiology, Hodgkin’s lymphoma, immunohistochemistry
INTRODUCTION
Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is an infrequently encountered B-cell neoplasm most commonly involving the cervical, axillary or inguinal lymph nodes of young adult males. The neoplastic cells comprise a minor component of the lymphoid tissue on histological sections and are termed lymphocyte predominant (LP) cells. LP cells, previously known as lymphocytic and histiocytic (L&H) cells, are large with scant cytoplasm, a single multilobated or folded nucleus, and multiple small basophilic nucleoli. The cellular milieu consists of small lymphocytes and histiocytes with underlying expanded meshworks of follicular dendritic cells. Small B cells are typically numerous in early lesions but can decrease over time with replacement of the follicular structure (1). LP cells maintain a B-cell phenotype, with expression of CD20, CD79a, OCT-2, and BOB.1 as well as CD45 and BCL6. LP cells may also be positive for EMA, but CD30 and CD15 are typically negative (2-4). The prognosis of NLPHL patients is generally good, although the disease frequently relapses after prolonged periods of remission (5).
NLPHL and classical Hodgkin lymphoma (CHL) share some similarities but overall have disparate epidemiologic, clinical, pathological, and prognostic features, justifying their separation into discrete entities. One helpful feature is the frequent detection of Epstein-Barr virus (EBV) in the neoplastic Hodgkin and Reed-Sternberg cells (HRS) cells of CHL, while EBV is reported to be absent in most cases of NLPHL (4). While cases of EBV-positive NLPHL have been reported in the literature, mainly originating from Asia and Latin America (6-20), but no large-scale study has been performed to determine the occurrence of EBV-positive NLPHL in a North American patient population. Thus, we aimed to investigate the frequency of EBV infection in LP cells of NLPHL cases submitted to 3 large reference centers in the United States and Canada.
MATERIALS & METHODS
Following approval by the institutional review board at each location, the archives of the National Cancer Institute (NCI), the Massachusetts General Hospital (MGH) and the British Columbia Cancer Agency (BCCA) were searched for cases diagnosed as NLPHL in which EBER-ISH had been performed, or for which material was available to perform this test. Available clinical data, histological features, and IHC results were recorded for each case. IHC stains and EBER had been performed by standard methods at each institution and included stains for CD20, OCT-2, CD30, CD15, and CD3 in most cases. All EBV-positive cases underwent central review at the NCI by a panel of 4 pathologists (ARH, AN, SP, ESJ). Two cases initially identified as possible EBV-positive NLPHL were later excluded after central review because they were felt to be aggressive B-cell lymphomas. A total of 302 NLPHL cases (220 NCI, 51 MGH, 31 BCCA) diagnosed between the years 2000 and 2013 were included in the study; 30 of these cases have been previously published by one of the institutions (21). All EBV-positive cases with available unstained slides or paraffin-embedded blocks were subjected to an expanded IHC panel including CD20, PAX5, CD79a, OCT-2, BOB.1, CD30, CD15, BCL6, EMA, IgD, CD3, PD-1, CD57, CD21, and LMP1. Double staining for CD20/EBER and PD-1/EBER was performed on one case. For double staining EBER was performed first followed by CD20 and PD-1 respectively according to the standard procedure of a single staining. The EBV-positive cases were further categorized according to the architectural and immuno-histological features described by Fan et al.(1) The patterns were assigned by consensus of the panel of 4 pathologists based on review of all available histological and IHC slides. Patterns were included if they comprised at least 5% of the total lymphoma surface area in the available slides.
Patient clinical characteristics were summarized as numbers and percentages for categorical variables, and median and range for continuous variables. Comparisons between categorical variables were analyzed by Fisher's exact test, and Mann-Whitney test was used for numerical comparisons between groups. Statistical analyses were calculated using the Prism software package, version 5 (GraphPad Software, Inc, La Jolla, CA). P values of 0.05 or less were regarded as statistically significant.
RESULTS
Assessment of 302 NLPHL cases
The study included 145 pediatric (age ≤ 18 years) and 157 adult (age > 18 years) patients (Table 1). NLPHL affected predominantly males, with a significantly higher male:female ratio in children (11:1) versus adults (3.5:1) (p=0.0005). The median age was 12 years in pediatric patients and 35 years in adults. Biopsied lymph nodes in children were more commonly cervical (p<0.0001) and less commonly axillary (p<0.0001) or intra-abdominal (p=0.001) than in adults (data not shown).
Table 1.
Clinical features and immunophenotype of LP cells in 302 cases of NLPHL diagnosed in the US & Canada
| All patients (n=302) |
EBV+ (n=12) | EBV- (n=290) | Comparison of EBV+ vs EBV- |
|
|---|---|---|---|---|
| Age, median (range) | 19 (4-80) | 25.5 (4-62) | 19 (4-80) | NS |
| M:F ratio | 5.2:1 | 5:1 | 5.3:1 | NS |
| CD20 | 99% (299/301) | 100% (12/12) | 99% (287/289) | NS |
| CD79a | 98% (49/50) | 100% (6/6) | 98% (43/44) | NS |
| PAX5 | 100% (73/73) | 100% (8/8) | 100% (65/65) | NS |
| OCT-2 | 99% (166/167) | 100% (11/11) | 99% (155/156) | NS |
| BOB.1 | 90% (43/48) | 60% (3/5) | 93% (40/43) | NS |
| BCL6 | 96% (117/122) | 91% (10/11) | 96% (107/111) | NS |
| EMA | 42% (59/142) | 0% (0/8) | 44% (59/134) | p=0.02 |
| CD30 | 28% (75/270) | 75% (9/12) | 25% (66/258) | p=0.0007 |
| CD15 | 3% (8/266) | 0% (0/12) | 3% (8/254) | NS |
| IgD | 57% (119/208) | 20% (2/10) | 59% (117/198) | ND* |
LP = lymphocyte predominant; NLPHL = nodular lymphocyte predominant Hodgkin lymphoma; M = male; F = female; EBV = Epstein-Barr virus. NS =Not significant. ND = Not done.
Statistical analysis not performed because IgD staining was performed on mostly younger patients (median age 16), a population with a known higher incidence of IgD+ LP cells.
LP cells maintained a B-cell phenotype, with positive staining for CD20 in 99% of all cases, CD79a in 98%, PAX5 in 100%, OCT-2 in 99%, and BOB.1 in 90%. Additionally, LP cells expressed BCL6 in 97% and EMA in 42% of cases. LP cells were CD30-positive in 75/270 (28%) cases, but the staining was usually focal or heterogeneous in LP cells. Eight cases expressed CD15 in LP cells (8/266, 3%), all of which were EBV-negative, and no cases co-expressed CD30 and CD15. No differences were identified in the immunoprofile of LP cells between pediatric and adult age groups (data not shown) with the exception of IgD: 87/124 (70%) of pediatric cases versus 32/84 (38%) of adult (> 18 yrs) cases tested expressed IgD in LP cells (p<0.0001). Most of these patients were males, with a male:female ratio of 16:1. The median age of the IgD-positive cases was 14 years, compared to 21 years for the IgD-negative cases (p<0.0001); however, all patients with IgD-positive LP cells were 25 years or younger. The median age of all cases tested for IgD was 16, whereas the median age of those cases not tested for IgD was 37, indicating some selection bias in the performance of this test.
Occasional background small lymphocytes and/or immunoblasts were EBV-positive by EBER ISH in 21 (14%) pediatric and 23 (15%) adult cases. In addition, EBER ISH was positive in the LP cells of 5 (3.4%) pediatric and 7 (4.5%) adult patients, and these 12 cases were the main focus of our study.
Description of 12 cases of EBV-positive NLPHL
The patients’ ages ranged from 4-62 years (median 44 years) (Table 2). All 5 pediatric patients were males, while 5/7 (71%) adult patients were males. One pediatric patient was native to Saudi Arabia, a second was from Paraguay, and a third was of American Samoan ancestry but currently residing in Hawaii. The remaining patients were residents of the United States or Canada. Biopsied lymph nodes included cervical (4), axillary (4), submandibular (2), and supraclavicular (2) locations. Patient 12 had a history of stage IA CHL diagnosed 9 years earlier, but this material was not available for our review. Only limited information regarding EBV viral loads, serologic studies, and follow-up data was available for these 12 patients. Of the 6 patients with known clinical staging, 2 were Ann Arbor Stage IA, 1 was Stage II, 2 were Stage III, and 1 was Stage IV. Two patients were treated with combined chemotherapy and radiotherapy and two patients were treated with therapy of unknown type; the treatment status of the remaining 8 patients is unknown. Of the 6 patients with available followup information, 4 are free of disease 1.3, 2, 3.5, and 7.5 years after diagnosis; 1 patient relapsed 6 years after diagnosis, and 1 patient has reduced lymphadenopathy with therapy currently ongoing.
Table 2.
Clinical features and immunophenotype of LP cells in EBV-positive NLPHL
| Case | Age (yrs) |
Sex | Nationality | Anatomic site (LN) |
Architectural pattern (Fan 2003)(1) |
EBER | LMP1 | CD20 | CD79a | PAX5 | OCT-2 | BOB.1 | CD30 | BCL6 | IgD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | M | USA | Cervical | E + A | + | ND | + | ND | ND | ND | ND | + fw | ND | – |
| 2 | 4 | M | USA (American Samoa) |
Submandibular | B + E | + | ND | + | ND | ND | + | ND | – | + f | + f |
| 3 | 5 | M | Saudi Arabia |
Submandibular | E + C + A | + | + f | + | Unsat | ND | + | + | + f | + | + |
| 4 | 12 | M | Paraguay | Cervical | A | + | ND | + | ND | ND | + | ND | + f | + | – |
| 5 | 9 | M | USA | Axillary | A + C | + | + | + | + fw | + w | + | – | + v | + fw | – |
| 6 | 26 | F | USA | Axillary | A | + | – | + | +w | + v | + | ND | – | + f | – |
| 7 | 62 | F | Canada | Axillary | A | + | ND | + | ND | + w | + | ND | – | + | – |
| 8 | 49 | M | USA | Supraclavicular | A + C | + | + f | + | + fw | + w | + | – | + f | + fw | ND |
| 9 | 44 | M | USA | Cervical | A | + | ND | + v | ND | + w | + | + | + fw | + f | ND |
| 10 | 35 | M | USA | Supraclavicular | B + A | + | + | + | + fw | + w | + | ND | + v | + fw | – |
| 11 | 25 | M | USA | Cervical | C + A | + | ND | + | + w | + | + | ND | + v | + f | – |
| 12 | 44 | M | USA | Axillary | C + A + E | + | + | + | + w | + v | + | + | + v | – | – |
LP = lymphocyte predominant; EBV = Epstein-Barr virus; NLPHL = nodular lymphocyte predominant Hodgkin lymphoma; yrs = years; M = male; F = female; LN = lymph node; + = positive; – = negative; ND = not done; f = focal; v = variable intensity; w = weak; Unsat = technically unsatisfactory for interpretation; Fan et al. architectural patterns: A, “Classic” B-cell-rich nodular pattern. B, Serpiginous/interconnected nodular pattern. C, Nodular with prominent extranodular LP cells. D, Nodular with T-cell-rich background. E, Diffuse, T-cell-rich B-cell lymphoma-like pattern. F, Diffuse “moth-eaten” with B-cell-rich background.
On review by the panel, all 12 EBV-positive cases met diagnostic criteria for NLPHL based on the histologic pattern and IHC expression profile. All cases displayed at least a partially nodular architectural pattern (Fig. 1A); cases from 3 children (cases 1-3) and 1 adult (case 12) also exhibited diffuse areas. However, none of the EBV-positive cases showed progression to T-cell/histiocyte-rich large B-cell lymphoma-like areas (THRLBCL) in the material submitted for review. The nodular areas contained small lymphocytes, histiocytes, and large atypical cells consistent with LP cells (Figs. 1B, 2A). Epithelioid histiocytes were identified in 11/12 patients, including necrotizing granulomas in one case (case 6) (Fig. 3B). One case (case 2) exhibited geographic areas of necrosis. Residual germinal centers were seen in 8 cases, and 3 of these demonstrated partially involuted germinal centers within the nodules. Focal capsular sclerosis was identified in 5/12 (42%) cases, and 3 of these also contained focal bands of nodular fibrosis (Fig. 3A). The LP cells were not only seen within nodules but were also present between the nodules in 3 adult patients as well as the 4 previously described cases with diffuse areas (1). All of the pediatric cases with diffuse areas contained many mummified and/or apoptotic cells, a feature also noted in three purely nodular adult cases (8, 9 and 10). Tumor cells displayed marked pleomorphism in 2 cases (8 and 10) (Fig. 3D) and had morphologic features reminiscent of HRS cells in 4 cases (3, 5, 7 and 12).
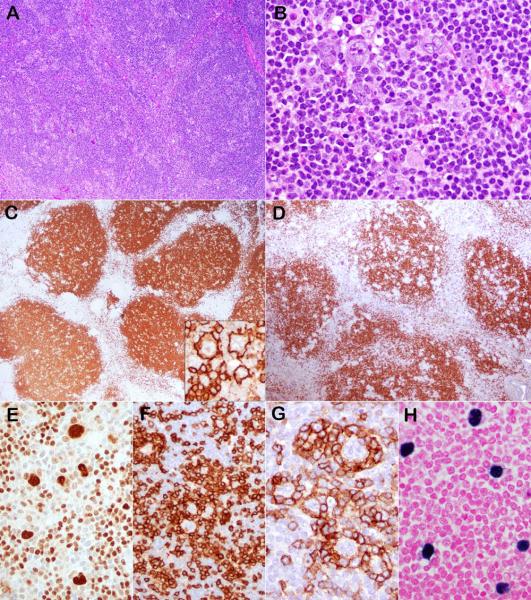
Figure 1.
Case 6 had the typical morphology and immunophenotype for NLPHL but was positive for EBV. (A) The normal lymph node architecture was effaced by a nodular infiltrate consisting of small lymphocytes, epithelioid histiocytes and scattered LP cells (B) with multilobated nuclei. The small lymphocytes express CD20 (C) and IgD (D). The LP cells are strongly positive for CD20 (C inset) and OCT-2 (E), are rosetted by CD3-positive (F) and PD1-positive (G) T cells, and are EBV-positive by EBER (H).
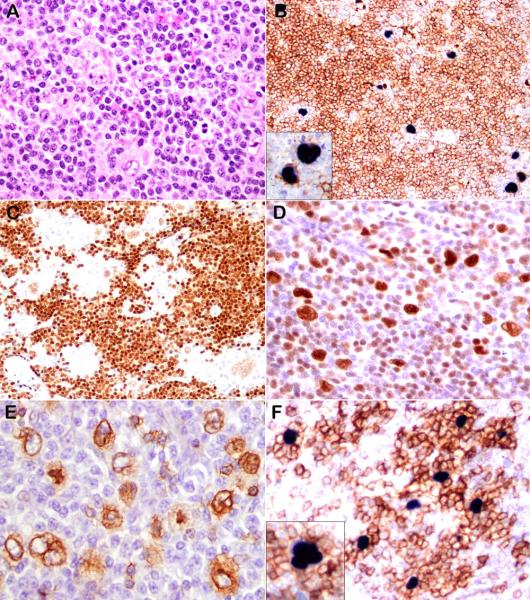
Figure 2.
Representative examples of EBV-positive NLPHL, cases 3 and 12. (A) LP cells are present in a background of small lymphocytes. (B) Double-staining demonstrates that the LP cells are strongly positive for CD20 and EBER. (C) PAX5 is weakly positive. LP cells also express BCL-6 (D) and IgD (E). PD-1 positive T cells rosette the EBER-positive cells (F, double stain) (case 3: A, D, E; case 12: B, C, F).
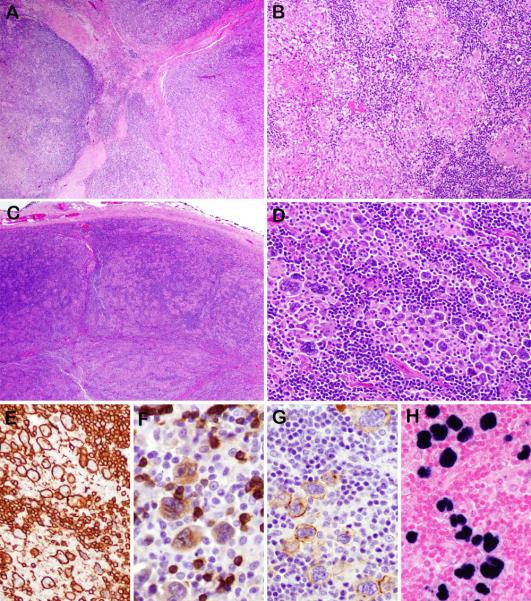
Figure 3.
Unusual morphological findings in some examples of EBV-positive NLPHL . Case 3, (also illustrated in Figure 2) demonstrates capsular fibrosis with focal fibrous septa (A). Case 6 (also illustrated in Figure 1) exhibits a prominent granulomatous reaction with focal areas of necrosis (B). In case 10 the low power nodular appearance is typical (C). However, the LP cells are larger than expected with more pleomorphic and polylobated nuclei arranged in clusters (D). They are strongly positive for CD20 (E), which also highlights the small B cells in the background. They are weakly positive for CD79a (F) and CD30 (G). EBER was positive (H).
The immunophenotypes of the EBV-positive and EBV-negative NLPHL cases are summarized in Table 2. Three EBER-positive cases were uniformly LMP1-positive, 2 cases were focally LMP1-positive, and one case was LMP1-negative. The LP cells expressed CD20 (12/12) (Figs. 1C inset, 3E), PAX5 (8/8), CD79a (6/6), and OCT-2 (11/11) (Fig. 1E) in all cases tested and BOB.1 (3/5, 60%) in the majority of tested cases. CD20 expression was strong and diffuse in 11/12 (92%) cases and OCT-2 was strongly and diffusely positive in all 11 cases tested. Confirmation that the EBV-positive cells were also strongly positive for CD20 was demonstrated on case 12 with double staining (Fig. 2B). PAX5 (Fig. 2C) and CD79a (Fig. 3F) were weak and/or variable in 7/8 (88%) and in all 6/6 (100%) cases tested, respectively. BCL6 was positive in LP cell nuclei in 10/11 cases (91%) (Fig. 2D), although the staining was focal and/or weak in 7 cases. Two pediatric but no adult cases were positive for IgD (Fig. 2E). Of note, CD30 was at least focally or weakly positive in 75% (9/12) of EBV-positive NLPHL (Fig. 3G), in contrast to 26% (66/258) EBV-negative NLPHL (p=0.0007). None of the EBV-positive cases demonstrated strong, uniform CD30 staining and all were negative for CD15. No EMA expression was seen in the 8 EBV-positive cases tested, versus in 59/134 cases (44%) of EBV-negative NLPHL cases tested (p=0.02). In the background cellularity, the nodular areas were composed mostly of CD20- and IgD-positive small B cells (Figs. 1C, 1D). CD21-positive follicular dendritic cell meshworks were identified in all 11 cases stained, with a notable expansion of the meshworks in 8 cases. CD3-positive T-cell rosettes surrounding the LP cells were observed in 11/12 cases (92%) (Fig.1F). Three of 4 tested cases contained increased CD57-positive cells, including 2 with rosettes around LP cells. Increased PD1-positive cells were present in 8/10 cases (80%), and these PD1-positive cells formed rosettes around LP cells in 9/10 cases (90%) (Fig. 1G). Double-staining with PD-1 and EBER confirmed rosetting of EBER-positive LP cells by the PD1-positive cells (Fig. 2F).
DISCUSSION
EBV, a linear 172 kbp double-stranded DNA herpesvirus, preferentially infects human B-cells via the CD21 surface antigen and establishes life-long persistent infection in more than 90% of the world’s population (22). Evidence of EBV infection can be found in the neoplastic cells of several hematopoietic and non-hematopoietic malignancies. In particular, EBV is frequently identified in the neoplastic cells of CHL (predominantly of mixed cellularity type), in some T-cell lymphomas, in diffuse large B-cell lymphomas (affecting elderly or immunocompromised patients), and in Burkitt lymphoma. However, the malignant cells of NLPHL are, in general, considered to be negative for EBV (4) and indeed, none of the 3 centers involved in this study routinely perform testing for EBV in NLPHL. Nevertheless, all 3 institutions have received EBV-positive NLPHL cases in consultation, and thus we sought to identify whether EBV positivity in NLPHL may be under-recognized. We found EBV in slightly less than 4% of the 302 cases analyzed. Since we included EBV-positive cases sent in consultation, the incidence in unselected NLPHL cases may be even lower.
Based on a comprehensive literature review we identified 39 cases of NLPHL reported to be positive for EBV in prior studies (6-20) (Table 3). Most positive cases were identified in reports from South America, Asia, or Africa, where there is a greater prevalence of EBV-related lymphoproliferative disorders and a higher prevalence of EBV in CHL as well. Only one positive case from the United States has been reported, in which a single positive case was encountered in a study of 16 pediatric cases (17). Another confounding factor in assessing the EBV status of NLPHL is that prior to the year 2000, cases diagnosed as NLPHL likely also included cases of lymphocyte-rich CHL (LRCHL) (4), as these two forms of Hodgkin lymphoma were not distinguished in older series. Only two reports identifying EBV in NLPHL have been published since 2000 (7, 18).
Table 3.
EBV-positive NLPHL reported in the literature
| Authors | Country | NLPHL*
cases total tested |
NLPHL cases positive, number (%) |
Patient ages studied |
EBV testing method |
|---|---|---|---|---|---|
| Pallesen et al. 1991 (8) | Denmark | 10 | 1 (10) | NR | LMP1 |
| Herbst et al. 1992 (9) | Germany | 2 | 2 (100) | NR | EBER, LMP1 |
| Murray et al. 1992 (10) | UK | 12 | 1 (8) | NR | LMP1 |
| Weinreb et al. 1992 (11) | UK | 13 | 4 (31) | Children | LMP1 |
| Ambinder et al. 1993 (12) | Honduras | 1 | 1 (100) | Child | EBER, LMP1 |
| USA | 2 | 0 | Children | ||
|
+Preciado MV et al. 1995 (20) |
Argentina | 6 | 1 (17) | Children | LMP1 |
|
+Preciado MV et al. 1995 (13) |
Argentina | 6 | 2 (33) | Children | LMP1, EBER |
| Huh J et al. 1996 (14) | Korea | 1 | 1 | Adult | EBER |
|
*Weinreb et al. 1996 (16) |
Multinational | 31 | 15 (48) | Children | LMP1 |
| *Weinreb et al. 1996 (15) | Kenya | 2 | 2 (100) | Children | LMP1 |
| 3 | 2 (67) | Adults | |||
| Andriko JA et al. 1997 (17) | USA | 16 | 1 (6) | Children | LMP1 |
|
+Preciado MV et al. 1997 (19) |
Argentina | 8 | 2 (25) | Children | LMP1, EBER |
| Chang et al. 2005 (7) | Vietnam | 3 | 3 (100) | Children | EBER |
| Nam-Cha et al. 2009 (18) | Spain | 57 | 1 (2) | NR | EBER |
| Current study | USA & Canada |
301 | 11 (4) | All ages | EBER, LMP1 |
NR = not reported; ISH = in-situ hybridization; IHC = immunohistochemistry.
, = It is unclear from the literature whether reports with the same first author include previously published case.
= It is unclear from the literature whether reports with the same first author include previously published case.
In CHL, EBV positivity is associated with the host’s geographic origin and immune status and is more common in developing countries. For example, upwards of 80% of CHL cases in Africa, 70-80% of cases in Latin America, and about 65% of reported cases in Asian populations are EBV-positive, while fewer than 40% of all cases in the United States (USA) and Europe are EBV-positive (16, 17, 23, 24). Two of the 12 patients in our study were native to the Middle East (Saudi Arabia) and South America (Paraguay), with a third patient originating from American Samoa but living in the USA. The ethnicity of all 302 of our cases is not known for certain, but most were residents of the USA or Canada at the time of biopsy. The geographic differences observed in CHL are thought to reflect genetic variation, socioeconomic status, age, and immune status. Complete histories with medical and social information to include socioeconomic status and immune function were not available for most patients in our study and thus could not be comprehensively assessed. Although the 2 non-Western cases account for 17% of our EBV-positive NLPHL, their exclusion would result in only a slightly lower overall rate in the studied population. No age predilection was identified in the EBV-positive patients, with a similar occurrence in both children (3.4%) and adults (4.5%). In contrast, EBV-positive CHL is reported to be more frequent in pediatric patients (children less than 10 years of age) (25).
Both the methods and interpretation of testing are critical considerations when evaluating EBV status in hematopoietic neoplasms. Previous reports describing EBV in NLPHL have mostly utilized IHC (LMP1 expression) and/or ISH (EBER); some studies confirmed results by PCR. However, PCR performed on tissue may produce confounding results if lymphocytes other than LP cells contain EBV. Indeed, we found scattered EBV-positive non-LP cells in 15% of our NLPHL cases, with similar incidences in both children and adults. This finding has also been reported in the literature for both NLPHL (26) and for CHL (9, 24, 27). The presence of EBV in non-neoplastic cells indicates prior EBV infection but may produce false-positive results when assessed only by PCR methods. Thus, visual examination to identify the cells that actually contain EBV is required to confirm that the virus is present within the neoplastic cells. EBV, when detected in CHL, has been shown to be clonal, indicating infection of the neoplastic clone early in disease evolution (28). Clonality testing for EBV requires isolation of DNA from fresh or frozen tissue samples and this material was not available in our study cohort. Therefore, we could not assess at what point the LP cells acquired the EBV infection.
The diagnosis of NLPHL in some older series is difficult to confirm, since LRCHL was first proposed as distinct from NLPHL in 1994 (29) and was not reliably segregated in clinical series until some years later (30). While these two entities are usually distinct and can be reliably distinguished based on morphology and IHC, a few of our cases did demonstrate overlapping features. For example, regressed germinal centers, often visualized at the periphery of the nodules in lymphocyte-rich CHL, were identified in 3 of the 12 EBV-positive NLPHL cases. LP cells with a Reed-Sternberg cell-like appearance were seen in a subset of the cases. Not only are morphologic features of NLPHL and LRCHL sometimes similar, but their immunophenotypes may also overlap. CD15 expression has been reported in some cases of NLPHL (3, 18) and was identified in a few EBV-negative cases (8/302, 2.7%) in our study, but not in any that were EBV-positive. A significant percentage (29%) of our NLPHL cases showed some staining of LP cells for CD30, although they did not typically have the diffuse and strong expression seen in the HRS cells of CHL. In contrast, none of the NLPHL cases tested by Nam-Cha et al. (18) were reported to be positive for CD30. While this disparity may be due to differences in antibody choice or staining techniques, CD30 positive cases were identified from all 3 participating institutions in our study. Moreover, a recent study found a relatively high incidence of variable CD30 expression in LP cells (2).
Infection with EBV can alter the B-cell immunophenotype, an observation that may help to explain the staining patterns in some of our EBV-positive cases. For example, PAX5 and CD79a were weakly and variably expressed respectively in 88% and 100% of the EBV-positive NLPHL cases in our series. LMP2A has been shown to interfere with the expression of transcription factors, including PAX5, during the development of B cells (31). LMP1 changes the gene expression profile of germinal center B-cells to resemble classical Hodgkin lymphoma cell lines, induces the expression of ID2 (a negative regulator of B-cell differentiation), and down-regulates expression of B-cell receptor components, including CD79a (32). EBNA2 downregulates BCL6 expression in non-Hodgkin lymphoma cell lines (33). Furthermore, a study of diffuse large B-cell lymphoma (DLBCL) in HIV-positive patients found increased CD30 expression and reduced BCL6 expression in EBV-positive as compared to EBV-negative cases (34). The EBV-positive NLPHL cases in our series were significantly more likely to be CD30-positive and weakly express BCL6 than the EBV-negative cases.
Since the differential diagnosis of CHL versus NLPHL may be raised by EBV-positive cases, particularly when atypical morphologic and/or immunophenotypic features are present, how does one render a final diagnosis of EBV-positive NLPHL? This distinction is clinically relevant, since NLPHL and CHL have different prognoses and clinical behavior and may have differing treatments (35). An IHC pattern indicating overall maintenance of B-cell phenotype is one helpful feature. LP cells are readily identified using OCT-2, which is strongly expressed in comparison to the background small B cells and was consistently expressed in both EBV-positive and EBV-negative cases. IgD expression in NLPHL, identified in some young patients (21), should exclude most cases of CHL, although rare cases of LRCHL have been reported to express IgD in HRS cells (18). Rosetting of malignant cells by PD-1 positive T cells is another feature universally seen in NLPHL, although noted by some authors to also occur in a subset of cases of LRCHL(18), (36). Unexpected IHC results such as weak and/or variable PAX5 in LP cells may provide one indication that EBV could be present. If testing for EBV is desired, EBER ISH is recommended rather than LMP1 IHC, as the latter can have more variable or absent staining.
A few of the cases we report were sent to one of our institutions for a second diagnostic opinion only because they were found to be positive for EBV. While this may have increased the number of positive cases in our series, we controlled for this factor by examining a large number of NLPHL cases from our files. Relapse and survival data comparing EBV-positive and EBV-negative groups would be of interest, but we were unable to obtain sufficient follow-up information to reliably assess outcome in our patient cohort.
In summary, EBV-positivity in the LP cells of NLPHL is may occasionally occur in North American patients, in both adults and children. Distinguishing these cases from LRCHL can be diagnostically challenging, since these cases can have partial expression of CD30, weak PAX5 staining, and an atypical appearance of LP cells. However, the architecture and characteristics of the background cells as well as overall maintenance of B-cell phenotype in the LP cells (with strong and diffuse CD20 and OCT-2 expression) support a diagnosis of NLPHL and help avoid an erroneous diagnosis of CHL.
ACKNOWLEDGEMENTS
We wish to thank the staff of the immunohistochemistry laboratories at all 3 participating institutions for their diligent performance of the stains used to complete this study. We also wish to thank the following individuals who contributed cases and follow up information for this study: R. Eisen, Greenwich Hospital, Greenwich, CT; K. Mehta, Monmouth Medical Center, Long Branch, NJ; Y.K. Hirata, Queen’s Medical Center, Honolulu, Hawaii; S. Ahmed, Alhada and Taif Military Hospitals, Taif, Saudi Arabia; L. Galluzzo and C. Sanchez-La Rosa, Hospital Nacional de Pediatría Dr. Prof J. P Garrahan, Buenos Aires, Argentina; L.D. Grant, Professional Pathology Services, Columbia, SC; I. Auer, Calgary Laboratory Services, Calgary, Alberta, Canada; J.R. Rushton, Northeast Baptist Hospital, San Antonio, TX; P. Cong, Integrated Oncology, New York, NY; W. Zhu, Seidman Cancer Center, Sandusky, OH; A. Reese, Ireland cancer Center, Sandusky, OH.
Footnotes
Disclosures: The authors have no relevant conflicts of interest or funding to disclose.
REFERENCES
- 1.Fan Z, Natkunam Y, Bair E, et al. Characterization of variant patterns of nodular lymphocyte predominant hodgkin lymphoma with immunohistologic and clinical correlation. The American journal of surgical pathology. 2003;27:1346–1356. doi: 10.1097/00000478-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Seliem R, Ferry JA, Hasserjian RP, et al. Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) with CD30-positive lymphocyte predominant (LP) cells. Journal of Hematopathology. 2011;4:175–181. doi: 10.1007/s12308-011-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkataraman G, Raffeld M, Pittaluga S, et al. CD15-expressing nodular lymphocyte-predominant Hodgkin lymphoma. Histopathology. 2011;58:803–805. doi: 10.1111/j.1365-2559.2011.03829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostopoulos I, Hansmann ML, Franssila K, et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96:1889–1899. [PubMed] [Google Scholar]
- 5.Swerdlow SH, Harris NL. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; Lyon, France: 2008. CE. e. [Google Scholar]
- 6.Khalidi HS, Lones MA, Zhou Y, et al. Detection of Epstein-Barr virus in the L & H cells of nodular lymphocyte predominance Hodgkin's disease: report of a case documented by immunohistochemical, in situ hybridization, and polymerase chain reaction methods. American journal of clinical pathology. 1997;108:687–692. doi: 10.1093/ajcp/108.6.687. [DOI] [PubMed] [Google Scholar]
- 7.Chang KC, Khen NT, Jones D, et al. Epstein-Barr virus is associated with all histological subtypes of Hodgkin lymphoma in Vietnamese children with special emphasis on the entity of lymphocyte predominance subtype. Human pathology. 2005;36:747–755. doi: 10.1016/j.humpath.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Pallesen G, Hamilton-Dutoit SJ, Rowe M, et al. Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet. 1991;337:320–322. doi: 10.1016/0140-6736(91)90943-j. [DOI] [PubMed] [Google Scholar]
- 9.Herbst H, Steinbrecher E, Niedobitek G, et al. Distribution and phenotype of Epstein-Barr virus-harboring cells in Hodgkin's disease. Blood. 1992;80:484–491. [PubMed] [Google Scholar]
- 10.Murray PG, Young LS, Rowe M, et al. Immunohistochemical demonstration of the Epstein-Barr virus-encoded latent membrane protein in paraffin sections of Hodgkin's disease. The Journal of pathology. 1992;166:1–5. doi: 10.1002/path.1711660102. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb M, Day PJ, Murray PG, et al. Epstein-Barr virus (EBV) and Hodgkin's disease in children: incidence of EBV latent membrane protein in malignant cells. The Journal of pathology. 1992;168:365–369. doi: 10.1002/path.1711680405. [DOI] [PubMed] [Google Scholar]
- 12.Ambinder RF, Browning PJ, Lorenzana I, et al. Epstein-Barr virus and childhood Hodgkin's disease in Honduras and the United States. Blood. 1993;81:462–467. [PubMed] [Google Scholar]
- 13.Preciado MV, De Matteo E, Diez B, et al. Presence of Epstein-Barr virus and strain type assignment in Argentine childhood Hodgkin's disease. Blood. 1995;86:3922–3929. [PubMed] [Google Scholar]
- 14.Huh J, Park C, Juhng S, et al. A pathologic study of Hodgkin's disease in Korea and its association with Epstein-Barr virus infection. Cancer. 1996;77:949–955. doi: 10.1002/(sici)1097-0142(19960301)77:5<949::aid-cncr22>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Weinreb M, Day PJ, Niggli F, et al. The consistent association between Epstein-Barr virus and Hodgkin's disease in children in Kenya. Blood. 1996;87:3828–3836. [PubMed] [Google Scholar]
- 16.Weinreb M, Day PJ, Niggli F, et al. The role of Epstein-Barr virus in Hodgkin's disease from different geographical areas. Archives of disease in childhood. 1996;74:27–31. doi: 10.1136/adc.74.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andriko JA, Aguilera NS, Nandedkar MA, et al. Childhood Hodgkin's disease in the United States: an analysis of histologic subtypes and association with Epstein-Barr virus. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1997;10:366–371. [PubMed] [Google Scholar]
- 18.Nam-Cha SH, Montes-Moreno S, Salcedo MT, et al. Lymphocyte-rich classical Hodgkin's lymphoma: distinctive tumor and microenvironment markers. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2009;22:1006–1015. doi: 10.1038/modpathol.2009.54. [DOI] [PubMed] [Google Scholar]
- 19.Preciado MV, Diez B, Grinstein S. Epstein Barr virus in Argentine pediatric Hodgkin's disease. Leukemia & lymphoma. 1997;24:283–290. doi: 10.3109/10428199709039015. [DOI] [PubMed] [Google Scholar]
- 20.Preciado MV, De Matteo E, Diez B, et al. Epstein-Barr virus (EBV) latent membrane protein (LMP) in tumor cells of Hodgkin's disease in pediatric patients. Medical and pediatric oncology. 1995;24:1–5. doi: 10.1002/mpo.2950240102. [DOI] [PubMed] [Google Scholar]
- 21.Prakash S, Fountaine T, Raffeld M, et al. IgD positive L&H cells identify a unique subset of nodular lymphocyte predominant Hodgkin lymphoma. The American journal of surgical pathology. 2006;30:585–592. doi: 10.1097/01.pas.0000194741.87798.45. [DOI] [PubMed] [Google Scholar]
- 22.Rickinson A. Epstein-Barr virus. Virus research. 2002;82:109–113. doi: 10.1016/s0168-1702(01)00436-1. [DOI] [PubMed] [Google Scholar]
- 23.Quintanilla-Martinez L, Gamboa-Domnquez A, Gamez-Ledesma I, et al. Association of Epstein-Barr virus latent membrane protein and Hodgkin's disease in Mexico. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1995;8:675–679. [PubMed] [Google Scholar]
- 24.Chan JK, Yip TT, Tsang WY, et al. Detection of Epstein-Barr virus in Hodgkin's disease occurring in an Oriental population. Human pathology. 1995;26:314–318. doi: 10.1016/0046-8177(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 25.Glaser SL, Lin RJ, Stewart SL, et al. Epstein-Barr virus-associated Hodgkin's disease: epidemiologic characteristics in international data. International journal of cancer Journal international du cancer. 1997;70:375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.Weiss LM, Chen YY, Liu XF, et al. Epstein-Barr virus and Hodgkin's disease. A correlative in situ hybridization and polymerase chain reaction study. The American journal of pathology. 1991;139:1259–1265. [PMC free article] [PubMed] [Google Scholar]
- 27.Kanavaros P, Sakalidou A, Tzardi M, et al. Frequent detection of Epstein-Barr virus (EBV), EBER transcripts and latent membrane protein-1 (LMP-1) in tumor cells in Hodgkin's disease arising in childhood. Pathology, research and practice. 1994;190:1026–1030. doi: 10.1016/s0344-0338(11)80897-6. [DOI] [PubMed] [Google Scholar]
- 28.Weiss LM, Movahed LA, Warnke RA, et al. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin's disease. N Engl J Med. 1989;320:502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- 29.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 30.Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin's disease and lymphocyte-rich classical Hodgkin's disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin's Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:776–783. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 31.Portis T, Longnecker R. Epstein-Barr virus LMP2A interferes with global transcription factor regulation when expressed during B-lymphocyte development. Journal of virology. 2003;77:105–114. doi: 10.1128/JVI.77.1.105-114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vockerodt M, Morgan SL, Kuo M, et al. The Epstein-Barr virus oncoprotein, latent membrane protein-1, reprograms germinal centre B cells towards a Hodgkin's Reed-Sternberg-like phenotype. J Pathol. 2008;216:83–92. doi: 10.1002/path.2384. [DOI] [PubMed] [Google Scholar]
- 33.Boccellato F, Anastasiadou E, Rosato P, et al. EBNA2 interferes with the germinal center phenotype by downregulating BCL6 and TCL1 in non-Hodgkin's lymphoma cells. Journal of virology. 2007;81:2274–2282. doi: 10.1128/JVI.01822-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao C, Silverberg MJ, Martinez-Maza O, et al. Epstein-Barr virus infection and expression of B-cell oncogenic markers in HIV-related diffuse large B-cell Lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4702–4712. doi: 10.1158/1078-0432.CCR-11-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shankar A, Daw S. Nodular lymphocyte predominant Hodgkin lymphoma in children and adolescents - a comprehensive review of biology, clinical course and treatment options. Br J Haematol. 2012;159:288–298. doi: 10.1111/bjh.12055. [DOI] [PubMed] [Google Scholar]
- 36.Nam-Cha SH, Roncador G, Sanchez-Verde L, et al. PD-1, a follicular T-cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. The American journal of surgical pathology. 2008;32:1252–1257. doi: 10.1097/PAS.0b013e318165b0d6. [DOI] [PubMed] [Google Scholar]