Abstract
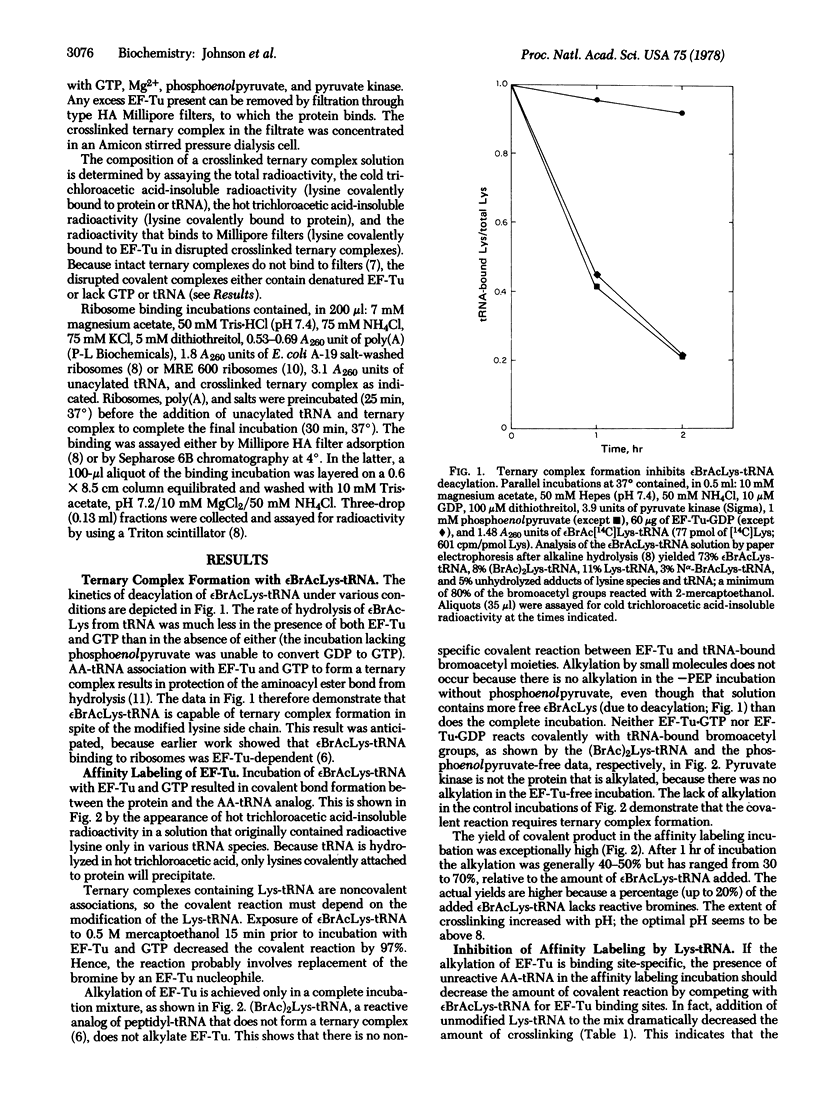
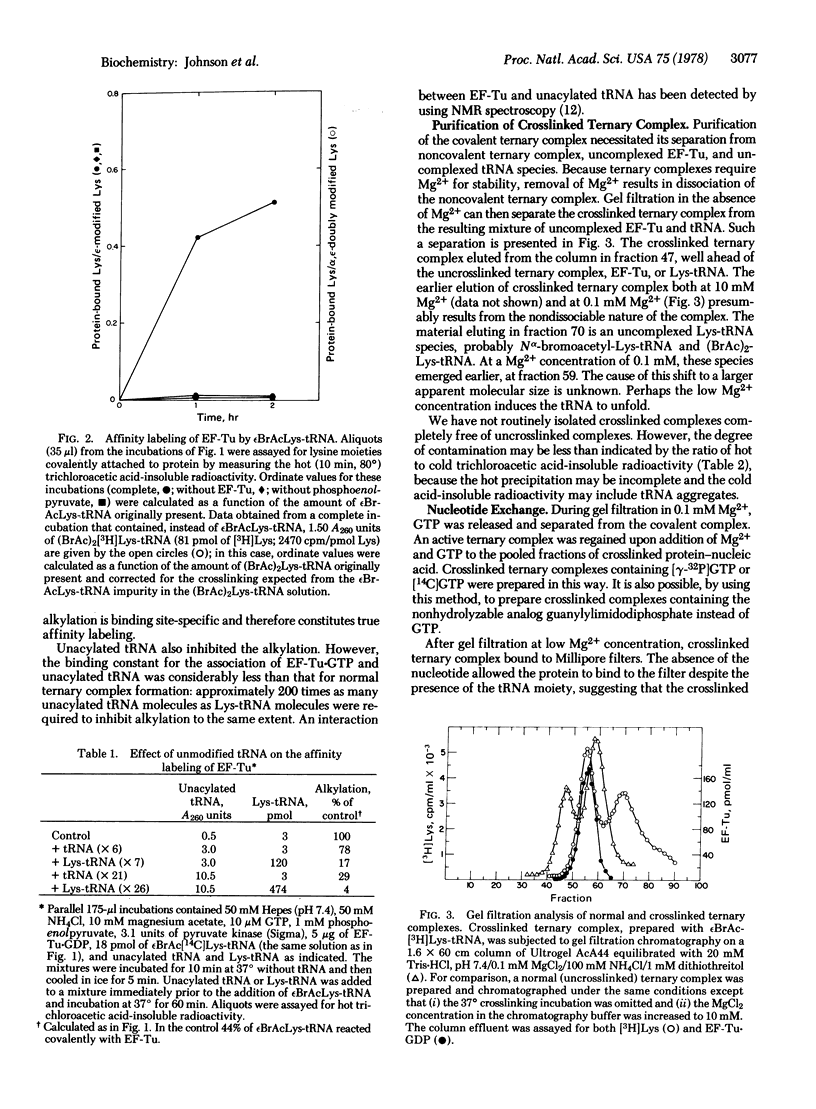
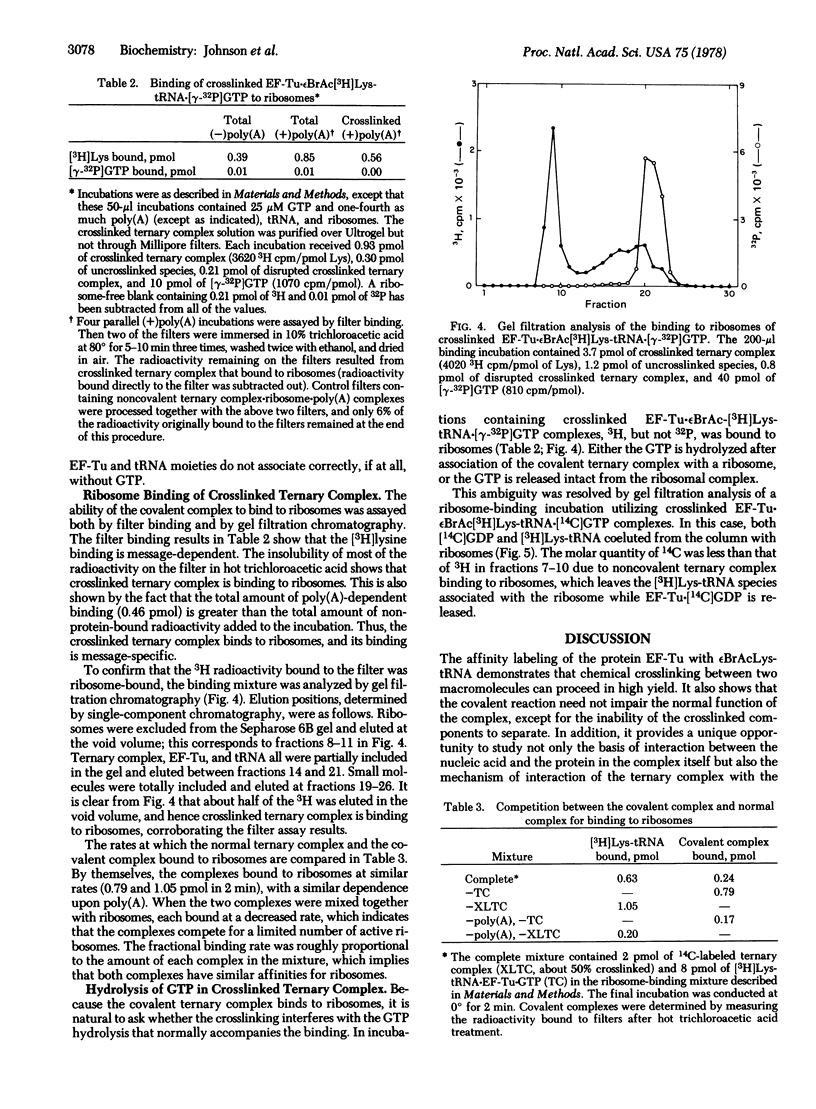
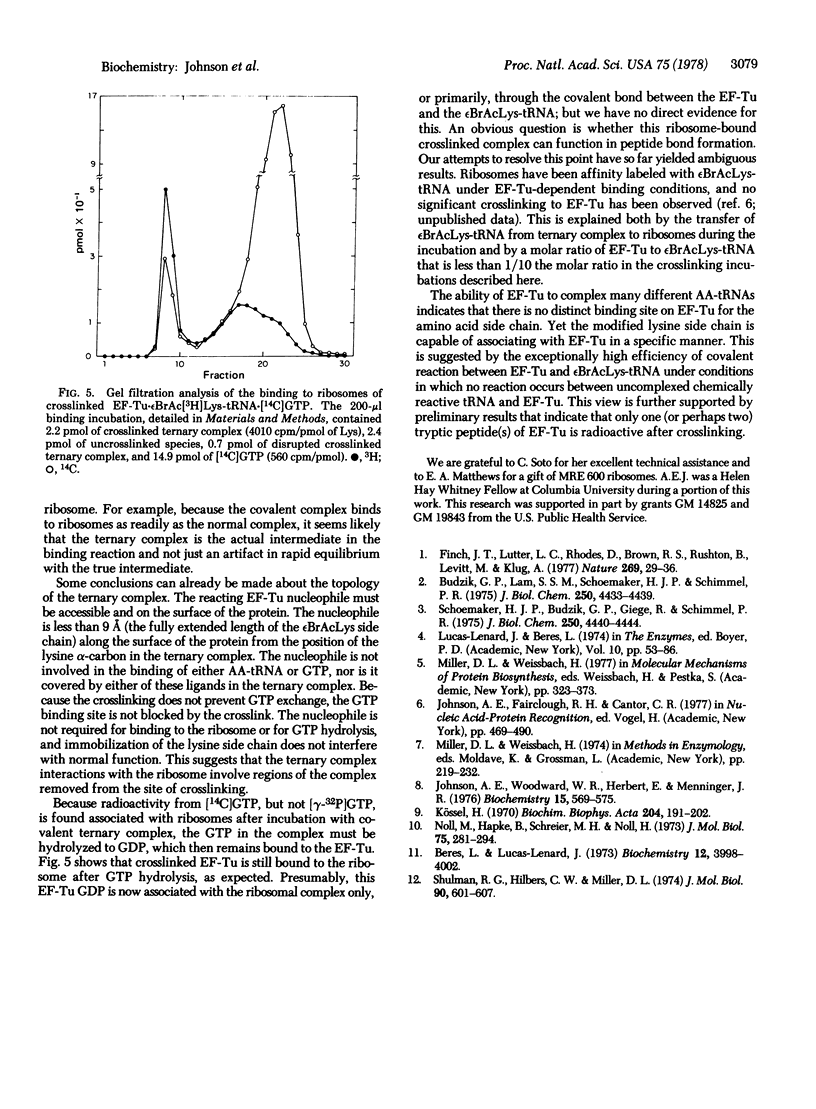
Complex formation between elongation factor Tu, GTP, and Nepsilon-bromoacetyl-Lys-tRNA results in the cross-linking of the protein and the modified Lys-tRNA. The efficiency of affinity labeling is greater than 50%. In the presence of unmodified Lys-tRNA, the amount of crosslinking is greatly decreased. There is no covalent reaction with elongation factor Tu in the absence of complex formation. Substantial purification of the crosslinked ternary complex can be achieved by gel filtration at low Mg2+ concentration and passage through nitrocellulose filters. The crosslinked complex exhibits message-dependent binding to ribosomes which is accompanied by the hydrolysis of the associated GTP, as shown by both filter assays and gel filtration profiles. The crosslinked complex therefore appears to function normally except for its inability to dissociate. These experiments demonstrate that the ternary complex is the true intermediate in the binding of aminoacyl-tRNA to the ribosomes.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beres L., Lucas-Lenard J. Studies on the fluorescence of the Y base of yeast phenylalanine transfer ribonucleic acid. Effect of pH, aminoacylation, and interaction with elongation factor Tu. Biochemistry. 1973 Sep 25;12(20):3998–4002. doi: 10.1021/bi00744a033. [DOI] [PubMed] [Google Scholar]
- Budzik G. P., Lam S. S., Schoemaker H. J., Schimmel P. R. Two photo-cross-linked complexes of isoleucine specific transfer ribonucleic acid with aminoacyl transfer ribonucleic acid synthetases. J Biol Chem. 1975 Jun 25;250(12):4433–4439. [PubMed] [Google Scholar]
- Finch J. T., Lutter L. C., Rhodes D., Brown R. S., Rushton B., Levitt M., Klug A. Structure of nucleosome core particles of chromatin. Nature. 1977 Sep 1;269(5623):29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Woodward W. R., Herbert E., Menninger J. R. Nepsilon-acetyllysine transfer ribonucleic acid: a biologically active analogue of aminoacyl transfer ribonucleic acids. Biochemistry. 1976 Feb 10;15(3):569–575. doi: 10.1021/bi00648a018. [DOI] [PubMed] [Google Scholar]
- Noll M., Hapke B., Schreier M. H., Noll H. Structural dynamics of bacterial ribosomes. I. Characterization of vacant couples and their relation to complexed ribosomes. J Mol Biol. 1973 Apr 5;75(2):281–294. doi: 10.1016/0022-2836(73)90021-1. [DOI] [PubMed] [Google Scholar]
- Schoemaker H. J., Budzik G. P., Giegé R., Schimmel P. R. Three photo-cross-linked complexes of yeast phenylalanine specific transfer ribonucleic acid with aminoacyl transfer ribonucleic acid synthetases. J Biol Chem. 1975 Jun 25;250(12):4440–4444. [PubMed] [Google Scholar]
- Schulman R. G., Hilbers C. W., Miller D. L. Letters to the editor: Nuclear magnetic resonance studies of protein-RNA interactions. I. The elongation factor Tu-GTP aminoacyl-tRNA complex. J Mol Biol. 1974 Dec 15;90(3):601–607. doi: 10.1016/0022-2836(74)90237-x. [DOI] [PubMed] [Google Scholar]


