Summary
Much progress has been made towards understanding the mechanistic basis of transplantation tolerance in experimental models, which includes clonal deletion of alloreactive T and B cells, induction of cell-intrinsic hyporesponsiveness, and dominant regulatory cells that mediate infectious tolerance and linked-suppression. Despite encouraging success in the laboratory, achieving tolerance in the clinic remains challenging, but the basis for these challenges are beginning to be understood. Heterologous memory alloreactive T cells generated by infections prior to transplantation have been shown to be a critical barrier to tolerance induction. Furthermore, infections at the time of transplantation and tolerance induction provide a pro-inflammatory milieu that alters the stability and function of regulatory T cells as well as the activation requirements and differentiation of effector T cells. Thus infections can result in enhanced alloreactivity, resistance to tolerance induction, and destabilization of the established tolerance state. We speculate that these experimental findings have relevance to the clinic, where infections have been associated with allograft rejection and may be a causal event precipitating the loss of grafts after long-periods of stable operational tolerance. Understanding the mechanisms by which infections prevent and destabilize tolerance can lead to therapies that promote stable lifelong tolerance in transplant recipients.
Keywords: infections, transplantation tolerance, innate immunity, T and B cells, alloreactivity
Introduction
The Nobel Prize in Medicine in 1960 was awarded jointly to Sir Frank Macfarlane Burnet and Peter Brian Medawar for the discovery of acquired immunological tolerance. Burnet and Fenner (1) put forward a monograph on the theory of antibodies, arguing that the ability to develop immunity to myriads of microorganisms and antigens simultaneously required a mechanism to prevent reactions to one’s own body. In other words, a mechanism must exist that enabled the organism to acquire the ability to distinguish between ‘self’ and ‘foreign’ and to respond appropriately. However, these mechanisms are occasionally overcome, leading the organism into accepting foreign as self. The first evidence suggesting the phenomenon of tolerance to allogeneic cells emerged from the curious observation described by Owen (2), where dizygotic cattle twin retain long-term the red blood cells of dizygotic origin. Since red blood cells have a finite lifespan, Owens inferred that there must have been an exchange of red-cell precursors as a consequence of vascular anastomoses in the placenta that resulted in stable life-long chimerism. Anderson et al. (3) subsequently reported that such twins accepted a skin ‘homograft’ from each other, thereby linking exposure to allogeneic cells in the fetal setting to the development of life-long tolerance to those cells and transplanted tissues of that donor origin. These observations of naturally acquired transplantation tolerance set the stage for the seminal experiments by Billingham et al. (4) in mice, whereupon the deliberate exposure to foreign homologous tissue cells during fetal life resulted in the prolonged acceptance of skin grafts from the same donor in adult life, a state they referred to as actively acquired tolerance. In contrast, exposure to the same foreign tissue for the first time as an adult resulted in graft rejection.
The concept that exposure of alloantigens to an immature immune system can result the acquisition of allo-specific tolerance fueled research over the next 50 years aimed at achieving transplantation tolerance in adult mice. Inducing tolerance in adult animals was considered a critical step, because tolerance induction through fetal or neonatal exposure to alloantigens could not be applied to clinical transplantation, where most recipients would be adults with fully developed and mature immune systems. Successful induction of transplantation tolerance was initially demonstrated using allogeneic or semi-allogeneic bone marrow chimeras generated following total lymphoid irradiation of the adult recipient to ablate pre-existing alloreactivity (5). However, observations of peripheral immune incompetence in full major histocompatibility complex (MHC)-incompatible bone marrow chimeras prompted a modification of the protocol towards the generation of mixed chimeras, where lethally irradiated mice were reconstituted with a mixture of donor and recipient bone marrow cells (6) (Fig. 1). In such mixed bone marrow chimeras, the presence and persistence of donor bone marrow was subsequently demonstrated to be essential for clonal deletion of donor-reactive T cells in the thymus (7, 8), while the presence of recipient APCs was necessary to allow appropriate priming of recipient-restricted CD4+ and CD8+ T cells in the periphery (9–11). Nevertheless, subtle immune defects can be observed for fully MHC-disparate mixed hematopoietic chimeras during persistent or chronic viral infections, because of ineffective priming of effector cells leading to a reduced ability to clear infected donor cells (Fig. 1), which could be partially alleviated by T-cell adoptive therapy or selected MHC haplotype matching (12, 13).
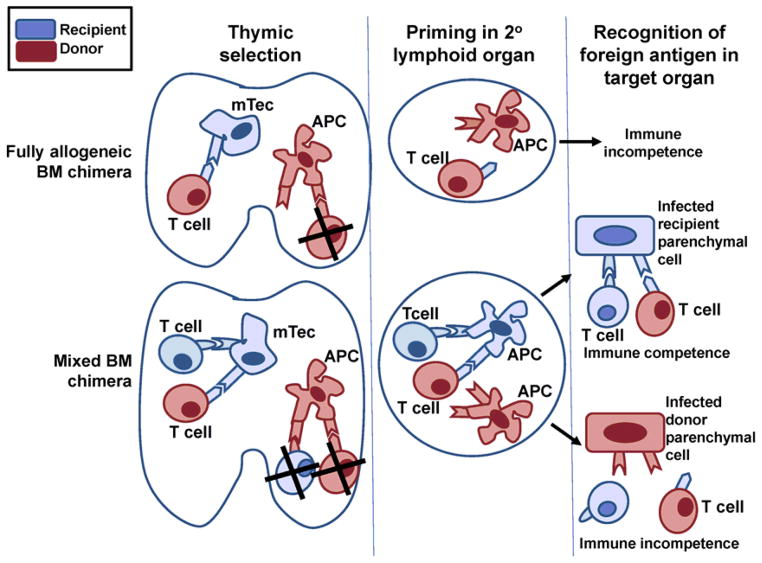
Fig. 1. Reduced peripheral immunity following tolerogenic regimens based on bone marrow chimerism.
In fully MHC mismatched allogenic bone marrow chimeras, recipient hematopoietic cells are completely eradicated and replaced by donor cells. Donor APCs in the thymus trigger negative selection of donor MHC-restricted T cells, while radioresistant thymic medullary epithelial cells (mTecs) of host origin drive positive selection of donor T cells restricted to recipient MHC. Because the peripheral APCs are all of donor origin, positively selected recipient-restricted T cells cannot be primed by donor APCs presenting microbial antigens, resulting in immune incompetence. In mixed bone marrow chimeras, donor APCs induce deletion of donor MHC-restricted host and donor T cells and recipient mTecs enable positive selection of donor and recipient T cells restricted to recipient MHC. In the periphery, these T cells can react with recipient APCs presenting microbial peptides and will therefore be able to recognize infected recipient target cells. However, they cannot recognize donor APCs presenting microbial peptides nor infected donor cells, thus resulting in partial immune incompetence.
Despite these successes in mouse models of tolerance through the reprogramming of the immune system, the translation of these findings to the clinic has not been straightforward, and a number of important barriers have been identified (14, 15). First, the ablation of the existing immune system in adults, to recapitulate an immature immune system, leads to significant loss of protective immunity and heightened susceptibility to infections and malignancies until immune reconstitution by the transplanted bone marrow is established. Second, it is difficult to achieve long-term stable mixed chimerism with MHC-disparate donor-recipient combinations because of rejection of the donor hematopoietic stem cells by residual radio-resistant recipient natural killer (NK) cells and T cells. Third, if full donor-chimerism is achieved in the setting of MHC-incompatibility, graft versus host disease (GVHD), which is mediated by donor mature T cells contaminating the hematopoietic stem cell preparation, becomes a significant problem in the situation where there is no residual host immune system to control them. Finally, as discussed above, in full MHC mismatched donor-recipient hematopoietic chimeras, immunodeficiency can arise even after full hematopoietic cell reconstitution. This immunodeficiency arises, in part, as a result of positive selection of the emerging T-cell repertoire being largely mediated by stromal elements in the recipient thymus bearing recipient MHC, while priming of the T cells in the periphery is by donor-derived antigen-presenting cells (APCs) that repopulated the recipient. Additionally donor class I-restricted CD8+ T cells have to recognize virally infected cells expressing recipient MHC class I molecules (Fig. 1). A recent report by Leventhal et al. (16) suggested that some of these issues may be overcome by the co-transplantation of a ‘facilitator’ cell population (17) together with allogeneic hematopoietic stem cells, as these cells enable full donor chimerism associated with tolerance without overt evidence of immunodeficiency or GVHD. However, the lineage of these ‘facilitator’ cells and how they function remains elusive. Finally, the duration of tolerance and the quality of immune competence in these patients are unclear as their long-term follow-up is still ongoing.
The challenges in translating the early findings of tolerance through fetal introduction of alloantigen to adult recipients and through the induction of fully MHC-incompatible mixed bone marrow chimerism prompted the exploration of new approaches for preventing rejection. This has been a remarkably productive area of investigation that led to the discovery of pharmacological agents that have facilitated transplantation to be the life saving procedure it currently is. Indeed, early studies by Billingham et al. (18) reported on the ability of corticosteroids to convert acute rejection of skin allografts to a feeble, chronic rejection of delayed onset and intermittent progress. Subsequently, immunosuppressive agents such as Cyclosporine A, FK506, prednisone, either alone or in combination, were shown in rodents and dog models to induce long-term graft survival even after pharmacological immunosuppression had been stopped (19–23). Tolerance was formally demonstrated by the acceptance of donor-matched skin or hearts or by the adoptive transfer of lymphocytes from tolerant into naive mice to prevent rejection of fresh donor-matched grafts. Despite these promising observations, the inability of pharmacological immunosuppression to induce long-term tolerance in the vast majority of transplant recipients in the clinic resulted in these findings being forgotten or ignored. Instead, new research focused on discovering biological agents that could rationally induce allograft acceptance and/or tolerance (24, 25). Additionally, research in the past 20 years has moved away from rats to focus on mouse models of allograft tolerance, as immunological tools became available and microsurgical techniques made solid organ transplantation in mice possible. Nonetheless, despite success in tolerance induction with new biological agents in mouse models, the translation from mice to humans has continued to be challenging, recapitulating the experience with pharmacological agents in rats.
We now recognize that the ability of pharmacological immunosuppression to induce transplantation tolerance in rat models in the 1980s is, in fact, prescient of recent emerging evidence of long-term drug-free allograft acceptance, or operational tolerance, in humans that was revealed by deliberate weaning of immunosuppression. While this is not a universal occurrence, the frequency of transplant recipients successfully weaned off conventional immunosuppression and acquiring operational tolerance has been reported to be as high as 38–60% for pediatric liver transplant recipients (26–29). However, the follow-up times were relatively short (median of 23.4–35.7 months). In a prospective multi-center clinical trial of immunosuppressive drug withdrawal of stable adult liver transplant recipients, 41 of 98 (41%) recipients successfully discontinued all immunosuppressive drugs with follow-up of 3 years post-drug withdrawal (26, 27). Thus, despite these promising observations with this cohort of operational tolerant patients, the major caveat was their relatively short follow up times, and indeed, reports of the operationally tolerant patients that exhibited normal liver function presented with a greater extent of fibrosis compared to patients on maintenance immunosuppression (30). The immunopathology observed could be explained by an incomplete state of tolerance in these liver transplant recipients, or by the state of tolerance being metastable and eroded over time.
In contrast to the impressive rates of successful drug weaning for liver transplant recipients, tolerance is a much more infrequent event for kidney transplant recipients, and, even when it occurs, can have variable stability (31–33). The loss of tolerance after years of a stable graft acceptance has been recently described by Brouard et al. (31), who followed a cohort of 27 patients who met the criteria of operational tolerance, namely stable kidney transplant function after weaning off immunosuppressive drugs for at least 1 year. A subset (30%) of these patients subsequently presented with graft dysfunction, with a median duration of operational tolerance of 10 ± 5 years (range 2–16), whereas the rest of the 19 patients remained operationally tolerant for a median drug-free period of 9 ± 4 years. Loss of tolerance was associated with transplant glomerulopathy or IF/TA for the 6 patients for which biopsies were available, with only two of these patients developing donor-specific antibodies (DSA) post-weaning.
The instability of tolerance was not only observed in this cohort of operationally tolerant patients but also is supported by a recent follow-up report by Kawai et al. (34) in patients achieving tolerance through a mixed bone marrow chimerism approach. In the first 4 patients that had follow-up of over 7 years, chronic humoral rejection was diagnosed at 5 years in one patient, donor-specific antibodies and transplant glomerulopathy were observed at 6.8 years in a second patient, while recurrence of original disease was observed in a third at 7 years, with only one of four patients remaining stably tolerant for more than 10 years in these patients. The next cohort consists of 3 operationally tolerant patients who are successfully off immunosuppression without evidence of rejection or donor-specific antibodies at 3–4 years of follow up; it remains uncertain whether these grafts will have the same long-term outcomes as the first 4 recipients.
These recent observations in operationally tolerant humans emphasize a need for critical inquiries into strategies that complement standard immunosuppression and that facilitate the acquisition of tolerance. A means to identify the best transplant candidates for weaning off immunosuppression is also needed, as is the ability to accurately diagnose and monitor the state of tolerance. Finally, despite the limited long-term follow-up of operationally tolerant recipients, the available data suggest that this tolerance may not permanent and completely protect the allograft of immunologically induced injury, so it is now imperative to identify the barriers that prevent the induction of robust tolerance and also those that destabilize established tolerance. In the latter case, it is possible that the state of tolerance was never optimally induced, the optimally induced state of tolerance eroded spontaneously, or specific triggers, such as infections, prompted the erosion. Here we discuss the possibility that infections are a significant barrier to both the induction and the maintenance of transplantation tolerance, focusing first on the known mechanisms of tolerance and on how infections themselves or the pro-inflammatory events they trigger affect these mechanisms of tolerance.
Cell-intrinsic mechanisms of T-cell transplantation tolerance and their stability
The range of therapeutic approaches that successfully induce long-term graft acceptance raises the question of whether each treatment results in a distinct mechanistic basis for tolerance. Much of our understanding on the mechanistic basis of tolerance comes from two main types of experimental models in rodents. As discussed above, the first involves tolerance mediated by transplanted donor hematopoietic stem cells, resulting in central tolerance mechanisms and the ‘reeducation’ of the adaptive immune system to recognize donor antigens as ‘self’. The second involves the induction of peripheral tolerance without hematopoietic stem cell transplantation through the use of either pharmacological immunosuppression or biological agents that target co-receptors on T cells (anti-CD4, anti-CD8)(35–37), costimulatory molecules [e.g. cytotoxic T-lymphocyte antigen-4 immunoglobulin fusion protein (CTLA-4Ig), anti-CD154, or anti-CD40] and adhesion molecules [e.g. anti-leukocyte function-associated antigen-1 (anti-LFA-1), anti-intercellular adhesion molecule-1 (anti-ICAM1), anti-very late antigen-4 (anti-VLA-4)] (24, 25, 38–42). One conclusion from these mouse models is that tolerance mediated by stable hematopoietic cell chimerism is more robust compared to the hyporesponsiveness induced by peripheral mechanisms, based on a more consistent allogeneic skin acceptance following the chimerism mode of tolerance induction. Nevertheless, rodent models indicate that donor hematopoietic stem cell migration to and persistence in the recipient thymus is necessary to continuously induce the deletion of alloreactive T cells that are constantly being replenished by precursors from the bone morrow (8), while the persistence of donor hematopoietic cells resident in the bone marrow is necessary for the deletion of newly generated graft-reactive B cells (43–45). Evidence for the latter comes from studies of B cells specific for the carbohydrate antigen, α 1,3Gal, which is not considered a classical alloantigen.
Despite the critical role for deletion and mixed bone marrow chimerism in rodent models, mixed hematopoietic chimerism across MHC-incompatibility has been notably transient in humans, whereas mixed chimerism is stable when MHC is matched (46–48). So the tolerance in these transient hematopoietic chimeras may be dependent on peripheral mechanisms. Furthermore, studies of self-reactive T and B cells suggest that central mechanisms are leaky and permit significant escape of autoreactive cells into the periphery and that peripheral mechanisms of tolerance are required to restrain these cells (49). Furthermore, depending on the conditioning regimen and how it affects the pre-existing alloreactive repertoire, mechanisms of peripheral tolerance may additionally be necessary. For example, Strober and colleagues (50, 51) demonstrated that in a model of tolerance in mice, conditioning with total lymphoid irradiation and anti-thymocyte globulin (ATG) resulted in the relative sparing of natural killer T (NKT) cells that develop a T-helper 2 (Th2) bias and then direct the polarizing of conventional T cells to Th2 and promote the expansion of interleukin-10 (IL-10)-producing regulatory T cells (Tregs). In that system, NKT cells, IL-4, Tregs, and IL-10 were all necessary for tolerance to develop. Similarly, studies by Sykes and colleagues (52–56) demonstrated that peripheral mechanisms are critical for the early establishment of tolerance induced by bone marrow transplantation and costimulation blockade. Furthermore, CD8+ T cells undergoing tolerance in that model intrinsically required CTLA-4 and programmed death-1 (PD-1) (55) and the nuclear factor for activation of T cells 1 (NFAT1) pathway (56) but also recipient B cells, recipient CD4+ T cells, recipient MHC class II, and recipient dendritic cells (52, 53). These studies demonstrated an unexpectedly complex mechanism of tolerance that involves T-cell-intrinsic and -extrinsic mechanisms for controlling alloreactive CD8+ T cells.
Mechanisms of T-cell peripheral tolerance can be categorized into cell-intrinsic and cell-extrinsic, the latter involving cells with regulatory activity (49, 57, 58) (Fig. 2). Cell-intrinsic T-cell tolerance can be achieved through deletion or hyporesponsiveness that is the result of anergy, exhaustion, or both. Deletion in the periphery can be induced by a combination of Fas-dependent and Fas-independent mitochondrial-dependent apoptosis pathways (reviewed in 59, 60). Insights into the critical role for Fas-dependent T-cell deletion in the periphery came from the Faslpr MRL-stain of mice that have a mutant allele of Fas that fails to transmit a death-inducing signal resulting in spontaneous T-cell lympoproliferative disease and autoimmunity (61). Lau et al. (62) reported on the successful translation of these basic science observations into an effective strategy for inducing allograft acceptance, whereupon islet allograft rejection was prevented by the co-transplantation of myoblasts genetically engineered to express Fas ligand to kill approaching alloreactive T cells. However, this approach was subsequently reported to dominantly induce the infiltration of neutrophils and the rapid destruction of the allogeneic islets (63); thus, alternative approaches to exploiting this pathway are being explored currently (64). Similarly the inhibition of the mitochondrial pathway of death, with the use of recipients expressing a Bcl-xL transgene in T cells, results in animals that are resistant to peripheral tolerance induction with costimulation blocking molecules (65), suggesting that this pathway to delete alloreactive T cells also contributes to tolerance induction. There is an extensive set of checkpoints that regulate T-cell survival and death, and the activation of nuclear factor-κB (NF-κB) downstream of TCR/CD28 ligation has been shown to be essential for the survival of activated T cells (66). In fact, cardiac allograft rejection cannot proceed in mice with impaired NF-κB in T cells (67, 68), unless T-cell survival is restored in these animals by the concomitant transgenic expression of Bcl-xL (69) or the genetic inactivation of Fas (70). Notably, stimulation of innate immune receptors such as Toll-like receptors and cytokine receptors such as those in the tumor necrosis factor receptor (TNFR) family also lead to NF-κB activation in T and B cells (71). Thus, it is likely that infections that stimulate these innate immune receptors and the production of inflammatory cytokines also impede the deletion of alloreactive T and B cells, at least transiently, and antagonize the induction of tolerance (72) (Fig. 3).
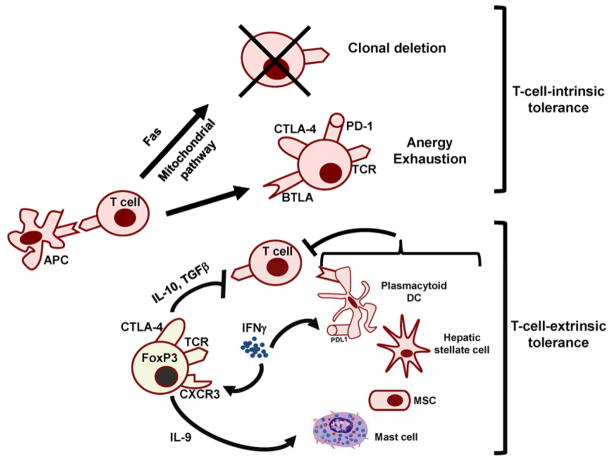
Fig. 2. Mechanisms of peripheral T-cell tolerance implicated in allograft acceptance.
In addition to thymic negative selection, several mechanisms of peripheral tolerance have been shown to operate in-models of transplantation tolerance. These include T-cell-intrinsic and T cell-extrinsic mechanisms.
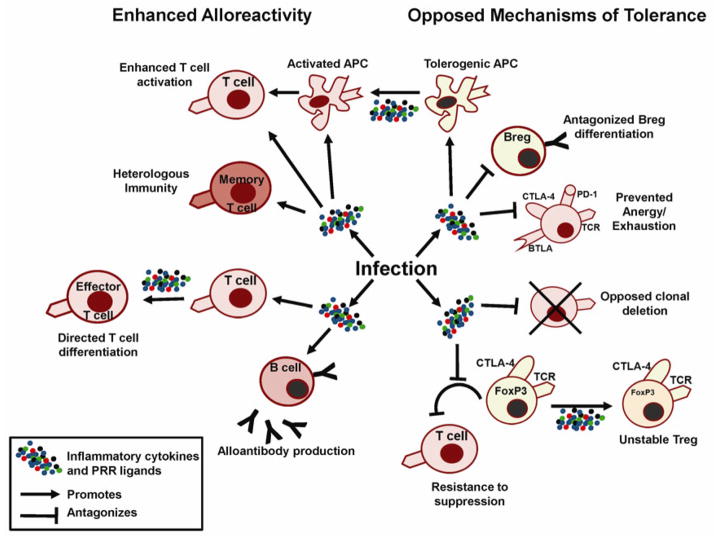
Fig. 3. Impact of infections on alloimmunity and tolerance mechanisms.
Infections result in the production of inflammatory cytokines, chemokines, and ligands for PRRs, resulting in signals that can enhance APC, T and B-cell activation, as well as antagonize adaptive cell-intrinsic and cell-extrinsic mechanisms of tolerance. Both result in augmented alloimmune responses within secondary lymphoid structures during priming as well as within the graft, ultimately leading to graft rejection.
Anergy is defined as a state that develops in T cells following antigen encounter in the absence of costimulatory signals, resulting in their functional inactivation for an extended period of time (73–75). First described with antigen-experienced T-cell lines and clones, signaling through CD28 upon binding to its ligands CD80 and CD86 was shown to prevent the induction of anergy, whereas the absence of this signaling pathway resulted in the induction of anergy (76, 77). These observations led to the hypothesis that blockade of this and another costimulatory pathway, CD40–CD154, would be effective at inducing transplantation tolerance. Seminal observations of tolerance by Lenschow et al. (24) in a xenogeneic pancreatic islet transplantation and Lin et al. (78) in a rat cardiac transplantation models using CTLA-4Ig, and by Larsen et al. (25) in allogeneic skin and cardiac allograft setting with CTLA4-Ig in combination with anti-CD154 were considered harbinger of the long awaited means to achieving tolerance in the clinic. However, while CTLA-4Ig has been reported effective at preventing rejection in the clinic, it failed to induce tolerance in non-human primates or humans (reviewed in 79).
Another distinct state of T-cell hyporesponsiveness termed exhaustion has more recently been described. Exhaustion was first identified by Hosokawa et al. (80) and referred to the hyporesponsiveness of B-cell responses following repeated immunizations in vivo and of T-cell responses as a result of virus persistence (81). The mechanistic basis for the functional impairment in cell exhaustion has recently become better described (82–84). Specifically, the genes for several inhibitory receptors including PD-1 (or Pdcd1) were found to be overexpressed, while genes for chemotaxis, adhesion, and migration were altered. Moreover, T-cell receptor (TCR) and cytokine signaling, metabolic and bioenergetics were significantly modified compared to naive and anergic T cells. The persistence of the allograft raises the possibility that a degree of T-cell exhaustion may also be induced in tolerant transplant recipients. However, without clear markers that discriminate the states of anergy and exhaustion (85), the importance of these mechanisms to the induction and maintenance of transplantation tolerance in various models remains to be definitively established.
Some states of T-cell anergy can be overcome by exogenous IL-2 or by costimulation with 4-1BB or CD134 (OX40) (86–88), while some instances of T-cell exhaustion are reversible following the blockade of the PD-1:PD-L1 inhibitory pathway (82, 89). PD-L1 is constitutively expressed on many cell types including vascular endothelial cells, pancreatic islets and keratinocytes, and indeed blockade of PD-L1 can prevent and reverse established self- and transplantation tolerance (90–93). Interestingly, PD-L1 can be induced in response to inflammatory cytokines and chemokines (94), and thus may be provide increased negative signals during inflammation and infection. Taken together, it is likely that during infection, a complex crosstalk of innate immune stimulation, inflammatory cytokines and costimulatory molecules may modulate T-cell anergy and exhaustion in vivo and thus affect the induction and maintenance of tolerance (Fig. 3).
Regulatory mechanisms of T-cell transplantation tolerance and their stability
The presence of a dominant cell-extrinsic mechanism of tolerance was inferred from early experiments with rats neonatally tolerized to allogeneic skin (95, 96) and subsequently with other models of tolerance including those induced by monoclonal antibodies that targeted T-cell subsets (36, 37, 97, 98). With these newer models, the processes of infectious tolerance and linked suppression became identified (99, 100), and were attributed to a specific subset of CD4+ T cells (20, 101–103). The identity of these suppressor T cells responsible for infectious tolerance was further narrowed down to the CD4+CD25+ subset (104–106) and subsequently to CD4+CD25+Foxp3+ Tregs (107–109). A number of mechanisms by which these Tregs control alloreactive T cells have been identified, including the secretion of IL-10 and/or transforming growth factor-β(TGFβ), as well as the expression of CTLA-4 (104, 105, 110). While natural Tregs may play a critical role in the initiation of transplantation tolerance, more recent studies indicate that induced Tregs (iTregs) also contribute to the induction and maintenance of donor-specific tolerance (111, 112).
The stability of the Treg lineage and of its regulatory activity have come into question based on adoptive transfer and lineage tracing demonstrating that FoxP3 expression can be reduced or FoxP3+ cells can start producing inflammatory cytokines when TGFβ is absent or pro-inflammatory cytokines are present (113–116). Because a putative instability of Tregs at sites of inflammation could have profound implications on their function in vivo and their use in cellular therapies, a number of recent studies have reexamined this issue in greater detail. There is now considerable evidence that natural Tregs generated in the thymus belong to a stable lineage of cells with persistent regulatory activity (117–119), and multiple mechanisms have been identified that confer epigenetic and functional stability to these cells. First identified was the demethylation of a CpG islet located in the regulatory element of the conserved non-coding sequence 2 (CNS2) of the Foxp3 locus (120–123). Rudra et al. (124) identified the transcription factors that regulated Foxp3 expression, while Samstein et al. (125) reported that TCR activation in CD4+ T cells prior to FoxP3 expression generated an enhancer landscape that was later utilized by the Foxp3 gene in Foxp3+ cells. More recently, Ohkura et al. (117, 118) reported that epigenetic changes in hypomethylation patterns downstream of TCR signaling together with Foxp3 expression regulated Treg development. Hypomethylation after TCR stimulation of genes encoding Treg function or Treg-associated molecules such as Foxp3, Tnfrsf18 (glucocorticoid-induced TNRF-related protein), Ctla4, and Ikaros family zinc finger 4 (Ikzf4 or Eos) were observed in Tregs but not in conventional T cells. Furthermore, there is evidence that a small subset of latent Tregs can transiently downregulate FoxP3 expression or function but robustly re-express FoxP3 and reacquire suppressive function upon TCR stimulation (126). Collectively, the data suggest that FoxP3 expression is not sufficient for marking or maintaining the function of Tregs and that an array of Treg-cell-specific epigenetic changes is also critical. Tregs that exhibit the full complement of these epigenetic changes and Foxp3 expression are the most stable.
There is evidence of iTregs that originate from CD4+Foxp3− T cells and acquire FoxP3 expression in the periphery but do not undergo the full epigenetic modification observed in natural Tregs; these cells can go on to lose their Foxp3 expression and give rise to Foxp3− helper T cells under inflammatory or lymphopenic conditions (113, 115, 116, 126). The plasticity of such iTregs can have significant implications for transplantation tolerance, especially since iTregs are induced during tolerance and play critical roles in the induction and maintenance of tolerance (111, 112, 127), and may be generated ex vivo for use in adoptive cellular therapy. Thus, there is considerable interest in identifying demethylating agents that may promote the stabilization of Tregs generated ex vivo for cellular therapy (121, 123, 128). Finally, in addition to the effects of inflammatory cytokines diminishing the regulatory function of Tregs, earlier studies by Pasare and Medzhitov (129) indicated that cytokines, specifically IL-6 produced by dendritic cells (DCs) upon TLR stimulation, may act directly on effector T cells to allow them to escape the suppressive effects of Tregs. Taken together, the pro-inflammatory conditions elicited during infections may trigger the loss of regulatory activity of iTregs as well as stimulate effector alloreactive T cells to override regulation. Both these mechanisms could therefore contribute to resistance to tolerance induction and to the instability of the established tolerant state that is mediated by Tregs (Fig. 3).
In addition to the potential ability of inflammatory cues to destabilize Tregs, there is also evidence that environmental signals can cause Tregs to further differentiate and express transcription factors normally associated with Th cell subsets (reviewed 118). T-bet+ Tregs develop in response to IFNγ to express the Th1-associated chemokine receptor CXCR3 which permits their migration to the same sites as Th1 effector cells to regulate their function in target tissues (130, 131). In addition, IRF4+ Tregs cells have been reported to control Th2 inflammation and the specific deletion of Irf4 in Tregs leads to uncontrolled Th2 immune responses (132). Similarly, loss of STAT3 in Tregs results in uncontrolled Th17 responses and fatal intestinal inflammation (133). STAT3 is a transcription factor downstream of IL-6 that is critical for Th17 differentiation. These observations suggest another layer of complexity with regards to the role pro-inflammatory cytokines play in tolerance induction and maintenance, and may explain in part the unexpected necessity of cytokines associated with rejection, such as IFNγ, in the acquisition of tolerance (134, 135) (Fig. 2).
In addition to canonical CD4+FoxP3+ Tregs, other T-cell subsets with suppressor activity have been implicated in transplantation tolerance, including CD8+CD28− and CD8+FoxP3+ (136–138), TCRαβ+CD3+CD4−CD8−NK1.1− [double negative (DN)] T cells (139, 140) and NKT cells (50, 51, 141). NK cells can also promote islet allograft tolerance following treatment with anti-CD154 or anti-LFA-1, through a perforin-dependent mechanism (142). Studies by Yu et al. (143) suggested that NK cells promoted tolerance by killing donor APCs thereby regulating alloreactive T-cell priming. Most recently, van der Touw et al. (144) reported on a role of NK cells in regulating monocyte or macrophage activation and infiltration in a model of cardiac allograft tolerance mediated by anti-CD154 plus donor-specific transfusion (DST). It is currently unclear how the non-canonical CD4+ and CD8+ regulatory T cells exert their tolerogenic function and what are the effects of pro-inflammatory cytokines on the maintenance of their suppressor state. These are important issues in light of the ability of these cells to secrete pro-inflammatory cytokines and to enhance allospecific T-cell responses and promote allograft rejection (144–146).
DCs are highly efficient antigen-presenting cells and, depending on their activation status, may be potent stimulators of naive T cells or potent mediators of central and peripheral tolerance by inducing clonal deletion, iTregs, and inhibiting memory T-cell responses (147). Indeed tolerogenic DCs have been used to successfully promote tolerance in rodent models and prolong donor-specific allograft survival in non-human primate models (148–150). The plasmacytoid DC subset has been particularly implicated in tolerance induction (151, 152). The mechanisms for the ability of DCs to induce tolerance have been attributed to their production of IL-10 and indoleamine 2,3-dioxygenase (IDO) that catalyzes the rate-limiting step in tryptophan degradation (153), expression of PD-L1 (154) or of the inhibitory receptors of the immunoglobulin-like transcript family, ILT3/IL4 (155). Furthermore, while early studies suggest that immature DCs have tolerogenic properties, it is now accepted that antigen uptake and presentation by DCs require partial maturation, and approaches are being explored to promote and stabilize the tolerogenic properties of DCs, including cytokine modulation with TGFβ, IL-10, and GM-CSF (reviewed 156) and treatment with rapamycin (157, 158). Nevertheless, despite promising experimental observations, there are significant risks and limitations to the translation of this approach in the clinic. For instance, the ligation of pattern recognition receptors (PRRs) on tolerogenic DCs promotes their activation and production of pro-inflammatory cytokines such as IL-6, IL-12, and TNFα (159), which may activate instead of suppress the immune response (Fig. 3). More recently, Wang et al. (160) and Smyth et al. (161) reported that the in vivo administration of drug-treated tolerogenic donor DCs resulted in their rapid death and the uptake and presentation of donor DC-derived MHC alloantigens by recipient APCs that could ultimately result in the promotion of alloimmune responses. These observations thus call into question the robustness of the approach of utilizing adoptively transferred DCs for tolerance induction, even though tolerogenic DCs may be critical in the de novo generation and maintenance of tolerance.
A range of cells of the myeloid lineage, including mast cells, myeloid-derived suppressor cells, alternatively activated macrophages and regulatory macrophages, have also been implicated in the induction and maintenance of transplantation tolerance (reviewed 58). The heterogeneous nature of suppressor cells from the myeloid lineage makes it challenging to definitively track them in vivo and delineate their function, stability, and roles in promoting tolerance to allografts. Some insights have come from the investigation of mast cells, which were reported by Noelle and colleagues (162) to be essential intermediaries in skin allograft tolerance, as mast cell-deficient mice were unable to develop tolerance. Specifically, Tregs secreted IL-9 to recruit mast cells into the tolerated graft, while mast cells produced GM-CSF to induce the accumulation and promote the survival of tolerogenic graft-derived DCs into the draining lymph node (163). More recently Nowak et al. (164) reported that mast cells secreted tryptophan hydroxylase-1 to regulate immune tolerance to skin allografts induced by anti-CD154 plus DST. However, degranulation by mast cells, either within the graft or systemically, causes a transient reduction in intragraft Tregs and a loss of Treg suppressor activities that is then accompanied by acute T-cell dependent rejection. Thus if mast cells are critical to the induction and stability of tolerance, their potential to convert to an immunostimulatory phenotype provides another mechanism by which tolerance can be destabilized (165).
Bone marrow-derived mesenchymal stromal or stem cells (MSCs) have also been implicated as potential mediators of transplantation tolerance. MSCs are a subpopulation of multipotent cells from the bone marrow that can differentiate into adipocytes, chondrocytes, and osteoblasts and can be rapidly expanded in vitro. Their immune modulatory (166) as well as their reparative properties have allowed MSCs to emerge as promising candidates for cell-based immunotherapy for inducing transplantation tolerance. MSCs have been shown to modulate toward alternatively activated macrophages or tolerogenic DCs that have downregulated TNF, IL-1α, IL-6, and IL-12p70, and upregulated IL-10 and phagocytic activity. MSCs have also been reported to directly suppress T-cell activation (reviewed in 167). Because MSCs mediate at least part of their suppressive effects through the generation of alternatively activated macrophages and tolerogenic DCs, it is possible this fate commitment to pro-tolerogenic cells could be suppressed and indeed, allogeneic as well as syngeneic MSCs have been reported to accelerate graft rejection (168–170). Another stromal cell type, the hepatic stellate cell, has been implicated in regulating liver transplant outcome and promoting islet allograft survival (171). The mechanisms by which these cells mediate tolerance are incompletely understood but appear to involve PD-L1, apoptotic death of graft-infiltrating effector T cells, and accumulation of Tregs. The stability of their regulatory function in the face of infections and inflammation and the critical role for IFNγ signaling for the regulatory effects of these hepatic stellate cells suggest possible maintenance of their function under inflammatory conditions.
The expression of PD-L1 by the graft has been demonstrated to be critical for the induction and maintenance of tolerance to islet and cardiac allografts (90, 172). Furthermore, PD-L1 expression can be enhanced by IFNγ, suggesting a reinforcement of this negative pathway during inflammation (173) (Fig. 2). It is possible that other molecules that negatively signal to T or B cells and that are expressed in the graft may play a similar role to PD-L1. For instance, the herpesvirus-entry mediator (HVEM) binds to the negative regulator, B and T cell lymphocyte attenuator (BTLA), which share structural homology with PD-1 and CTLA-4 (174, 175). BTLA expression is induced by the activation of CD4+ and CD8+ T cells and is highly expressed on anergic CD4+ T cells (176). BTLA-deficient T cells proliferate more vigorously than wildtype cells, consistent with the role of BTLA as a negative regulator on T cells (174). In addition to BTLA, HVEM also binds to another co-inhibitory molecule CD160, a glycosylphosphatidylinositol-anchored member of the immunoglobulin superfamily that is expressed on T cells and NK cells (177). However, HVEM can bind to the costimulatory molecule LIGHT [lymphotoxin-related, inducible ligand that competes with herpes simplex virus (HSV) glycoprotein D for HVEM expressed on T cells], a TNF superfamily member. Thus, immunomodulation via HVEM-BTLA interactions to preserve a tolerogenic microenvironment may be difficult to control under pro-inflammatory conditions because of the ability of HVEM to bind to LIGHT, an activating molecule. Collectively, it is clear that the graft microenvironment contributes to the maintenance of tolerance and that infection and subsequent release of pro-inflammatory cytokines can have profound effects on the graft microenvironment and the stability of tolerant state.
Alloreactive B-cell tolerance mechanisms and their stability
While much of the focus on the mechanisms of transplantation tolerance has been on controlling alloreactive T cells, there is increasing evidence that alloantibodies can promote graft loss through acute or chronic antibody-mediated rejection in clinical transplantation without T-cell infiltration into the allograft (178, 179). Thus for long-term stable graft acceptance, alloantibody production ideally also has to be prevented. The production of alloantibodies in mouse models has been shown to be dependent on T-cell help, so one important strategy to achieving B-cell tolerance is through the inhibition of T-cell help. However, recent studies indicate that the specialized lineage of T-follicular helper (Tfh) cells providing help to B cells has distinct activation requirements compared to Th1 or T ‘emigrant’ effector cell lineages (180, 181), with Tfh cells deriving from precursors that have higher TCR affinity or greater TCR-p:MHCII dwell time or p:MHCII availability. While it is unclear whether these features result in Tfh cells being more or less susceptible to immunosuppression and tolerance-inducing protocols compared to other T-effector lineages, the observations of de novo DSA and chronic antibody-mediated rejection in the absence of overt acute T-cell-mediated rejection are consistent with the notion that Tfh-alloantibody axis may be differentially regulated. In clinical donor hematopoietic stem cell-mediated tolerance, the inability to achieve or the loss of established tolerance are most consistently associated with uncontrolled alloantibody production and acute or chronic humoral rejection despite apparent T-cell tolerance (34, 46, 182, 183). In patients as well as in mouse models, treatment with alemtuzumab that resulted in profound depletion of T cells promoted de novo allospecific B-cell expansion and alloantibody formation, and ultimately chronic allograft rejection (184, 185). Elevated serum levels of the B-cell activating factor (BAFF) also referred to as B-lymphocyte stimulator (BLyS) is proposed to be the proximal cause for B-cell expansion and alloantibody production following T-cell depletion (186). Taken together, these observations support the conclusion that attention must also be paid to B-cell tolerance if stable tolerance is to be achieved.
Compared to investigations into the mechanisms of T-cell tolerance, there are considerably fewer studies on B-cell tolerance in the transplantation setting. In spite of this, there is clear evidence that specific B-cell tolerance can be achieved clinically. Studies of Fan et al. (187) took advantage of the immaturity in infants of immune responses to carbohydrate (T-independent type 2) antigens, namely the A and B blood group antigens, to study the antibody response to these antigens in the 13 ABO-incompatible heart transplants performed in recipients aged 2 days-14 months. Importantly, since recipients were on standard immunosuppression such that the absence of response to the incompatible blood groups antigens expressed by the allograft could be the result of global immunosuppression, they demonstrated that this absence of response was specific. They focused their analysis on a subset of 9 blood group O recipients that have the ability to produce both anti-A and anti-B antibodies and who received A or B incompatible hearts. They reported that these recipients specifically lost the ability to respond to the incompatible blood group antigens expressed by the donor heart while spontaneously developing antibodies to blood group antigens not expressed by themselves or the donor graft. Furthermore, the lack of production of anti-A or anti-B antibodies was dependent on the presence of the graft. Ex vivo studies revealed an absence of antibody-secreting cells, which was consistent with clonal B-cell deletion as the mechanism of tolerance. Nevertheless a contribution of peripheral B-cell tolerance mechanisms was not definitively ruled out. Of note, these observations of B-cell tolerance in a clinical transplantation setting recapitulated the observations of Owen (2) and Medawar and colleagues (3, 4, 188) on fetal tolerance made almost 50 years previously in cattle, rabbits, and rodents.
The models of transplantation tolerance induced by mixed bone marrow chimerism demonstrate the existence of mechanisms of B-cell tolerance independently of T-cell tolerance mechanisms (43, 189–191). Kawahara et al. (191) reported that mixed bone hematopoietic chimerism in a congenic setting led to unresponsiveness to the α 1,3Gal carbohydrate epitope. This unresponsiveness was the result of anergy of the pre-existing Gal-specific B-1 cells in the early post-transplantation period and clonal deletion and/or receptor editing at later times post-transplantation. By tracking adoptively transferred alloreactive B cells expressing an alloreactive 3–83 B-cell receptor (BCR) transgene, clonal deletion, and possibly receptor editing were dominantly induced when B cells encountered alloantigen at an immature state in the bone marrow (190). This central deletion of alloreactive B cells is considered to promote robust tolerance and the impact of infections and inflammation on central B-cell tolerance mechanisms has not been reported. However, the observations by Nagaoka et al. (192) of changes in the numbers of developing and circulating immature B cells expressing the recombination activating gene-2 (Rag2) following the immunization with alum or malaria infection supports possible modulation of central B-cell tolerance mechanisms in the setting of infection.
The escape of significant populations of autoreactive B cells from the bone marrow into the periphery as well as their generation during the mature naive to memory IgG+ B-cell transition in both mouse and human systems have been documented (193, 194). The efficiency of central B-cell tolerance mechanisms in eliminating the alloreactive B-cell repertoire has been exhaustively delineated in the context of hematopoietic chimerism; however, whether a low frequency of alloreactive B cells, especially those with low affinity, emerge in the periphery has not been comparably investigated. Furthermore, even if central tolerance mechanisms are stringent, new alloreactive B cells may inadvertently be generated during the germinal center response. Finally, in models of peripheral tolerance, pre-existing alloreactive B cells have to be controlled. Early studies addressing peripheral mechanisms of B-cell tolerance by Li et al. (193–196) have used the alloreactive 3–83 BCR-transgenic mice to track alloreactive B cells in vivo. Peripheral deletion of alloreactive B cells and suppression of T-cell help were reported to be the bases for B-cell hyporesponsiveness in the model cardiac allograft tolerance induced by anti-CD154/DST. B-cell anergy associated with a developmentally arrested transitional phenotype (IgM+CD21/35lo) with a high turnover rate was reported in a model of B-cell tolerance, where alloreactive B cells emerged in the presence of a heart allograft (190).
Early studies by Mollov et al. (197) in a model of mixed chimeras prepared by anti-CD154 conditioning regimen suggested that B cells were necessary for CD8+ T-cell tolerance. The mechanism was not clarified but shown to be independent of B-cell antigen presentation, expression of MHC class I or class II, IDO, and IL-10. More recently, Parsons et al. (198) reported on the induction of islet allograft tolerance upon blockade with anti-BAFF antibody in combination with rapamycin. This treatment was associated with the depletion of mature follicular and marginal zone B cells, a relative sparing of transitional B cells, and a gradual increase in the percentage of Foxp3+ Tregs and IL-10 in the peripheral blood of long-term tolerant recipients. These observations raised the intriguing possibility that transitional immature B cells may play a critical role in the induction and maintenance of T-cell tolerance, instead of simply being passive recipients of instruction from T-helper cells. The possibility of transitional B cells promoting T-cell tolerance is supported by a number of recent reports on regulatory B cells modulating autoimmunity (199, 200), and of an enriched B cell gene signature in operationally-tolerant renal transplant recipients (32, 33, 201). A critical role for regulatory B cells (Bregs) has also been reported for models of tolerance to islet as well as cardiac allografts mediated by anti-TIM-1 alone or in combination with anti-CD45RB (202, 203). The phenotype of Bregs is controversial and identified either as a Tim-1+ population enriched for IL-4 and IL-10 expression or as immature B cells overexpressing CD1d, CD5, BANK-1, and FcγR2b, although immature B cells overexpressing CD1d, CD5, BANK-1, and Fcγr2b have also been implicated (201, 204). Despite a recent demonstration that a class of Bregs, the IL-10-producing, CD1dhiCD5+B10 cells, can be expanded ex vivo with CD154, IL-4, and BAFF followed by IL-21 (205), the lineage and functional stability of these cells under inflammatory conditions has still not been definitively tested.
A number of studies suggest the possibility that TLR engagement and cytokines may facilitate the loss of peripheral B-cell tolerance. Early studies by Marshak-Rothstein and colleagues (206, 207) demonstrated that chromatin-IgG complexes activate autoreactive B cells by the dual engagement of the B-cell receptor and TLRs, in particular MyD88-dependent TLR-9, prompting the production of rheumatoid factor autoantibodies. This concept of dual BCR and TLR activation of B cells was extended to autoantigens associated with RNA (208), in addition to DNA, and the release of IFNα, a cytokine that is linked to systemic lupus erythematosus. It is reasonable to extrapolate from these findings that alloantigens presented in association with comparable TLR ligands released during infection or tissue damage would also result in enhanced B-cell activation and the production of alloantibodies.
Another way in which infections may enhance B-cell activation is through sustained upregulation of BAFF. There is evidence that BAFF in the serum is increased during chronic hepatitis C virus infection, and especially in the individuals who also develop an autoimmune, mixed cryoglobulinaemia syndrome (209). Epstein-Barr virus infection, through the latent membrane protein 1, can induce BAFF production by B cells (210) and a T-cell-independent production of pathogenic class-switched antibodies. Elevated BAFF production by lung epithelia during bacterial pneumonia (211, 212) and by endothelial cells during dengue virus infection (213) have also been documented. Collectively, these observations delineate a pathway in which infections, through the engagement of TLRs and/or through BAFF production may be able to activate B cells and stimulate the T-independent production of antibodies. How these processes affect the stability of regulatory B cells, and the net effect of these pro-inflammatory events on B and T-cell activation especially in the context of peripheral B-cell tolerance remains to be investigated.
Impact of infections on alloreactive T-cell repertoire and transplantation tolerance: heterologous memory
While antigen recognition by TCRs has been shown to be exquisitely specific, TCRs can also be promiscuous and can bind different peptide/MHC combinations with different affinities (214–216). What portion of TCRs in a normal T-cell repertoire in both humans and rodents exhibits promiscuous activity is not fully defined but may be fundamentally important in ensuring sufficient numbers of T cells capable of recognizing the breadth of peptide-MHC combinations that might be encountered and in supplementing TCR diversity generated by VDJ recombination (reviewed in 217). As discussed in a recent review by Su et al. (217) the frequency of cross-reactive peptide-MHC ligand recognition by three different TCRs ranged from one in 104 to one in 108, such that T cells expressing TCRs with promiscuous activity may be biologically significant (218). They reported that uninfected blood donors had a high proportion of CD4+ cells with a memory phenotype that recognized a range of virus-derived peptide-MHC tetramers, including peptides from human immunodeficiency virus, cytomegalovirus, and herpes simplex virus. These T cells are postulated to have acquired a memory phenotype from cross-reactivity to environmental antigens encountered in their hosts’ lifetime, because these T-cell populations specific for the same viruses were all naive in neonates (218). Several TCRs cross-reacting between viral peptides presented on self-MHC and donor or self-peptides presented on allogeneic MHC have been reported in both mice and humans (219). Taken together, these observations suggest that viral, bacterial, and environmental antigens as well as actual alloantigens (through pregnancies, blood transfusions, or prior transplantations) can generate the significant percentage of alloreactive T cells with a memory phenotype observed in adults. This is considered significant, as memory T cells are now accepted as being more resistant to immunosuppressive strategies and constituting a major barrier to transplantation tolerance, by virtue of increased cell numbers, alterations in signaling, and epigenetic changes, which result in relaxed activation requirement compared to naive T cells.
Compelling evidence is available from mouse models that sequential exposure to different viruses generates a biologically significant population of alloreactive memory CD8+ T cells (220) that confers resistance to costimulation blockade-induced tolerance. Furthermore, LCMV-reactive memory CD8+ T cells generated with live virus infection can drive skin allograft rejection following adoptive transfer into T-cell-deficient recipients (221). In addition to viral infections generating heterologous immunity to alloantigens, exposure to the parasite Leishmania major was reported to have comparable effects and also prevented tolerance induction to subsequent skin grafts (222). Bacterial infections, especially those that produce superantigens capable of stimulating whole families of T cells expressing a particular TCRβ chain, may be particularly effective at generating heterologous alloreactivity in both an antigen-dependent and -independent manner, and thus increase the repertoire of alloreactive T cells affected. In humans, the presence of IFNγ-producing anti-donor T cells has been correlated with increased risk of post-transplant rejection episodes (223), and virus-specific lymphocytes have been shown to contribute significantly to the alloresponse assayed in vitro for certain responder-stimulator HLA combinations (224). Thus collectively, there is significant data supporting the notion that infections generate memory T cells that can cross-react with alloantigens and that these T cells have fundamental differences in activation requirements and susceptibility to tolerance induction from naive T cells.
Heterologously generated alloreactive memory appears in non-human primates to reside predominantly in the IFNγ-producing CD8+ T-cell subset (225). By selecting donor-recipient combinations that have reduced frequencies of donor-specific IFNγ-producing memory T cells, successful tolerance induction was achieved in 4 of 5 recipients with an induction protocol for achieving HSC chimerism, whereas those combinations with high donor-specific memory T-cell frequencies developed donor-specific antibodies and acutely rejected their grafts (225). Importantly, depleting CD8+ memory T cells together with minimizing the levels of pro-inflammatory cytokines at the time of HSC transplantation promoted tolerance to kidney allografts in this non-human primate model (226). Collectively, these studies underscore the barrier memory T cells exert on tolerance induction, and link observations of TCR cross-reactivity, memory and reduced susceptibility to tolerance induction.
In addition to selection of donor-recipient pairs with low frequencies of donor-specific memory T cells to favor the development of tolerance, a number of strategies are being tested that can mitigate the effects of memory T cells. Inhibition of these T cells with reagents that block the adhesion molecules LFA-1 or VLA-4 have been demonstrated to be successful in sensitized mouse recipients (41). Furthermore, anti-LFA-1 in combination with anti-IL-2Rα and sirolimus or belatacept resulted in prolonged islet allograft survival in non-human primates, supporting the use of LFA-1-targeted induction therapies, such as anti-LFA-1 or LFA-3-Ig, in combination with co-stimulation blockade to inhibit resistant T cells and prolonging allograft survival (40, 227). While these adjunct therapies are proposed for the induction of tolerance, there are nonetheless significant caveats associated with the non-specific inhibition of memory T-cell function early post-transplantation when the infection risks are highest. Indeed, the use of LFA-3-Ig in combination with belatacept and sirolimus resulted in cytomegalovirus reactivation in 4 of 6 rhesus macaque recipients of renal allografts while providing no significant survival benefit (228). The concern of over-immunosuppression is further underscored by the voluntary withdrawal from the market of alefacept (LFA-3-Ig) and efalizumab (blocks LFA-1-ICAM-1 interaction), with the latter being associated with increased risk of developing progressive multifocal leukoencephalopathy. Thus, the benefits gained towards transplantation tolerance induction by a more effective blunting of memory alloreactive T cells have to be balanced with the possible morbidity of reduced protective memory against pathogens resulting in a more severe or extended infection.
Impact of infections on the alloreactive B-cell repertoire and transplantation tolerance
While much of the focus has been on the generation of memory alloreactive T cells by prior infections, there has long been evidence of sensitization of B cells to blood group ABO antigens. Human anti-B and anti-A antibodies were demonstrated to be readily elicited by feeding Escherichia coli O (86), which express high human blood group B antigen and faint group A antigen, to healthy humans infants within the first year of life (229). Other bacteria such as group B Streptococcus type II and Proteus mirabilis that are pathogenic to humans have also been reported to express blood group antigens and are capable of eliciting antibody responses in individuals that are not expressing those specific blood group antigens and are therefore not tolerant to those antigens (230, 231). While these blood group antigens are not considered to be classical alloantigens, they nonetheless are able to induce hyperacute graft rejection and illustrate the situation whereby antigens expressed by bacteria, either from normal microbiota or through infections, sensitize the donor-reactive B-cell repertoire.
B-cell receptor specificity for bacterial lipopolysaccharide (LPS) has been recently identified in B cells infiltrating and expanding within human renal allografts undergoing chronic rejection (232). Since LPS can react in these cells with both the BCR and TLR4, these observations led the authors to speculate on the possibility that infection and the attendant exposure to LPS play a role in the chronic rejection of human kidney transplants. Zorn and his colleagues reported on the appearance during acute renal allograft rejection of polyreactive antibodies of the IgM and IgG isotypes that are cross-reactive to donor HLA. By examining monoclonal antibodies generated from immortalized B cells isolated from patients undergoing kidney rejection, they conclusively demonstrated simultaneous reactivity to HLA, major-histocompatibility-complex (MHC) class I-related chain A (MICA), self-antigens and lysates of a kidney cell line (233, 234). Furthermore, the VH sequence of the B-cell clone producing this polyreactive antibody showed evidence of somatic mutations that were consistent with a memory phenotype. These data on polyreactive antibodies that have specificity for HLA antigens are therefore consistent with the notion that some antibodies with documented alloreactivity may actually be polyreactive and may have been generated by antigens expressed during infections. In addition, whether antibodies with polyreactivity are the bases for the observation of high panel-reactive antibody titers in sensitized patients warrants further investigation.
While definitive proof is lacking, it is reasonable to infer from available data that presensitization of alloreactive humoral responses can occur during infection or exposure to environmental antigens as a result of cross-reactivity or polyreactivity. The impact of preformed antibodies in preventing graft acceptance is well-recognized, based on correlative studies in humans and in vivo models in mice (235–237) whereby the pro-inflammatory effects of antibody binding to graft endothelium results in complement activation and the recruitment of FcγR+ mononuclear cells and NK cells and of T cells that mediate graft rejection (238–241). Studies by Burns et al. (242, 243) further showed that the presence of memory alloreactive B cells or pre-formed alloantibodies prevented the induction of tolerance to skin and cardiac allografts in mice treated with anti-CD154 plus DST. The mechanism appeared to be dependent on the ability of the alloantibodies to bind to donor DST, converting the DST treatment from tolerogenic to immunogenic (242, 243). Thus it is likely that infections generate memory B cells and induce the production of allograft-reactive antibodies that serve as barriers to tolerance induction and long-term graft acceptance.
Infections prevent tolerance induction: effects of bystander inflammation
The notion of bystander inflammation enhancing immune responses has its roots in the pattern-recognition theory proposed by the late Charles Janeway Jr. over 20 years ago (242, 243), which provided a conceptual framework for our current understanding of the critical interplay between innate and adaptive immunity. Discovery of several families of pattern-recognition receptors (PRRs) and definition of their critical role in stimulating both innate and adaptive immune cells have resulted in a more complete understanding of how immune responses to foreign antigen are elicited (244, reviewed in 245). While early studies focused on ligands from pathogens engaging PRRs to orchestrate protective immunity, endogenous ligands often referred to as damage-associated molecular patterns (DAMPs) that are generated from cellular stress and tissue damage have also been implicated (246, 247). Both types of physiological agonists have been proposed to engage TLR pathways in transplant recipients: endogenous ligands may be released by injured tissues during the surgical processes of harvesting and implantation of the graft as well as during the cold ischemia storage period, while exogenous ligands expressed by microbes may be introduced during surgery and infection (248–251). The identity of the endogenous ligands and the effects of DAMPs on alloreactivity have been the subject of a number of reviews (252, 253) and are not be further discussed here.
The importance of the TLR pathway in the stimulation of alloreactivity and transplantation rejection was first demonstrated by Goldstein et al. (254), in studies showing that the absence of the universal TLR signal adapter protein MyD88 resulted in the prolonged acceptance of minor antigen-mismatched (HY-mismatch) skin grafts. The inability to reject these allografts was demonstrated to be associated with a reduced number of mature DCs in the draining lymph nodes and impaired generation of anti-graft-reactive Th1 immunity. These studies thus provided proof-of-principle evidence that MyD88 and, by inference, TLRs (as a role for IL-1R family members that also use MyD88 was excluded in these studies) were critical for the initiation of adaptive alloimmune responses. Nevertheless, the critical need for MyD88 in the stimulation of alloreactivity was called into question when, in a follow-up study of skin transplantation across a full MHC-mismatch, only modest delay in rejection was observed even in the absence of MyD88 in both graft and recipient (255) or when both MyD88 and TRIF, another adapter downstream of TLR signaling, were absent in the donor skin (256). These data point to alternative means of activating alloreactivity when the MHC is fully mismatched and there is therefore a larger repertoire of T cells engaged, but the triggers of innate immunity that license the alloreactive T-cell response following transplantation have not been definitively identified.
Subsequent to these observations on the modest role of MyD88 in the stimulation of unmodified alloreactivity and rejection, a number of laboratories investigated the effect of TLR engagement on tolerance induction in skin, cardiac, islet, and HSC transplantation, where the alloreactivity at the time of transplantation is blunted by the tolerogenic protocol (257–260). Those studies reported that the introduction of TLR agonists opposed the development of transplantation tolerance, whereas the absence of donor and recipient MyD88 in animals receiving suboptimal therapy with costimulation blockade promoted the long-term acceptance of fully-mismatched skin grafts (260, 261). The mechanisms by which TLR agonists prevented costimulation blockade-mediated graft acceptance have also been described. In the case of the TLR4 agonist LPS or the TLR3 agonist polyinosinic:polycytidylic acid (poly I:C), TLR signaling prevented the alloreactive CD8+ T-cell deletion that is necessary for transplantation tolerance to be established. Both TLR agonists mediated their effects through type 1 IFN receptor, and the administration of IFNβ recapitulated the effects of the TLR agonists (258, 259). The TLR9 agonist CpG was reported to prevent the intragraft recruitment of CD4+Foxp3+ Tregs and the development of linked-suppression (260). These effects were mediated by the combination of IL-6 and IL-17 (262). CpG also interferes with natural Treg function and promoted the differentiation of Th1 in an IL-6-dependent manner (263). Finally, in studies investigating how the absence of MyD88 facilitated tolerance induction, impaired inflammatory DC responses that resulted in reduced T-cell activation and increased T-cell susceptibility to suppression mediated by CD4+CD25+ Tregs were identified to be critical (261). Collectively these observations suggest that TLR agonists signaling through MyD88 induce the production of specific cytokines and co-stimulatory molecules that then regulate alloreactive T-cell and Treg function.
The impact of live infections with microbes that express multiple TLR agonists as well as ligands of other pattern-recognition receptors has been examined in a number of models of transplantation tolerance. The earliest investigations used viral infections. Lymphocytic choriomeningitis virus (LCMV) infection induced allograft rejection in mice treated with anti-CD154/DST if infected shortly after transplantation, and rejection was CD8-dependent. Pichinde virus (PV) had similar effects but murine cytomegalovirus (MCMV) and vaccinia (VV) virus infection did not abrogate the induction of allograft acceptance in these anti-CD154/DST-treated recipients (264). These observations suggested that T-cell cross-reactivity between virus-specific antigens and alloantigens or bystander stimulation of allospecific T cells could explain the observed transplant outcomes; however, the inability of MCMV, VV, or poly I:C to replicate the effects of LCMV and PV led the authors to favor the former possibility. Bacterial infections at the time of transplantation and tolerance induction with anti-CD154/DST had strikingly similar effects as viral infections (251, 265). Notably, infection with the intracellular Gram-positive bacteria Listeria monocytogenes or Staphylococcus aureus prevented tolerance induction, whereas infection with the Gram-negative bacterium Pseudomonas aeruginosa did not. Listeria infection prevented transplantation tolerance via mechanisms that were independent of MyD88 but dependent on Type I Interferon signaling, and IFNβ alone was sufficient to replicate the effects of Listeria infection. In contrast, the ability of Staphylococcus infections to prevent tolerance was dependent on MyD88 and IL-6 but independent of IL-17/Th17 (251). Furthermore, neutralizing IL-6 or administering a single pulse of methylprednisolone to modulate IL-6 production during Staphylococcus infections succeeded in facilitating skin allograft acceptance. These observations suggested the possibility of a therapeutic option of modulating select pro-inflammatory cytokines to facilitate the development of tolerance, while having modest effects on protective anti-microbial immunity. Thus, as for viral infections, bacterial infections elicit specific pro-inflammatory cytokine signatures that serve as barriers to tolerance induction.
Metastable tolerance: effects of infection after tolerance is established
Recent clinical observations indicate that some patients who achieve operational tolerance, namely the long-term acceptance of allografts, may eventually acquire alloreactivity and reject their grafts (31, 266). While the underlying cause is not known, some states of tolerance may be metastable and reversible. The term ‘metastable’ tolerance was first used by Bickerstaff and Orosz (267) to define a state of continued graft acceptance but whose cellular mechanisms to maintain tolerance changed over time. In their model, graft acceptance was initially mediated by inhibitory/regulatory T cells that resided within the CD25+CD4+ subset and that produced anti-inflammatory cytokines such as TFGβ or IL-10. These Tregs developed slowly, taking up to 30–60 days to reach maximum regulatory activity within the spleen as tested in a trans vivo delayed type hypersensitivity (DTH) assay. However, by day 150 post-transplantation, these inhibitory T cells were no longer detectable despite continued graft acceptance, leading the authors to conclude that a regulatory state of tolerance is metastable but that other mechanisms, such as deletion or cell-intrinsic hyporesponsiveness, must eventually become dominant. A similar observation of metastable tolerance, although over a much condensed time span, was reported by Sykes and her colleagues (53) in a murine model of tolerance involving mixed bone marrow chimerism, where regulation was replaced by clonal deletion as the predominant mechanism.
The term metastable tolerance has since been used more broadly to define a situation where operational tolerance that is fully established is then lost and allograft rejection ensues. From a theoretical point of view, there are a number of reasons why established regulatory tolerance may become abrogated. It has been proposed that Tregs have to reach a sufficient percentage of 35–50% of CD4+ T cells (268), to prevent T-effector cell activation within the lymph node and T-effector function within the peripheral tissue (269–271). Furthermore, as discussed above, inflammatory cytokines can transiently destabilize the function of Tregs, especially those Tregs that are induced from non-regulatory T cells, as well as render T-effector cells less susceptible to regulation. These observations collectively suggest that regulatory transplantation tolerance may be overcome by pro-inflammatory cytokines that affected Tregs and also promoted the activation of effector cells that then migrated into the graft to mediate rejection. Despite this reasoning, it has been remarkably difficult to abrogate tolerance in vivo, once it is fully and robustly established. Agents that were able to prevent tolerance, including TLR agonists, viral and bacterial infections, could not precipitate rejection of tolerant cardiac allografts. The reasoning for these observations was that the repertoire of alloreactive T cells may be pruned during the tolerance process to be intrinsically less reactive compared to naive mice, and that regulatory processes that maintain tolerance have to also be overcome. It was therefore noteworthy when Wang et al. (248) reported on the ability of an acute infection with L. monocytogenes to abrogate tolerance to cardiac allografts following anti-CD154/DST induction. This rejection was dependent on IL-6 and IFNαR signaling, as well as the presence of CD4+ and CD8+ T cells. In contrast, only IFNαR and either CD4+ or CD8+ T cells were necessary for the prevention of tolerance in this model. These observations suggest that for rejection to occur in tolerant recipients, alloreactive cells may have to overcome the established mechanisms of tolerance to expand and acquire effector functions necessary for rejection. Indeed, ex vivo experiments showed that IL-6 facilitated cell proliferation, whereas IFNβ promoted the secretion of IFNγ. Finally, as a potential therapeutic approach, the inhibition of IFNβ-signaling or of IL-6 expression was sufficient to preserve tolerance during Listeria infection.
The resilience of an established regulatory tolerance bodes well for long-term graft acceptance, and it may be that only infections that elicit both Type I IFNs and IL-6 are able to abrogate a robust model of tolerance. Whether combinations of viral and bacterial infections that individually elicit each of these cytokines are able to replicate the effects of Listeria infection is not known. Furthermore, whether other combinations of cytokines are able to abrogate tolerance and what the impact is of infections on different models of tolerance such as those elicited by pharmacological immunosuppression are also not known and require further investigation. This latter question is of particular interest in light of recent reports of late loss of operational tolerance in patients following weaning off pharmacological immunosuppression or tolerance induction with a hematopoietic stem cell regimen (31, 34). In those clinical studies, the underlying immunological cause for graft loss after a long period of tolerance could not be determined because of the small cohort of patients in both studies, although we noted that of the 6 operationally tolerant patients off conventional immunosuppression that reported a bacterial infection, 4 went on to reject their grafts. Furthermore, of the 9 viral or bacterial infections noted, 6 occurred in the 8 patients that went on to reject their grafts while only 3 were reported for the 19 patients that retained their tolerant state (31). In the studies by Leventhal et al. (16), one patient that established full chimerism at two months post-transplantation developed a febrile illness that progressed to sepsis and loss of donor chimerism at three months post-transplantation. In the studies of Kawai et al. (34), the only patient who succumbed to acute cellular rejection 2 month after immunosuppression withdrawal had an acute pyelonephritis just prior to rejection (272, M. Sykes, personal communication). Whether these are meaningful correlations will require a larger cohort of tolerant patients as well as a closer examination of the consequences of infection and alloreactivity both in experimental models and in the clinic.
Conclusions
We are at an exciting time in transplantation tolerance, when a number of studies in the clinic have identified an increasing cohort of patients that have achieved operational tolerance to their renal or liver allografts. These patients provide an opportunity to test whether the mechanisms of tolerance identified in experimental models are relevant in humans and also highlight new areas of investigation, such as whether distinct mechanisms maintain tolerance to different organs and whether regulatory B cells play a prominent role in this tolerance. Furthermore, in the real world, patients are exposed to infections and other pro-inflammatory situations that may impact alloreactivity and the stability of tolerance, thus a sensitive means of monitoring the robustness of tolerance over time is essential as is the identification of therapies that can reinforce metastable tolerance.
Acknowledgments
This work was supported in part by grants, NIAID P01AI-97113 to ASC and MLA; R01 AI071080 to MLA, and ROTRF 979162997 and NIAID R01 AI072630 to ASC.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Burnet FM, Fenner F. The production of antibodies. Macmillan; Melbourne: 1949. [Google Scholar]
- 2.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 3.Anderson D, Billingham RE, Lampkin GH, Medawar PB. The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity. 1951;5:379–397. [Google Scholar]
- 4.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 5.Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 7.Sharabi Y, Abraham VS, Sykes M, Sachs DH. Mixed allogeneic chimeras prepared by a non-myeloablative regimen: requirement for chimerism to maintain tolerance. Bone Marrow Transplant. 1992;9:191–197. [PubMed] [Google Scholar]
- 8.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62:380–387. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 9.Zinkernagel RM, Sado T, Althage A, Kamisaku H. Anti-viral immune response of allogeneic irradiation bone marrow chimeras: cytotoxic T cell responsiveness depends upon H-2 combination and infectious agent. Eur J Immunol. 1984;14:14–23. doi: 10.1002/eji.1830140104. [DOI] [PubMed] [Google Scholar]
- 10.Ruedi E, Sykes M, Ildstad ST, Chester CH, Althage A, Hengartner H, et al. Antiviral T cell competence and restriction specificity of mixed allogeneic (P1 + P2----P1) irradiation chimeras. Cell Immunol. 1989;121:185–195. doi: 10.1016/0008-8749(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 11.Sykes M, Sachs DH. Bone marrow transplantation as a means of inducing tolerance. Semin Immunol. 1990;2:401–417. [PubMed] [Google Scholar]
- 12.Koehn BH, et al. Fully MHC-disparate mixed hemopoietic chimeras show specific defects in the control of chronic viral infections. J Immunol. 2007;179:2616–2626. doi: 10.4049/jimmunol.179.4.2616. [DOI] [PubMed] [Google Scholar]
- 13.Williams MA, et al. Primary and secondary immunocompetence in mixed allogeneic chimeras. J Immunol. 2003;170:2382–2389. doi: 10.4049/jimmunol.170.5.2382. [DOI] [PubMed] [Google Scholar]
- 14.Strober S, Spitzer TR, Lowsky R, Sykes M. Translational studies in hematopoietic cell transplantation: treatment of hematologic malignancies as a stepping stone to tolerance induction. Semin Immunol. 2011;23:273–281. doi: 10.1016/j.smim.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol. 2012;12:403–416. doi: 10.1038/nri3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leventhal J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Science Trans Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colson YL, Shinde Patil VR, Ildstad ST. Facilitating cells: novel promoters of stem cell alloengraftment and donor-specific transplantation tolerance in the absence of GVHD. Crit Rev Oncol/Hematol. 2007;61:26–43. doi: 10.1016/j.critrevonc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Billingham RE, Krohn PL, Medawar PB. Effect of locally applied cortisone acetate on survival of skin homografts in rabbits. Br Med J. 1951;2:1049–1053. doi: 10.1136/bmj.2.4739.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakamoto K, Ochiai T, Shinohara N, Sato H. Enforcement of specific unresponsiveness by the long-term presence of allogeneic heart grafts. Transplantation. 1984;37:97–100. doi: 10.1097/00007890-198401000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Hall BM. Mechanisms maintaining enhancement of allografts. I. Demonstration of a specific suppressor cell. J Exp Med. 1985;161:123–133. doi: 10.1084/jem.161.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochiai T, Nakajima K, Nagata M, Hori S, Asano T, Isono K. Studies of the induction and maintenance of long-term graft acceptance by treatment with FK506 in heterotopic cardiac allotransplantation in rats. Transplantation. 1987;44:734–738. doi: 10.1097/00007890-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Todo S, et al. Immunosuppression of canine, monkey, and baboon allografts by FK 506: with special reference to synergism with other drugs and to tolerance induction. Surgery. 1988;104:239–249. [PMC free article] [PubMed] [Google Scholar]
- 23.Ozer K, et al. Induction of tolerance to hind limb allografts in rats receiving cyclosporine A and antilymphocyte serum: effect of duration of the treatment. Transplantation. 2003;75:31–36. doi: 10.1097/00007890-200301150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lenschow DJ, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 25.Larsen CP, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 26.Feng S, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. J Am Med Assoc. 2012;307:283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 27.Benitez C, et al. Prospective multi-center clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824–1835. doi: 10.1002/hep.26426. [DOI] [PubMed] [Google Scholar]
- 28.Ohe H, et al. Factors affecting operational tolerance after pediatric living-donor liver transplantation: impact of early post-transplant events and HLA match. Transpl Int. 2012;25:97–106. doi: 10.1111/j.1432-2277.2011.01389.x. [DOI] [PubMed] [Google Scholar]
- 29.Takatsuki M, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. doi: 10.1097/00007890-200108150-00016. [DOI] [PubMed] [Google Scholar]
- 30.Yoshitomi M, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation. 2009;87:606–614. doi: 10.1097/TP.0b013e318195a7cb. [DOI] [PubMed] [Google Scholar]
- 31.Brouard S, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant. 2012;12:3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- 32.Newell KA, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagoo P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–61. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013;368:1850–1852. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood MJ, Sloan DJ, Wood KJ, Charlton HM. Indefinite survival of neural xenografts induced with anti-CD4 monoclonal antibodies. Neuroscience. 1996;70:775–789. doi: 10.1016/s0306-4522(96)83014-4. [DOI] [PubMed] [Google Scholar]
- 36.Cobbold SP, Martin G, Qin S, Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986;323:164–166. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- 37.Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 38.Kirk AD, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 39.Kirk AD, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badell IR, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120:4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitchens WH, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant. 2012;12:69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arefanian H, Tredget EB, Rajotte RV, Gill RG, Korbutt GS, Rayat GR. Short-term administrations of a combination of anti-LFA-1 and anti-CD154 monoclonal antibodies induce tolerance to neonatal porcine islet xenografts in mice. Diabetes. 2010;59:958–966. doi: 10.2337/db09-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aksentijevich I, Sachs DH, Sykes M. Humoral tolerance in xenogeneic BMT recipients conditioned by a nonmyeloablative regimen. Transplantation. 1992;53:1108–1114. doi: 10.1097/00007890-199205000-00025. [DOI] [PubMed] [Google Scholar]
- 44.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087–1098. [PubMed] [Google Scholar]
- 45.Yang YG, et al. Tolerization of anti-Galalpha1-3Gal natural antibody-forming B cells by induction of mixed chimerism. J Exp Med. 1998;187:1335–1342. doi: 10.1084/jem.187.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawai T, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. N Engl J Med. 2011;365:1359–1360. doi: 10.1056/NEJMc1107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spitzer TR, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011;91:672–676. doi: 10.1097/TP.0b013e31820a3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 50.Hongo D, Tang X, Dutt S, Nador RG, Strober S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood. 2012;119:1581–1589. doi: 10.1182/blood-2011-08-371948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol. 2007;178:6242–6251. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells, and MHC class II. J Immunol. 2008;181:165–173. doi: 10.4049/jimmunol.181.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4+CD25- T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8+ T cells, precluding a role for sustained regulation. Eur J Immunol. 2005;35:2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 54.Lucas CL, Sykes M. Layers of regulation in induction of mixed chimerism by anti-CD40L. Chimerism. 2011;2:111–113. doi: 10.4161/chim.2.4.19053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucas CL, et al. LAG-3, TGF-beta, and cell-intrinsic PD-1 inhibitory pathways contribute to CD8 but not CD4 T-cell tolerance induced by allogeneic BMT with anti-CD40L. Blood. 2011;117:5532–5540. doi: 10.1182/blood-2010-11-318675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fehr T, et al. A CD8 T cell-intrinsic role for the calcineurin-NFAT pathway for tolerance induction in vivo. Blood. 2010;115:1280–7. doi: 10.1182/blood-2009-07-230680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood KJ, Ushigome H, Karim M, Bushell A, Hori S, Sakaguchi S. Regulatory cells in transplantation. Novartis Found Symp. 2003;252:177–88. discussion 88–93, 203–210. [PubMed] [Google Scholar]
- 58.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 59.Mueller DL, Jenkins MK. Molecular mechanisms underlying functional T-cell unresponsiveness. Curr Opin Immunol. 1995;7:375–381. doi: 10.1016/0952-7915(95)80113-8. [DOI] [PubMed] [Google Scholar]
- 60.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 62.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 63.Kang SM, Hoffmann A, Le D, Springer ML, Stock PG, Blau HM. Immune response and myoblasts that express Fas ligand. Science. 1997;278:1322–1324. doi: 10.1126/science.278.5341.1322. [DOI] [PubMed] [Google Scholar]
- 64.Yolcu ES, et al. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice. J Immunol. 2011;187:5901–5909. doi: 10.4049/jimmunol.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wells AD, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 66.Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finn PW, Stone JR, Boothby MR, Perkins DL. Inhibition of NF-kappaB-dependent T cell activation abrogates acute allograft rejection. J Immunol. 2001;167:5994–6001. doi: 10.4049/jimmunol.167.10.5994. [DOI] [PubMed] [Google Scholar]
- 68.Zhou P, et al. Impaired NF-kappaB activation in T cells permits tolerance to primary heart allografts and to secondary donor skin grafts. Am J Transplant. 2003;3:139–147. doi: 10.1034/j.1600-6143.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhou P, Balin SJ, Mashayekhi M, Hwang KW, Palucki DA, Alegre ML. Transplantation tolerance in NF-kappaB-impaired mice is not due to regulation but is prevented by transgenic expression of Bcl-xL. J Immunol. 2005;174:3447–3453. doi: 10.4049/jimmunol.174.6.3447. [DOI] [PubMed] [Google Scholar]
- 70.Molinero LL, Wang Y, Zhou P, Yagita H, Alegre ML. Fas mediates cardiac allograft acceptance in mice with impaired T-cell-intrinsic NF-kappaB signaling. Transpl Int. 2009;22:845–852. doi: 10.1111/j.1432-2277.2009.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill GR, et al. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. J Immunol. 2000;164:656–63. doi: 10.4049/jimmunol.164.2.656. [DOI] [PubMed] [Google Scholar]
- 73.Safford M, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz RH, Mueller DL, Jenkins MK, Quill H. T-cell clonal anergy. Cold Spring Harb Symp Quant Biol. 1989;54:605–610. doi: 10.1101/sqb.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- 76.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 77.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 78.Lin H, et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229:294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hosokawa T, Amagai T, Muramatsu S. Studies on B-cell memory. I. Generation and exhaustion of B-cell memory by thymus-dependent antigen in T-cell depleted mice. Immunology. 1979;38:283–289. [PMC free article] [PubMed] [Google Scholar]
- 81.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 82.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 83.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 85.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang SM, Beverly B, Tran AC, Brorson K, Schwartz RH, Lenardo MJ. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992;257:1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- 87.Habib-Agahi M, Phan TT, Searle PF. Co-stimulation with 4-1BB ligand allows extended T-cell proliferation, synergizes with CD80/CD86 and can reactivate anergic T cells. Int Immunol. 2007;19:1383–1394. doi: 10.1093/intimm/dxm106. [DOI] [PubMed] [Google Scholar]
- 88.Redmond WL, Gough MJ, Weinberg AD. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur J Immunol. 2009;39:2184–2194. doi: 10.1002/eji.200939348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fife BT, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang L, Han R, Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol. 2007;37:2983–2990. doi: 10.1002/eji.200737583. [DOI] [PubMed] [Google Scholar]
- 94.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 95.McCullagh P. Suppressor cells in homograft tolerant rats. Australian J Biol Med Sci. 1976;53:431–436. doi: 10.1038/icb.1975.48. [DOI] [PubMed] [Google Scholar]
- 96.Dorsch S, Roser B. Suppressor cells in transplantation tolerance. II. Identification and probable mode of action of chimeric suppressor T cells. Transplantation. 1982;33:525–529. [PubMed] [Google Scholar]
- 97.Benjamin RJ, Cobbold SP, Clark MR, Waldmann H. Tolerance to rat monoclonal antibodies. Implications for serotherapy. J Exp Med. 1986;163:1539–1552. doi: 10.1084/jem.163.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Darby CR, Morris PJ, Wood KJ. Evidence that long-term cardiac allograft survival induced by anti-CD4 monoclonal antibody does not require depletion of CD4+ T cells. Transplantation. 1992;54:483–490. doi: 10.1097/00007890-199209000-00019. [DOI] [PubMed] [Google Scholar]
- 99.Qin S, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 100.Davies JD, Leong LY, Mellor A, Cobbold SP, Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J Immunol. 1996;156:3602–3607. [PubMed] [Google Scholar]
- 101.Hall BM, Jelbart ME, Dorsch SE. Suppressor T cells in rats with prolonged cardiac allograft survival after treatment with cyclosporine. Transplantation. 1984;37:595–600. doi: 10.1097/00007890-198406000-00014. [DOI] [PubMed] [Google Scholar]
- 102.Quigley RL, Wood KJ, Morris PJ. Mediation of antigen-induced suppression of renal allograft rejection by a CD4 (W3/25+) T cell. Transplantation. 1989;47:684–688. doi: 10.1097/00007890-198904000-00022. [DOI] [PubMed] [Google Scholar]
- 103.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J Immunol. 1999;163:4805–4810. [PubMed] [Google Scholar]
- 104.Hara M, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 105.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25(+)CD4(+) regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 106.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cobbold SP, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 108.Feng G, et al. Functional regulatory T cells produced by inhibiting cyclic nucleotide phosphodiesterase type 3 prevent allograft rejection. Sci Trans Med. 2011;3:83ra40. doi: 10.1126/scitranslmed.3002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feng G, Wood KJ, Bushell A. Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 2008;86:578–589. doi: 10.1097/TP.0b013e3181806a60. [DOI] [PubMed] [Google Scholar]
- 110.VanBuskirk AM, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106:145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kendal AR, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011;208:2043–2053. doi: 10.1084/jem.20110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Francis RS, Feng G, Tha-In T, Lyons IS, Wood KJ, Bushell A. Induction of transplantation tolerance converts potential effector T cells into graft-protective regulatory T cells. Eur J Immunol. 2011;41:726–738. doi: 10.1002/eji.201040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 115.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ohkura N, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 118.Ohkura N, Sakaguchi S. Treg cells acquire new directions, cytokines navigate. Immunity. 2012;37:443–444. doi: 10.1016/j.immuni.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 119.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Polansky JK, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 122.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lal G, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rudra D, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol. 2012;13:1010–9. doi: 10.1038/ni.2402. Epub 2012/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Samstein RM, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 127.Ferrer IR, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc Natl Acad Sci USA. 2011;108:20701–20706. doi: 10.1073/pnas.1105500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 129.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 130.Hall AO, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Konieczny BT, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 135.Wei B, Baker S, Wieckiewicz J, Wood KJ. IFN-gamma triggered STAT1-PKB/AKT signalling pathway influences the function of alloantigen reactive regulatory T cells. Am J Transplant. 2010;10:69–80. doi: 10.1111/j.1600-6143.2009.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Manavalan JS, et al. Alloantigen specific CD8+CD28- FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16:1055–1068. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 137.Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, Luo X. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. Am J Transplant. 2012;12:2335–2347. doi: 10.1111/j.1600-6143.2012.04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beres AJ, Haribhai D, Chadwick AC, Gonyo PJ, Williams CB, Drobyski WR. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. J Immunol. 2012;189:464–474. doi: 10.4049/jimmunol.1200886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen W, Ford MS, Young KJ, Cybulsky MI, Zhang L. Role of double-negative regulatory T cells in long-term cardiac xenograft survival. J Immunol. 2003;170:1846–1853. doi: 10.4049/jimmunol.170.4.1846. [DOI] [PubMed] [Google Scholar]
- 140.Zhang ZX, et al. Adoptive transfer of DNT cells induces long-term cardiac allograft survival and augments recipient CD4(+)Foxp3(+) Treg cell accumulation. Transpl Immunol. 2011;24:119–126. doi: 10.1016/j.trim.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 141.Kohrt HE, Pillai AB, Lowsky R, Strober S. NKT cells, Treg, and their interactions in bone marrow transplantation. Eur J Immunol. 2010;40:1862–1869. doi: 10.1002/eji.201040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med. 2005;11:1059–1065. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 143.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203:1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van der Touw W, Burrell B, Lal G, Bromberg JS. NK cells are required for costimulatory blockade induced tolerance to vascularized allografts. Transplantation. 2012;94:575–584. doi: 10.1097/TP.0b013e318264d3c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maier S, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med. 2001;7:557–562. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 146.McNerney ME, et al. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6:505–513. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 147.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23:252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eto M, Hackstein H, Kaneko K, Nomoto K, Thomson AW. Promotion of skin graft tolerance across MHC barriers by mobilization of dendritic cells in donor hemopoietic cell infusions. J Immunol. 2002;169:2390–2396. doi: 10.4049/jimmunol.169.5.2390. [DOI] [PubMed] [Google Scholar]
- 149.O’Connell PJ, Li W, Wang Z, Specht SM, Logar AJ, Thomson AW. Immature and mature CD8alpha+ dendritic cells prolong the survival of vascularized heart allografts. J Immunol. 2002;168:143–154. doi: 10.4049/jimmunol.168.1.143. [DOI] [PubMed] [Google Scholar]
- 150.Ezzelarab MB, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13:1989–2005. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 153.Trabanelli S, Ocadlikova D, Evangelisti C, Parisi S, Curti A. Induction of regulatory T Cells by dendritic cells through indoleamine 2,3-dioxygenase: a potent mechanism of acquired peripheral tolerance. Curr Med Chem. 2011;18:2234–2239. doi: 10.2174/092986711795656054. [DOI] [PubMed] [Google Scholar]
- 154.Tokita D, et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85:369–377. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 155.Brenk M, et al. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2009;183:145–154. doi: 10.4049/jimmunol.0803277. [DOI] [PubMed] [Google Scholar]
- 156.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 157.Turnquist HR, Thomson AW. Taming the lions: manipulating dendritic cells for use as negative cellular vaccines in organ transplantation. Curr Opin Organ Transplant. 2008;13:350–357. doi: 10.1097/MOT.0b013e328306116c. [DOI] [PubMed] [Google Scholar]
- 158.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 159.Morelli AE, et al. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–1523. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 160.Wang Z, et al. Dendritic cell therapies in transplantation revisited: deletion of recipient DCs deters the effect of therapeutic DCs. Am J Transplant. 2012;12:1398–1408. doi: 10.1111/j.1600-6143.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Smyth LA, et al. Tolerogenic Donor-Derived Dendritic Cells Risk Sensitization In Vivo owing to Processing and Presentation by Recipient APCs. J Immunol. 2013;190:4848–4860. doi: 10.4049/jimmunol.1200870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu LF, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 163.de Vries VC, Pino-Lagos K, Nowak EC, Bennett KA, Oliva C, Noelle RJ. Mast cells condition dendritic cells to mediate allograft tolerance. Immunity. 2011;35:550–561. doi: 10.1016/j.immuni.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nowak EC, et al. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J Exp Med. 2012;209:2127–2135. doi: 10.1084/jem.20120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Vries VC, Pino-Lagos K, Elgueta R, Noelle RJ. The enigmatic role of mast cells in dominant tolerance. Curr Opin Organ Transplant. 2009;14:332–337. doi: 10.1097/MOT.0b013e32832ce87a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 167.English K, Wood KJ. Mesenchymal stromal cells in transplantation rejection and tolerance. Cold Spring Harbor Persp Med. 2013;3:a015560. doi: 10.1101/cshperspect.a015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Inoue S, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 169.Seifert M, et al. Crosstalk between immune cells and mesenchymal stromal cells in a 3D bioreactor system. Int J Artificial Organs. 2012;35:986–995. doi: 10.5301/ijao.5000118. [DOI] [PubMed] [Google Scholar]
- 170.Casiraghi F, et al. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant. 2012;12:2373–2383. doi: 10.1111/j.1600-6143.2012.04115.x. [DOI] [PubMed] [Google Scholar]
- 171.Yang HR, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tanaka K, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Watanabe N, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 175.Sedy JR, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 176.Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 177.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 178.Loupy A, et al. Very late heart transplant rejection is associated with microvascular injury, complement deposition and progression to cardiac allograft vasculopathy. Am J Transplant. 2011;11:1478–1487. doi: 10.1111/j.1600-6143.2011.03563.x. [DOI] [PubMed] [Google Scholar]
- 179.Mengel M, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tubo NJ, et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Porcheray F, et al. B-cell immunity in the context of T-cell tolerance after combined kidney and bone marrow transplantation in humans. Am J Transplant. 2009;9:2126–2135. doi: 10.1111/j.1600-6143.2009.02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kawai T, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767–1775. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]
- 184.Bartosh SM, Knechtle SJ, Sollinger HW. Campath-1H use in pediatric renal transplantation. Am J Transplant. 2005;5:1569–1573. doi: 10.1111/j.1600-6143.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 185.Kwun J, et al. Patterns of de novo allo B cells and antibody formation in chronic cardiac allograft rejection after alemtuzumab treatment. Am J Transplant. 2012;12:2641–2651. doi: 10.1111/j.1600-6143.2012.04181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bloom D, et al. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant. 2009;9:1835–1845. doi: 10.1111/j.1600-6143.2009.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fan X, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004;10:1227–1233. doi: 10.1038/nm1126. [DOI] [PubMed] [Google Scholar]
- 188.Billingham RE, Brent L, Medawar PB. Acquired tolerance of skin homografts. Ann NY Acad Sci. 1955;59:409–416. doi: 10.1111/j.1749-6632.1955.tb45955.x. [DOI] [PubMed] [Google Scholar]
- 189.Sykes M, Shimizu I, Kawahara T. Mixed hematopoietic chimerism for the simultaneous induction of T and B cell tolerance. Transplantation. 2005;79(Suppl):S28–29. doi: 10.1097/01.tp.0000153296.80385.e7. [DOI] [PubMed] [Google Scholar]
- 190.Parsons RF, et al. Acquisition of humoral transplantation tolerance upon de novo emergence of B lymphocytes. J Immunol. 2011;186:614–620. doi: 10.4049/jimmunol.1002873. [DOI] [PubMed] [Google Scholar]
- 191.Kawahara T, Shimizu I, Ohdan H, Zhao G, Sykes M. Differing mechanisms of early and late B cell hyporesponsiveness induced by mixed chimerism. Am J Transplant. 2005;5:2821–2829. doi: 10.1111/j.1600-6143.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 192.Nagaoka H, Gonzalez-Aseguinolaza G, Tsuji M, Nussenzweig MC. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J Exp Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 194.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li Y, Ma L, Shen J, Chong AS. Peripheral deletion of mature alloreactive B cells induced by costimulation blockade. Proc Natl Acad Sci USA. 2007;104:12093–12098. doi: 10.1073/pnas.0705240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li Y, Ma L, Yin D, Shen J, Chong AS. Long-term control of alloreactive B cell responses by the suppression of T cell help. J Immunol. 2008;180:6077–6084. doi: 10.4049/jimmunol.180.9.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mollov JL, et al. Recipient dendritic cells, but not B cells, are required antigen-presenting cells for peripheral alloreactive CD8+ T-cell tolerance. Am J Transplant. 2010;10:518–526. doi: 10.1111/j.1600-6143.2009.02967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Parsons RF, et al. Murine islet allograft tolerance upon blockade of the B-lymphocyte stimulator, BLyS/BAFF. Transplantation. 2012;93:676–685. doi: 10.1097/TP.0b013e318246621d. [DOI] [PubMed] [Google Scholar]
- 199.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 200.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6:636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- 201.Pallier A, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 202.Ding Q, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee KM, et al. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant. 2012;12:2072–2078. doi: 10.1111/j.1600-6143.2012.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Le Texier L, et al. Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant. 2011;11:429–438. doi: 10.1111/j.1600-6143.2010.03336.x. [DOI] [PubMed] [Google Scholar]
- 205.Yoshizaki A, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 207.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 208.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fabris M, et al. B-Lymphocyte stimulator (BLyS) up-regulation in mixed cryoglobulinaemia syndrome and hepatitis-C virus infection. Rheumatology. 2007;46:37–43. doi: 10.1093/rheumatology/kel174. [DOI] [PubMed] [Google Scholar]
- 210.He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. 2003;171:5215–5224. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Eddens T, Kolls JK. Host defenses against bacterial lower respiratory tract infection. Curr Opin Immunol. 2012;24:424–430. doi: 10.1016/j.coi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McNamara PS, et al. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax. 2013;68:76–81. doi: 10.1136/thoraxjnl-2012-202288. [DOI] [PubMed] [Google Scholar]
- 213.Dalrymple NA, Mackow ER. Endothelial cells elicit immune-enhancing responses to dengue virus infection. J Virol. 2012;86:6408–6415. doi: 10.1128/JVI.00213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gras S, Kjer-Nielsen L, Chen Z, Rossjohn J, McCluskey J. The structural bases of direct T-cell allorecognition: implications for T-cell-mediated transplant rejection. Immunol Cell Biol. 2011;89:388–395. doi: 10.1038/icb.2010.150. [DOI] [PubMed] [Google Scholar]
- 215.Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol. 2007;7:942–953. doi: 10.1038/nri2200. [DOI] [PubMed] [Google Scholar]
- 216.Gras S, et al. A structural basis for varied alphabeta TCR usage against an immunodominant EBV antigen restricted to a HLA-B8 molecule. J Immunol. 2012;188:311–321. doi: 10.4049/jimmunol.1102686. [DOI] [PubMed] [Google Scholar]
- 217.Su LF, Davis MM. Antiviral memory phenotype T cells in unexposed adults. Immunol Rev. 2013;255:95–109. doi: 10.1111/imr.12095. [DOI] [PubMed] [Google Scholar]
- 218.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Smith KA. Toward a molecular understanding of adaptive immunity: a chronology - part II. Front Immunol. 2012;3:364. doi: 10.3389/fimmu.2012.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 221.Brehm MA, et al. Allografts stimulate cross-reactive virus-specific memory CD8 T cells with private specificity. Am J Transplant. 2010;10:1738–1748. doi: 10.1111/j.1600-6143.2010.03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 223.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 224.Macedo C, et al. Contribution of naive and memory T-cell populations to the human alloimmune response. Am J Transplant. 2009;9:2057–2066. doi: 10.1111/j.1600-6143.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 225.Nadazdin O, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Trans Med. 2011;3:86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yamada Y, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12:330–340. doi: 10.1111/j.1600-6143.2011.03795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Weaver TA, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15:746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lowe MC, et al. Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy. Am J Transplant. 2013;13:312–319. doi: 10.1111/j.1600-6143.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Springer GF, Horton RE. Blood group isoantibody stimulation in man by feeding blood group-active bacteria. J Clin Invest. 1969;48:1280–1291. doi: 10.1172/JCI106094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Boyer KM, Theeravuthichai J, Vogel LC, Orlina A, Gotoff SP. Antibody response to group B streptococcus type III and AB blood group antigens induced by pneumococcal vaccine. J Ped. 1981;98:374–378. doi: 10.1016/s0022-3476(81)80698-1. [DOI] [PubMed] [Google Scholar]
- 231.Drach GW, Reed WP, Williams RC., Jr Antigens common to human and bacterial cells., 3.Autoagglutinin to blood group A stimulated by sepsis due to Proteus mirabilis. J Lab Clin Med. 1973;81:919–924. [PubMed] [Google Scholar]
- 232.Grover RK, et al. The costimulatory immunogen LPS induces the B-Cell clones that infiltrate transplanted human kidneys. Proc Natl Acad Sci USA. 2012;109:6036–6041. doi: 10.1073/pnas.1202214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Porcheray F, et al. Expansion of polyreactive B cells cross-reactive to HLA and self in the blood of a patient with kidney graft rejection. Am J Transplant. 2012;12:2088–2097. doi: 10.1111/j.1600-6143.2012.04053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Porcheray F, et al. Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant. 2013;13:2590–2560. doi: 10.1111/ajt.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wasowska BA, et al. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 236.Mizutani K, et al. The importance of anti-HLA-specific antibody strength in monitoring kidney transplant patients. Am J Transplant. 2007;7:1027–1031. doi: 10.1111/j.1600-6143.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 237.Smith JD, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplan. 2011;11:312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 238.Uehara S, et al. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol. 2005;175:3424–3430. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 239.Hirohashi T, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ding JW, et al. Hyperacute rejection by anti-Gal IgG1, IgG2a, and IgG2b is dependent on complement and Fc-gamma receptors. J Immunol. 2008;180:261–268. doi: 10.4049/jimmunol.180.1.261. [DOI] [PubMed] [Google Scholar]
- 241.Yin D, et al. Cutting edge: NK cells mediate IgG1-dependent hyperacute rejection of xenografts. J Immunol. 2004;172:7235–7238. doi: 10.4049/jimmunol.172.12.7235. [DOI] [PubMed] [Google Scholar]
- 242.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186:214–221. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Burns AM, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol. 2009;182:1314–1324. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
- 244.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 245.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 246.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6:2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 247.Duan L, et al. High-mobility group box 1 promotes early acute allograft rejection by enhancing IL-6-dependent Th17 alloreactive response. Lab Invest. 2011;91:43–53. doi: 10.1038/labinvest.2010.141. [DOI] [PubMed] [Google Scholar]
- 248.Wang T, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10:1524–1433. doi: 10.1111/j.1600-6143.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ahmed E, Alegre M, Chong A. Role of bacterial infections in allograft rejection. Exp Rev Clin Immunol. 2008;4:281–293. doi: 10.1586/1744666X.4.2.281. [DOI] [PubMed] [Google Scholar]
- 250.Ahmed EB, Daniels M, Alegre ML, Chong AS. Bacterial infections, alloimmunity, and transplantation tolerance. Transplant Rev. 2011;25:27–35. doi: 10.1016/j.trre.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ahmed EB, Wang T, Daniels M, Alegre ML, Chong AS. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant. 2011;11:936–946. doi: 10.1111/j.1600-6143.2011.03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Alegre ML, Goldstein DR, Chong AS. Toll-like receptor signaling in transplantation. Curr Opin Organ Transplant. 2008;13:358–65. doi: 10.1097/MOT.0b013e3283061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Land WG, Memer K. Tissue injury-induced initiation of the adaptive immune response. Transplantation. 2012;93:e38. doi: 10.1097/TP.0b013e31824e7642. [DOI] [PubMed] [Google Scholar]
- 254.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest. 2003;111:1571–1578. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tesar BM, Zhang J, Li Q, Goldstein DR. TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a toll-like receptor signal adaptor protein. Am J Transplant. 2004;4:1429–1439. doi: 10.1111/j.1600-6143.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 256.McKay D, Shigeoka A, Rubinstein M, Surh C, Sprent J. Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur J Immunol. 2006;36:1994–2002. doi: 10.1002/eji.200636249. [DOI] [PubMed] [Google Scholar]
- 257.Thornley TB, et al. TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. J Immunol. 2006;176:1561–1570. doi: 10.4049/jimmunol.176.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Thornley TB, et al. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J Immunol. 2007;179:6620–6629. doi: 10.4049/jimmunol.179.10.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Miller DM, et al. TLR agonists prevent the establishment of allogeneic hematopoietic chimerism in mice treated with costimulation blockade. J Immunol. 2009;182:5547–5559. doi: 10.4049/jimmunol.0802077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen L, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6:2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 261.Walker WE, Nasr IW, Camirand G, Tesar BM, Booth CJ, Goldstein DR. Absence of innate MyD88 signaling promotes inducible allograft acceptance. J Immunol. 2006;177:5307–5316. doi: 10.4049/jimmunol.177.8.5307. [DOI] [PubMed] [Google Scholar]
- 262.Chen L, et al. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Porrett PM, et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181:1692–1699. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Welsh RM, et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210–2218. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang T, et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180:5991–5999. doi: 10.4049/jimmunol.180.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kawai T, Sachs DH. Tolerance induction: hematopoietic chimerism. Curr Opin Organ Transplant. 2013;18:402–407. doi: 10.1097/MOT.0b013e328363621d. [DOI] [PubMed] [Google Scholar]
- 267.Bickerstaff A, Orosz C. Evidence for a limited contribution of immune regulation to cardiac allograft acceptance. Hum Immunol. 2002;63:935–947. doi: 10.1016/s0198-8859(02)00447-0. [DOI] [PubMed] [Google Scholar]
- 268.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: how many cells do we need? Curr Opin Organ Transplant. 2012;17:349–354. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 269.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tadokoro CE, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203(3):505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Markmann JF, Kawai T. The quest for transplantation tolerance: have we finally sipped from the cup? Science translational medicine. 2012;4:124fs5. E. doi: 10.1126/scitranslmed.3003678. [DOI] [PMC free article] [PubMed] [Google Scholar]